Abstract
The beneficial effect of seed priming in improving critical growth stages like seed germination and early growth phases has been accepted by Plant Physiologists for many important field crops. In the present investigation, studies were made to see the effect of heavy metal stress imposed during germination using solution of HgCl2 in four different concentrations (0.0, 0.50, 0.75 and 1.00 mM) in Petri dishes on primed and non-primed seeds of wheat. Priming has been done with distilled water (hydro), Mg(NO3)2 and Ca(NO3)2 (halo) salts. Different germination parameters, such as germination percentage, radicle and plumule lengths, seedling emergence, soluble and insoluble sugar contents and activity of α-amylase in endosperm were studied at different study periods. Primed seeds increased all the germination parameters except insoluble sugar content in respect to non-primed control in the absence of HgCl2. However, the use of primed seeds has shown to overcome the inhibitory effects of heavy metal stress imposed in the form of HgCl2 solution during the period of germination. Hence, the work concludes the mitigating effects of priming under heavy metal stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals like cadmium, cobalt, nickel, copper and mercury, have toxic effects on plants and act as persistent pollutants in the atmosphere; these may be added either by natural or anthropogenic activities. Some metals precipitate readily in soil, some are immobilized in nature, in which the later considered more hazardous to plants. Among the various phases of plant’s life, seed germination represents the most important phase. Wang et al. (2003) reported that heavy metals affect seed germination which resultantly reduces root and shoot elongation, their dry weight and level of total soluble protein. Pourrut et al. (2011) reviewed that lead causes oxidative damage and loss of nutrients, alteration in membrane permeability and modulates sugar and protein metabolism, whereas Sethy and Ghosh (2013) studied the role of various heavy metals like Pb, Ni, Cd, Co, Cr and Hg on the process of seed germination of various crops and noted the toxic effects of all these metals on the loss in productivity. Seed germination inhibition by heavy metals applied in the form of lead nitrate, cadmium nitrate and mercuric chloride was observed in Cassia siamea L. and Zea mays L. crops, respectively (Shafiq and Iqbal 2005; Bose et al. 2008). Increased lead (lead nitrate) concentrations were found to inhibit seed germination, early growth of seedling, fresh and dry weights, length and total protein content of root/shoot in Zea mays L. (Hussain et al. 2013). Further, maize plants while treated with various heavy metals, Pb was found most toxic and decreased the growth and plant’s protein content (Ghani 2010). Although relative toxicity of different metals to plant can vary with the genotype and experimental conditions, most of the heavy metals act through the changes in the permeability of cell membrane, reactions of sulfhydryl (–SH–) groups with cations (Bose et al. 1983), affinity for reacting with phosphate group of ATP or ADP, replacement of essential ions, oxidative stress, etc. Germination and seedling growth of Arabidopsis were affected by the use of heavy metals like Cu, Hg, Cd and Pb, where the level of toxicity was greater with Cu followed by Hg, Cd and Pb (Li et al. 2005). However, mercury is one of the best known toxic metals discharged from human activities. The toxic effects of Hg on seed germination, growth, biochemical constituents and yield have been studied by various workers (Munzuroglu and Geekil 2002; Muddarisna et al. 2013). Seed priming, as a pre-sowing technique can improve root emergence and increase germination rate by making changes in metabolic activities in seeds (Taylor and Harman 1990; Bose and Mishra 2001) during the process of germination. However, hydropriming (using water) and halopriming (using salts) were introduced to improve germination speed, uniformity and also the allometry (changes in different parts of a plant over time) in several horticultural and field crops (Ashraf and Foolad 2005; Krishnottar et al. 2009; Srivastava and Bose 2012). In the present investigation, studies were made to find out the effect of heavy metal (HgCl2) stress on the morphophysiological and biochemical parameters of germinating wheat variety HUW-468, using hydro and halo primed seeds.
Materials and methods
Wheat seeds (Triticum aestivum L. HUW-468) were procured from the Department of Genetics and Plant Breeding, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi. Healthy and bold seeds were surface sterilized with 0.01 % HgCl2 solution for 3 min and then washed 5–6 times thoroughly by distilled water. Three priming treatments were used for this experiment included hydropriming (using distilled water) and halopriming, using two common salts, i.e., Mg(NO3)2 and Ca(NO3)2 [7.5 mM concentration (Das and Bose 1998) of each salt was selected for priming] with a non-primed treatment as control. For priming purpose the surface sterilized seeds were soaked in distilled water, and in 7.5 mM concentration of Mg(NO3)2 and Ca(NO3)2 solutions for 10 h. After completion of soaking time, the seeds were washed 2–3 times using distilled water and then kept under fan for drying till it attains its initial weight, thereafter the dried seeds were kept in paper bags and used as per the necessity during experimentation within 1 month. Twenty-five seeds of primed and non-primed sets were placed in Petri dishes (diameter 3.0 in.) and they were provided with a shock of four different concentrations (0.0, 0.50, 0.75 and 1.00 mM) of mercuric chloride solutions for 48 h. Thereafter, germinated seed/seedlings were removed from HgCl2 solutions and washings were made 4–6 times, using distilled water, then kept for another 48 h in distilled water for germination study. After 96 h of germination, only fully germinated seedlings were maintained in experimental pots (having sufficient moisture content) and the rest were removed. These pots were kept in the laboratory to maintain the controlled conditions. The experiments were conducted using completely randomized design (CRD) in factorial arrangement with four treatments and each treatment was replicated three times. Experiment was carried out in the Seed Physiology Laboratory, Department of Plant Physiology, Institute of Agricultural Sciences, B.H.U., Varanasi (U.P.) India.
The germination studies were carried out in Petri dishes to measure germination parameters (germination percentage, plumule and radicle lengths). Germinated seeds were counted daily and percent germination was calculated using the following formula, considering the number of germinated seeds only at the end of studied period.
Radicle and plumule lengths of germinated seeds were measured using graph papers and they were calculated per seed basis using the following formula.
where, L 1 + L 2 + L 3 + ············ + L n : represents radicle length of the germinated seeds present in the Petri dish.
N is the number of seeds placed in the Petri dish for study.
Plumule length of all germinated seeds was measured using the following formula.
where, X 1 + X 2 + X 3 + ········· + X n : represents plumule length of the germinating seeds.
N is the number of seeds placed in the Petri dish for study.
The α-amylase, soluble and insoluble sugar contents were measured in the endosperm, obtained from 48 to 96 h germinated wheat seeds using the methods of Bernfeld (1955) and Dubios et al. (1956), respectively.
Statistical analysis
Mean values were taken from each treatment of three independent replications; and Statistical Package for Social Science (SPSS Version 16.0) was used for the analysis of variance. Significant differences among various treatments were determined using Duncan’s test.
Results
Study regarding percent germination of wheat (variety HUW-468) seeds was made using different concentrations (0.0, 0.50, 0.75, 1.00) of HgCl2, and the data were presented in Table 1. Result showed that primed seeds with Mg(NO3)2, Ca(NO3)2 and distilled water (hydro) significantly increased germination percentage; this study was conducted from 12 to 72 h. At 12 h, the germination was started in primed as well as in non-primed sets; among the primed sets Mg(NO3)2 (haloprimed) treatment (T3) showed 94 % germination and that was followed by Ca(NO3)2 (halo primed) (T4) and hydro primed (T2) one, whereas the non-primed control (T1) was found to be poor in this respect, the later showed only 34 % of germination. With the application of HgCl2 percent germination was found to reduce in primed as well as in non-primed sets; however, the concentrations of HgCl2 more than 0.5 mM, i.e., 0.75(H2) and 1(H3)mM failed to show any germination at 12 h. At 48 h, 25 % germination was recorded in 1 mM HgCl2 treated non-primed sets. The result suggested that 0.75 and 1 mM HgCl2 treated non-primed seeds showed only 68 and 47 % germination, respectively, whereas hydro primed as well as Mg(NO3)2 and Ca(NO3)2 primed sets showed 98–100 % germination at 72 h.
Tables 2 and 3 are representing the radicle and plumule lengths of the germinating seeds at 60, 72 and 84 h. Radicle and plumule lengths were found to be significantly affected by HgCl2. The seed priming with hydro, Mg(NO3)2 and Ca(NO3)2 were found to maintain significantly higher radicle and plumule lengths. The non-stressed control sets showed maximum radicle and plumule lengths, whereas with increased metal stress level it declined constantly. At 84 h, during non-stressed control condition maximum radicle length was recorded in Ca(NO3)2 primed set while during stressed condition Mg(NO3)2 primed set showed maximum value. Mean values also supported the data of main table and the trend was same in all the three studied hours regarding radicle length. The same pattern was also noted in the case of plumule length at all the mentioned study periods. However, at 84 h no remarkable differences were visualized with hydro and nitrate primed sets.
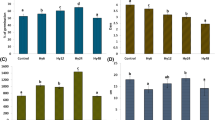
The activity of α-amylase enzyme of wheat endosperm was measured at different studied periods (Fig. 1a). The result clearly showed that at both the studied hours, i.e., 48 and 96, the increasing concentrations of HgCl2 significantly inhibited the activity of enzyme in respect to non-stressed control sets, however, when non-primed sets were compared with hydro and nitrate primed sets α-amylase activities were observed to improve significantly even in the presence of higher concentrations of HgCl2, i.e., at 0.75 and 1 mM. The mean data suggested that the Mg(NO3)2 was found best among all the sets in improving the α-amylase activity in respect to varying concentrations of HgCl2.
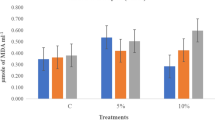
a–c Effect of varying concentrations of HgCl2 on α-amylase activity, insoluble sugar and soluble sugar content in the endosperm of hydro, nitrate salt primed and non-primed seeds of wheat variety HUW-468. Data presented are means from three replicates with standard errors. Within each treatment, different letters indicate significant differences by Duncan’s multiple range test at P < 0.05 where: (1) T 1, T 2,T 3 and T 4 are non-primed, hydro, Mg(NO3)2 and Ca(NO3)2 halo primed seeds, respectively. (2) H 0, H 1, H 2 and H 3 are different concentrations (0, 0.5, 0.75 and 1 mM) of HgCl2
It was noted from the Fig. 1b that insoluble sugar was found to decrease with the increasing hours of germination in both primed and non-primed sets. Regarding varying concentrations of HgCl2 it has been visualized that as the concentration of HgCl2 increased retaining of the insoluble sugar in the endosperm increased; this showed that HgCl2 interfered with the degradation of insoluble to soluble sugar during germination. However, the use of primed seeds, mainly Mg(NO3)2 was significantly found to improve the rate of degradation of sugar by decreasing its amount in the endosperm of germinating wheat seeds in the absence as well as in the presence of HgCl2. The same trend was observed from the mean values of 96 h germinated seeds (Fig. 1b).
Regarding changes in soluble sugar of endosperm during the germination of wheat seeds showed that in HgCl2 treated sets the soluble sugar content was found to be significantly decreased with increasing concentrations of this metal chloride (HgCl2) at both the studied hours, i.e., 48 and 96 h (Fig. 1c). In Mg(NO3)2, primed sets soluble sugar in endosperm significantly increased which has been clearly visualized in the 48 h of study, whereas at 96 h only magnesium nitrate treated set showed more soluble sugar content in respect to non-primed set in the presence of 1 mM HgCl2.
Study of seedling emergence has been presented in Table 4. In this case, after 48 h of HgCl2 treatment to the primed and non-primed wheat seeds followed by another 48 h in the absence of HgCl2, the seeds/seedlings were shifted to the pots and the study regarding emergence of seedling was observed at 6 DAS. The hydro and Mg(NO3)2 primed seeds showed very little difference in seedling emergence in respect to Ca(NO3)2 and non-primed control sets when they were not subjected to any phytotoxic treatment of HgCl2. It was observed that 0.5 mM concentration of HgCl2 had the capacity for higher seedling emergence in wheat variety HUW-468, but after that it was decreased with increasing concentration up to 1 mM of the HgCl2. The mean value suggested that Mg(NO3)2 treated sets were always found significantly better among all the primed sets in emerging the seedlings except at 0.75 mM of HgCl2(H2) treatment level where hydro primed set showed higher (82.67 %) seedling emergence.
Discussion
Results clearly suggested that the heavy metal HgCl2 showed its toxicity in inhibiting the germination in non-primed control sets; however, the inhibition caused due to the presence of HgCl2 was reduced, using hydro and haloprimed (nitrate salts primed) seeds. Yaksha et al. (2011) suggested that putrescine hardened (a kind of priming) seeds of Brassica showed a good percent of germination even in the presence of heavy metals. This report supports this study where the hydro and nitrate primed (a kind of hardening) seeds showed an ability to overcome the heavy metal toxicity by improving their percent germination. An inhibition in the seed germination and growth of plumule and radicle has been investigated and exhibited by number of workers regarding many crops with the application of HgCl2 (Ling et al. 2010; Lan-fang et al. 2004). Use of increasing concentration (0.0, 0.50, 0.75, 1 mM) of HgCl2 decreased radicle length, whereas primed sets showed more radicle length in comparison to non-primed one. The use of primed seeds had the capacity to enhance the radicle length in normal as well as in HgCl2 treated sets. However, it can be suggested that priming of seeds can overcome the inhibition of germination and radicle and plumule growth, even in the presence of phytotoxic metal like HgCl2. Bose et al. (2008) studied the phytotoxic effect of HgCl2 on seed germination and seedling growth of Maize. They also observed that HgCl2 inhibited α-amylase activity in maize endosperm during germination. However, this might be the first report where it has been observed that priming/hardening of wheat seed of variety HUW-468 may mitigate phytotoxic effect of HgCl2 during germination via improving α-amylase activity in germinating endosperm. Ling et al. (2010) reported that the low concentrations of HgCl2 had short term promoting action on wheat seedling emergence, which has been also observed in this study; this type of increase in seedling growth suggests that low concentration of toxic metal may induce the growth process as an eustress, which actually stimulates the internal metabolic activities for overcoming the low stressful situation. Yaksha et al. (2011) reported that harden seeds has better effect on the seedling growth than non hardened seeds of Brassica spp. Bose et al. (1982) while working with maize seed germination under the influence of various nitrogenous salts reported that nitrogenous salts were able to improve not only the degradation of the stored material but also it induces the rate of mobilization of degraded materials towards embryo. The same might be occurring in the present piece of work where the rate of degradation in nitrate primed seeds were more which has been realized from the data of the 48 h of study period and its mobilization might be higher which has been realized from the study of soluble sugar content at 96 h. Anayatullah and Bose (2007) also found that nitrate primed seeds improved the activity of α-amylase enzyme and soluble sugar content in germinating wheat seeds. However, Bose and Pandey (2003) reported that Mg(NO3)2 and Ca(NO3)2 both increased the electrical conductivity (dS m−1) of germinating Okra seeds as compared to distilled water treated one, and it also improves the activity of nitrate reductase enzyme in 24 h germinating seed/seedling (Cotyledon + Plumule + Radicle).They also suggested that the influx of ions of these salts inside the seed may increase during soaking (a kind of priming), as a result, the process of germination becomes fasten which may further overcome a number of stresses including heavy metal one as found in the present case. Hence, it may be suggested that the inhibitory effect of HgCl2 on seed germination and seedling growth of wheat can be alleviated by hydro and nitrate salt priming. Molecular mechanism of priming of nitrate salts verses HgCl2 in respect to stress alleviation can unveil the study in proper direction.
Conclusion
The study concludes that heavy metal stress, imposed using varying concentrations of HgCl2 caused a significant reduction in germination percentage, radical and plumule lengths and seedling emergence percentage in respect to control non-stressed wheat seeds. Halo priming of seeds with Mg(NO3)2 markedly reduced/mitigated the harmful effects of HgCl2 stress and also improved all the measured parameters significantly during germination. Effect of seed priming with Mg(NO3)2 appeared to improve germination at different period in the presence of HgCl2 may be influencing the rate of metabolism which may also differ with the changing ratio of heavy metal stress.
Author contribution statement
Experiments of the present piece of work were done by M. Kumar (first author) and analyses were performed by S. Mondal and B. Pant. However, compilation of the whole manuscript was done/written by Prof. B. Bose (Supervisor) and M. Kumar.
References
Anaytullah, Bose B (2007) Nitrate-hardened seeds increase germination, amylase activity and proline content in wheat seedlings at low temperature. Physiol Mol Biol Plants 13:199–207
Ashraf M, Foolad MR (2005) Pre-sowing seed treatment—a shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv Agron 88:223–271
Bernfeld P (1955) Amylases a and b. Methods Enzymol 1:149–158
Bose B, Mishra T (2001) Effect of seed treatment with magnesium salt on growth and chemical attributes of mustard. Indian J Plant Physiol 6 (4 new series):431–434
Bose B, Pandey MK (2003) Effect of nitrate pre-soaking of okra seeds on growth and nitrate assimilation of seedling. Physiol Mol Biol Plants 9:287–290
Bose B, Srivastava HS, Mathur SN (1982) Effect of some nitrogenous salt on nitrogen transfer and protease activity in germination of maize (Zea Mays L.) seeds. Biol Plant 24:89–95
Bose B, Srivastava HS, Mathur SN (1983) Partial purification and properties of protease from maize seed endosperm. Beitr Biol Pflanz 58:383–391
Bose B, Anaytullah KS, Srivastava AK, Kuril SK, Singh PK (2008) Effect of mercuric chloride on seed germination, seedling growth and enzyme activities in maize (Zea mays L.). Indian J Plant Physiol 13:284–290
Das GV, Bose B (1998) Influence of soaking of wheat seed in nitrates of magnesium and calcium on certain physiological characters of the germinating seed. Andhra Agric J 45:47–50
Dubios M, Gilles RA, Hamilton JK, Robers PA, Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal Chem 28:350–356
Ghani A (2010) Toxic effects of heavy metals on plant growth and metal accumulation in maize (Zea mays L.). Iran J Toxic 3:326–334
Hussain A, Abbas N, Arshad F, Akram M, Khan ZI, Ahmad K, Mansha M, Mirzaei F (2013) Effects of diverse doses of lead (Pb) on different growth attributes of Zea mays L. Agric Sci 4:262–265
Krishnotar Bose B, Srivastava AK, Shahi JP (2009) Response of rabi maize crop to seed invigoration with magnesium nitrate and distilled water. Indian J Plant Physiol 14:71–77
Lan-fang DU, Zong-gen S, Da YU, Wei-hong LIU, Yan DENG, Yin-fen Z (2004) Toxological effect of mercury stress on pea seed. Sinica 24:2266–2271
Li W, Mao R, Liu X (2005) Effects of stress duration and non-toxic ions on heavy metals toxicity to Arabidopsis seed germination and seedling growth. Ying Yong Sheng Tai Xue Bao 16:1943–1947
Ling T, Fangke Y, Jun R (2010) Effect of mercury to seed germination coleoptile growth and root elongation of four vegetables. Res J Phytochem 4:225–233
Muddarisna N, Krisnayanti BD, Utami SR, Hanayanto E (2013) Effects of mercury on growth and biochemical constituents of maize seedlings. Plant Sci Feed 3:53–56
Munzuroglu O, Geekil H (2002) Effect of metals on seed germination, root elongation and coleoptiles and hypocotyls growth in Triticum aestivum and Cucumis sativus. Arch Environ Contam Toxicol 43:203–213
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol 213:113–136. doi:10.1007/978-1-4419-9860-6_4
Sethy SK, Ghosh S (2013) Effect of heavy metals on germination of seeds. J Nat Sci Biol Med 4:272–275. doi:10.4103/0976-9668.116964
Shafiq M, Iqbal MZ (2005) The toxicity effects of heavy metals on germination and seedling growth of Cassia siamea Lamark. J New Seeds 7:95–105
Singh Y, Malik CP (2011) Phenols and their antioxidant activity in Brassica juncea seedlings growing under HgCl2 stress. J Microbiol Biotech Res 1:124–130
Srivastava AK, Bose B (2012) Impact of seed hardening treatment with nitrate salts on nitrogen and anti oxidant defence metabolisms in Triticum aestivam L under deference sowing conditions. Vegetos 25:292–299
Taylor AG, Harman GE (1990) Concepts and technologies of selected seed treatments. Ann Rev Phytopathol 28:321–339
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14. doi:10.1007/s00425-003-1105-5
Acknowledgments
Authors are grateful to Prof. Ram Dhari, Department of Genetics and Plant Breeding, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, for providing the seeds to conduct the present investigation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M Horbowicz.
Rights and permissions
About this article
Cite this article
Kumar, M., Pant, B., Mondal, S. et al. Hydro and halo priming: influenced germination responses in wheat Var-HUW-468 under heavy metal stress. Acta Physiol Plant 38, 217 (2016). https://doi.org/10.1007/s11738-016-2226-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2226-3