Abstract
Maize is an important crop worldwide. Its grain yield is susceptible to decrease under conditions of abiotic stress, such as shade in subtropical and temperate zones. The genetic basis of shade tolerance has not been determined in maize. MicroRNAs (miRNAs) are known to play critical roles in plant stress responses, including responses to environmental stress; but shade-associated miRNAs have not previously been identified in maize. In this study, the shade-sensitive inbred line 502 was used to examine miRNA expression differences in maize ear, after a 10-day treatment of either shade or exposure to natural light. A total of 130 known miRNAs belonging to 21 families were identified, of which 45 miRNAs were differentially expressed between shaded and natural light treatments. Twelve novel miRNAs were also predicted. In total, 94 miRNAs were upregulated and 48 downregulated in plants exposed to shaded conditions, compared with those exposed to natural light. These differentially expressed miRNAs may participate in regulating hormone homeostasis, metabolism, development and flower timing. These results suggest that the decrease of maize yield under shaded conditions may partly be determined by the differential expression of shade-induced miRNAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize depends on sunlight, being photosynthetic, but in many areas of the world, maize-growing regions are cloudy and overcast when crops are in their later growth stages, for example on the North China Plain (Jin et al. 1996). Pressure to increase grain yield leads to a greater density of planting, leading in turn to self-shading among maize plants. Persistent shading during flowering affects maize development, and reduces grain yield. Reed et al. have previously reported that photosynthesis decreases, and kernel abortion increases, when plants are shaded during flowering (Reed et al. 1988). Tollenaar et al. and Gerakis et al. have also reported on the effect of shade stress on grain yield in maize (Tollenaar and Daynard 1978; Gerakis and Papakosta-Tasopoulou 1980), and that kernel weight and yield decrease when plants are shaded during grain filling (Schmidt and Colville 1967; Kiniry and Ritchie 1985). Some quantitative trait loci for shading tolerance have previously been identified (Yuan et al. 2012). However, the genetic basis of the effect of shade on ear development in maize, particularly the role of regulatory miRNAs, has hitherto been unclear.
In recent years, numerous small RNAs have been discovered that have roles in post-transcriptional regulation of gene expression during development (Kozomara and Griffiths-Jones 2011), including microRNAs (miRNA). MicroRNAs are a class of endogenous small RNAs, 20–24 nt long, which play an important role in plant growth, development and stress responses (Bartel 2004). Allowing plants to adapt to environmental pressure, miRNAs can be up- or downregulated to cope with stresses such as nutrient starvation (Fujii et al. 2005), drought (Zhou et al. 2010), cold (Zhou et al. 2008), salinity (Sunkar et al. 2008), UV-B radiation (Zhou et al. 2007), and mechanical stress (Lu et al. 2005).
Achard et al. found that miR159 expression could regulate gibberellin (GA) or abscisic acid (ABA) to control floral organ development (Achard et al. 2004); and that miR160 was upregulated, and miR169 and miR167 were downregulated in response to ABA (Liu et al. 2007, 2009; Li et al. 2008). Expression of miR168, miR171 and miR396 were found to change in Arabidopsis thaliana plants coping with high salinity, drought and cold pressure; and miR397, miR165/166, miR393, miR396, miR169 and miR172 were found to have up- or downregulated expression in response to cold and heat stress (Sunkar and Zhu 2004; Liu et al. 2008; Lu et al. 2008). Currently, sequences of 321 maize microRNAs, 713 rice microRNAs and 427 Arabidopsis miRNAs have been deposited in miRBase21 (http://www.mirbase.org/). Although miRNAs from several plant species have been identified as having changes in expression related to environmental stress, no extensive studies exist of maize miRNAs in response to shading.
High-throughput sequencing technology has been proven to be useful and cost-effective in discovering stress-related miRNAs. To examine the differential accumulation of miRNAs in maize ears developing for 10 days under either shade or and natural light, high-throughput sequencing and qRT-PCR were performed. The differential expression of miRNAs was analyzed in the context of maize ear development under natural light and shading.
Materials and methods
Material and treatments
An inbred line of maize with shade-sensitive characteristics, 502, was selected from the local Chinese germplasm, Tangsipingtou (Liuzheng et al. 2008). In spring 2012, the inbred line was evaluated under shading and natural light treatments, at the farms of Henan Agricultural University (Zhengzhou, 34°48′N 113°42′E) in northern China. The farms have an average yearly temperature of 14.3 °C and an average annual rainfall of 640.9 mm. Sowing took place on 30 May 2012. The inbred line was sown in a single plot, consisting of six rows planted 60 cm apart; each row being 5 m long, with 22 plants, planted 23 cm apart. During the seedling stage, 175 kg N ha−1 (urea), 67.5 kg P2O5 ha−1 (calcium superphosphate), and 101.3 kg K2O ha−1 (potassium nitrate) were added to the soil, and an additional 175 kg N ha−1 (urea) was added before pollination. The shading treatment was as described by Fournier and Andrieu (2000), except that plants were planted in a 3.5 m high isolation chamber, and shaded from 7 days before tasseling, for 10 days. Shading was with black polypropylene fabric with 50 % light penetration while controls developed under natural light (light intensity 1538.6 μmol m−2 s−1). The only top of the shade shelf and the east–west sides 2 m from the laying down were shaded and there were 2 m distance from the top of shed to canopy in order to create the microclimate of shed according to field (Table 1). The data in the table are used Li-6400 Portable Photosynthesis system (LI-COR Inc. USA) in 11:00 am every day, and lasted 10 days. Ear samples from each treatment were collected on the tenth shaded day; ears from nine plants per treatment were pooled into two samples, and the samples were collected from 7 to 8 a.m. quick-frozen in liquid nitrogen, and stored at −80 °C.
RNA isolation and miRNA library construction and sequencing
Total RNA was isolated from the pooled samples of nine ears as three biological replicates for both shaded and natural-light treatments, using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNase-free DNase I was used to remove any residual DNA for 30 min at 37 °C. sRNAs 18–30 nt in length were purified from total RNA, on a 15 % polyacrylamide denaturing gel. The isolated sRNAs were sequentially ligated to RNA-DNA chimeric oligonucleotide adaptors, reverse transcribed, and amplified with 15 cycles of PCR to produce sequencing libraries (Lau et al. 2001). Sequencing was performed on a Solexa Illumina Genome Analyzer at BGI (Beijing Genomics Institute, Shenzhen, China).
Identification of known and novel miRNAs
Raw sequence processing was done with the PHRED and CROSS MATCH programs, as previously reported (Sunkar and Zhu 2004; Sunkar et al. 2005). After deletion of the vector sequence, any sequences of 18 nt or longer were used for further analysis. Initially, tRNA, snRNA, snoRNA, rRNA and poly-A tails were deleted from among the small RNA sequences. The remaining sequences were compared with the maize ncRNA collections in NCBI Genbank and Rfam. To identify conserved miRNAs in maize, unique small RNA sequences were used for searching the miRNA database using BLASTn. Only the small RNA sequences that had precursor and mature forms that exactly matched known maize microRNAs in miRBase 15.0, were regarded as being conserved microRNAs. To find possible novel miRNA precursor sequences in our dataset, mature miRNAs were used in a BLASTn search, with results including only sequences that met the standards of previously-described miRNA precursors (Ambros et al. 2003). These were mature sequences in the stem region of the stem-loop structure, 20–22 nt long, with a maximum free-folding energy of −20 kcal mol−1. A minimum of 50 reads at least in one library and a maximum of six unpaired nucleotides between the miRNA and miRNA* were allowed. If the distance between the miRNA and miRNA* was between 5 and 240 nt, the sequence was folded into a secondary structure using mFold 3.2. If a perfect stem-loop structure was formed, the small RNA sequence met the criteria and would be regarded as a possible novel maize miRNA candidate.
Real-time quantitative PCR
The expression levels of the predicted target genes and miRNAs were estimated by qPCR, Both RT-PCR and Solexa sequencing were done on the same RNA samples. Eight conserved nature miRNAs and a novel miRNA and eight target genes were selected for further real-time PCR analysis. Reverse transcription reactions were done with the One Step PrimeScript® miRNA cDNA Synthesis Kit (Takara). 20 μL RT reactions were incubated at 37 °C for 60 min, then at 85 °C for 5 s, then stored at 4 °C, following the manufacturer’s protocol. Real-Time PCR was performed with SYBR Premix Ex Taq II™ (Takara) on a Bio-Rad IQ5 Real-Time PCR Detection System (BIO-RAD, USA). Each 25 μl PCR reaction solution included 2 μl cDNA, 1 μl PCR forward primer (10 μM), 1 μl Uni-miR qPCR primer (10 μM), 12.5 μl SYBR premix EX TaqII (2×), and 8.5 μl dH2O. The reactions were incubated at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 20 s, and 72 °C for 10 s. All reactions were replicated three times for each sample. The relative expression level of the miRNAs was calculated using the 2−ΔΔCT, and these data were normalized on the basis of Actin-2 values, Actin-2 (act2) was used as the endogenous control, the miRNA used for normalization were those expressed during natural light. All primer sequences are listed in (S2 Table and S6 Table).
Novel miRNA target prediction
We used the Mireap tool to predict the target genes of newly identified miRNAs (Allen et al. 2005; Schwab et al. 2005). Briefly, the rules used were: (1) sRNA and target genes may not have more than four mismatches (G-U changes count as 0.5 mismatches); (2) in the miRNA/target duplex, there may not be more than two adjacent mismatches; (3) sites 2–12 of the miRNA/target duplex (5′ end of miRNA) must not have adjacent mismatches; (4) the miRNA/target duplex must not have mismatches in positions 10–11; (5) the miRNA/target duplex must not have more than 2.5 mismatches in positions 1–12; and (6) the minimum free energy (MFE) of the miRNA/target duplex should not be less than 75 % of the MFE of the miRNA bound to its exact complement. All newly-identified miRNAs predicted to target mRNA sequences met the above criteria. In order to validate the predicted target, then qPCR was carried to analysis the expression levels of the target genes of novel miRNAs, (all primer sequences are listed in S7 Table).
Gene ontology (GO) analysis
To further understand the function of the miRNAs that were differentially expressed between shading and natural light treatments, we used the GO analysis tool, AgriGO (http://bioinfo.cau.edu.cn/agriGO/analysis.php) (Du et al. 2010) with the TIGR maize V5a genome transcript IDs as a background/reference dataset. This assigned putative functions to target genes.
Results
The appearance of maize ears under shading and natural light
The inbred line 502 under Shading condition showed a slowdown of ear and tassel growth and development rate, delay the tasselling date, silking date, prolonged the anthesis-silking interval (ASI). The ears of inbred line 502 were significantly affected after 10 days shading (Fig. 1). In comparison with the natural light treatment, ears grown in the shade were 52.3 % shorter (average length reduction 6.7 cm); and their diameters were 16.7 % less (average diameter reduction 0.4 cm). All data were subjected to t test in SPSS statistical program. It’s obvious that the difference between the shade and the natural light treatment was significant as indicated by statistical test listed in Table 2.
Sequencing and annotation of maize miRNAs
As given in Table 3, Solexa sequencing produced 16,794,262 unfiltered sequence reads in the natural light library, and 17,729,648 unfiltered sequence reads in the shade library. Of these, 12.91 % of the reads (of which 37.49 % were unique) were found to be specific to natural light treatment; 14.32 % (of which 42.36 % were unique) were found to be specific to the shading treatment, and the remaining 72.77 % of the reads (of which 20.15 % were unique) were present in both libraries. The mean frequency of reads in the shade-specific library was 1.22, while reads in the natural light library had a mean frequency of 1.20, and the mean frequency of reads both in natural light library and shade-specific library was 12.79.
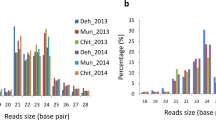
After filtering, a total of 16,489,436 reads (of which 5,521,450 were unique) remained in the natural light library; and 17,427,810 reads (of which 5,987,732 were unique) remained in the shade library. Plotting their size distribution (Fig. 2) shows that ~97 % of the sequences in both libraries were 18–21 nt long, with a mode length of 21 nt, which corresponds to the typical length distribution of mature plant miRNAs (Sunkar and Zhu 2004).
Known miRNAs
Non-protein coding sequences (including rRNA, tRNA, snRNA and snoRNA) were identified by searching the Rfam10.1 and NCBI Genbank databases. Comparing filtered sequences with known non-protein coding sequences, 0.64 % of the unique sequences in the natural light library, and 0.55 % of the unique sequences in the shade library had matches.
Comparing filtered reads with known miRNAs, 351,236 (of which 353 were unique) were identified in the natural light library, and 346,544 (of which 416 were unique) in the shade library, respectively accounting for 2.13 and 1.99 % of each library (Table 4). A BLASTN search of the generic miRNAs resulted in the identification of 130 sequences belonging to 21 families (S1 Table). Most miRNAs were more highly expressed under shading conditions than under natural light. The most abundant sequences expressed under shade conditions were miR528a-5p, miR528b-5p, miR390a-5p, miR390b-5p, miR156j-5p, miR1432-5p, miR166a-5p, miR166c-5p and miR168a-3p; while miR529-5p, miR319a-5p, miR319c-3p, miR319b-3p, miR319d-3p, miR156b-5p, and miR156d-5p were abundant under natural light conditions (Fig. 3a, b). In both libraries, miR1432-5p, miR390a-5p, miR390b-5p, miR319a-3p, miR319c-3p, miR319b, miR319d-3p, miR156b-5p and miR156d-5p were moderately abundant (S1 Table). Eleven miRNAs, including miR528a-5p, miR156b-5p, miR166a-5p, miR156j-5p, miR166c-5p, miR166 g-5p, miR166 m-5p, miR319a-5p, miR156c-5p, miR172c-5p and miR529-5p, differed in expression but were present in both libraries.
Comparison of different expressions of known miRNAs between natural light and shading treatments. a Ratio (shading/natural light) of normalized numbers of miRNA sequence reads (known miRNAs) with at least two times higher expression in shade than in natural light, and at least 20 reads in one of the data sets. b Ratio (natural light/shading) of the normalized numbers of miRNA sequence reads of (known miRNAs) with at least two times higher expression in natural light than shade ear, and at least 20 reads in one of the data sets
Novel miRNAs
The ability of their pre-miRNA sequences to adopt the canonical stem-loop hairpin structure is a differentiating feature of miRNAs, meaning that a set of precise rules can be used to identify potential miRNAs (Jones-Rhoades et al. 2006; Meyers et al. 2008). There were 12 novel miRNAs present in either or both libraries (Table 5). Their stem-loop hairpin structures were determined (S3 Table). Of these miRNAs, novel-mir-1918, novel-mir-2159, and novel-mir-2281 were present in the shade library; novel-mir-418, novel-mir-583, novel-mir-665 were present in the natural light library; and novel-mir-69, novel-mir-123, novel-mir-1274, novel-mir-720, novel-mir-762, and novel-mir-890 were present in both libraries.
Differentially expressed miRNAs and qRT-PCR
Eight conserved miRNAs and one novel miRNA were chosen to validate the sequencing results by qRT-PCR analysis. These results indicated that the relative expression levels of selected miRNAs in maize ear were consistent with the deep sequencing data. Evidence for the differential expression of miRNAs in the ear of the inbred line 502, under natural light and shading treatments, was sought by comparing the frequency of occurrence of the 130 conserved and 12 novel miRNAs, based on a normalized value of at least 10 in one library. The greatest degree of differential expression was shown in 16 conserved miRNAs and six novel miRNAs (Table 6). Of these, two showed a significantly greater than twofold difference in expression level, between natural light and shading treatments, which was further verified by by qRT-PCR (Fig. 4a).
Function of different expression of miRNAs under natural light and shading
From the set of 16 conservative mature miRNA sequences, which showed differential expression between natural light and shading treatments, 306 potential target genes were predicted (S4 Table). These targets include proteins, DNA binding domains, transcription factors (TFs), and ATP binding domains. Most of the targets of the conservative miRNAs have many TFs, including SBP, SPL, MYB, ARF and NAM, which are known to regulate plant development. All of these TFs are known to have roles controlling gene expression involved in flower timing, metabolism and development. Furthermore, monitoring the expression levels of eight predicted target genes by qPCR analysis revealed negative correlations with the levels of their corresponding miRNAs (Fig. 4b).These results implied that these miRNAs may be directly or indirectly involved in maize shading stress through regulation of target gene expression. To validate the target genes of novel miRNAs, qPCR was carried to analysis the expression levels of three novel miRNAs and target genes, showing that the expression trends of the target genes were contrary to the novel miRNAs (Fig. 5), it indicated that microRNAs targets are cleaved sequences by novel microRNAs. To analyze the functions of the novel miRNAs, 229 target mRNAs (S5 Table) were submitted to the AgriGO website (http://bioinfo.cau.edu.cn/agriGO/analysis.php). Compared with their respective background/reference sequences, the novel miRNAs were categorized as having diverse functions (Fig. 6). The most highly-represented functions with a significant difference from the background/reference sequences were metabolic processes (75.6 %), cellular processes (63.3 %), biological regulation (23.3 %), response to stimuli (18.9 %) regulation of biological processes (18.9 %) and signal processing (5.6 %). These functions are important to the development of maize ears both under shading and under natural light. Further analysis of their targets will clarify how these newly identified miRNAs function in maize under shade stress.
Distribution of differentially expressed novel miRNA target genes and their functional categories as determined by GO analysis. The x-axis shows categories of biological functions; the y-axis represents the proportion of gene function. Blue bars show the percentage of input genes that were expressed under natural light and shade; green bars show the percentage of background/reference target genes. Where input genes show a different percentage from background genes, the function may be regulated by novel miRNAs
Discussion
Here, we have sequenced and analyzed the miRNAs that are produced in the female floral organ (ear) of maize, under shaded and natural light conditions. The targets of some of the miRNAs identified, which are mainly TFs, are known to regulate physiological processes and genetic programs including metabolism, flowering, hormone signaling and stress responses. For example, some of the conserved miRNAs, miR156, miR172, miR171, miR166, miR159, miR319, miR160 and miR164 are known to function in flower development. The miR156 family regulates TFs of the SQUAMOSA promoter-binding like (SPL) family, which regulates the timing of development; and the change from vegetative to reproductive growth, in combination with miR172 (Gandikota et al. 2007). Curaba et al. have shown that in barley, miR171 and its targets in the miR156 pathway, combined with miR156 and miR172, have a role in creating a pleiotropic phenotype that includes short vegetative phytomers and late flowering (Curaba et al. 2013).
A number of miRNAs have been shown to participate in regulation of responses to abiotic stress, such as salinity (Zhou et al. 2010), cold (Sunkar et al. 2008) and dehydration (Zhou et al. 2008). Banu et al. showed that Osa-miR528 is highly-expressed under salt stress, where superoxide radicals, hydroxyl radicals and H2O2, and reactive oxygen species are scavenged by superoxide dismutase (SOD) (Banu et al. 2009). The predicted target in maize of miR528 is Cu/Zn SOD, and it is reported to be upregulated in salt-stressed rice leaves (Kim et al. 2007). In this study miR528 was upregulated in the shade treatment compared with the natural light treatment. Thus, the scavenging of reactive oxygen species may be occurring under shade in the ear of inbred line 502. TCP TFs regulate the development of leaf size and form, and flower symmetry (Banu et al. 2009). The miR159 and miR319 families control the production of TFs of the MYB family, which are known to play an important role in development, metabolism and responses to biotic and abiotic stresses (Palatnik et al. 2007). In this study, the expression of miR319 and miR166, which target TCP TFs, was downregulated under shade; which indicates the induction of their target TF transcripts, and thus enhanced shade tolerance. This regulation of miR166 and miR319, as well as their target TF transcripts, may explain the development of maize ears in a shaded environment.
The shade response was not only regulated by TFs. miR167 and miR164 were found to be upregulated after shading, suggesting that these miRNAs were involved in the initiation of receipt of the shading stress signal. The target of miR167 is ARF8, which is an auxin-response factor; the transcription of AUX/IAA genes is regulated by ARFs (Ulmasov et al. 1997). Auxin induces the targeted ubiquitinylation and degradation of specific AUX/IAA proteins (Gray et al. 2001). A decrease in ARF transcripts, causing the upregulation of miR167, may weaken the auxin response and thus also affect ear development. If miR167 does not interact with ARF8, this will affect the development of gynoecium, stamens and immature flowers, which in turn affects ovule development and anther indehiscence (Wu et al. 2006); which may explain maize delay ASI (flowering interval of male and female spike) under shading.
The transcripts of NAC-domain proteins are the predicted targets of zma-miR164. In Arabidopsis thaliana, NAC1-overexpressing lines are bigger, with larger leaves and thicker stems than wild type (Xie et al. 2005). Here, with upregulation of miR164 a decrease in NAC-domain proteins could explain why maize ears were smaller under the shade treatment (Fig. 1). In Arabidopsis, the miR396 family regulates several transcription factors that are expressed in inflorescences and pollen grains, and are involved in maturation (Chambers and Shuai 2009); it also regulates cell proliferation in leaves (Rodriguez et al. 2010).
It has been reported that miR172, miR159 and miR166 have essential roles in flowering time and floral organ identity (Aukerman and Sakai 2003; Chen 2004). The target gene of miR172 is AP2, which is an important transcription factor for regulating plant flowering and photoperiod. MiR164, miR167, miR159, miR528 and miR396 were increased under shade treatment (Fig. 7), therefore their target genes expression were decreased, which are important for auxin-responsive gene function, develop metabolism, superoxide dismutase and photosynthesis, their expression were increase or decreased maybe influenced the morphological physiological and metabolic of the maize. Here, its expression was decreased in the shade treatment, correlating with a decrease in maize yield under shade stress and prolonged maize ASI (flowering interval of male and female spike) under shaded conditions.
Availability of supporting data
The generated raw reads of two small RNA libraries in this study are available in NCBI SRA database. The information can be found at the following links: http://www.ncbi.nlm.nih.gov/sra/?term=SRP057673. The accession numbers of shading and natural light, are SRR1997865, SRR1997866, respectively.
Author contribution statement
Liuzheng Yuan, Chaohai Li, Jihua Tang and Jiayou Liu conceived and designed the experiments; Liuzheng Yuan, Hang Song, Moubiao Zhang and Hongping Li performed the experiments; Liuzheng Yuan and Moubiao Zhang analyzed the data; Liuzheng Yuan wrote the paper, and Jihua Tang modified the paper.
References
Achard P, Herr A, Baulcombe DC, Harberd NP (2004) Modulation of floral development by a gibberellin-regulated microRNA. Development 131(14):3357–3365. doi:10.1242/dev.01206
Allen E, Xie Z, Gustafson AM, Carrington JC (2005) microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121(2):207–221
Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T (2003) A uniform system for microRNA annotation. RNA 9(3):277–279
Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 15(11):2730–2741. doi:10.1105/tpc.016238
Banu NA, Hoque A, Watanabe-Sugimoto M, Matsuoka K, Nakamura Y, Shimoishi Y, Murata Y (2009) Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J Plant Physiol 166(2):146–156. doi:10.1016/j.jplph.2008.03.002
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Chambers C, Shuai B (2009) Profiling microRNA expression in Arabidopsis pollen using microRNA array and real-time PCR. BMC Plant Biol 9:87. doi:10.1186/1471-2229-9-87
Chen X (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303(5666):2022–2025. doi:10.1126/science.1088060
Curaba J, Talbot M, Li Z, Helliwell C (2013) Over-expression of microRNA171 affects phase transitions and floral meristem determinancy in barley. BMC Plant Biol 13:6. doi:10.1186/1471-2229-13-6
Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38(Web Server issue):W64–W70. doi:10.1093/nar/gkq310
Fournier C, Andrieu B (2000) Dynamics of the elongation of internodes in maize (Zea mays L.). Effects of shade treatment on elongation patterns. Ann Bot 86(6):1127–1134
Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK (2005) A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol 15(22):2038–2043. doi:10.1016/j.cub.2005.10.016
Gandikota M, Birkenbihl RP, Hohmann S, Cardon GH, Saedler H, Huijser P (2007) The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J 49(4):683–693. doi:10.1111/j.1365-313X.2006.02983.x
Gerakis PA, Papakosta-Tasopoulou D (1980) Effects of dense planting and artificial shading on five maize hybrids. Agric Meteorol 21(2):129–137
Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414(6861):271–276
Jin Z, Ge D, Zheng X, Chen H (1996) Assessing the potential impacts of global climate change on maize production in China. Acta Agronomica Sinica 05:513–524
Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol 57:19–53. doi:10.1146/annurev.arplant.57.032905.105218
Kim DW, Shibato J, Agrawal GK, Fujihara S, Iwahashi H, Kim DH, Shim I, Rakwal R (2007) Gene transcription in the leaves of rice undergoing salt-induced morphological changes (Oryza sativa L.). Mol Cells 24(1):45–59
Kiniry JR, Ritchie JT (1985) Shade-sensitive interval of kernel number of maize. Agron J 77(5):711–715
Kozomara A, Griffiths-Jones S (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 39:D152–D157. doi:10.1093/nar/gkq1027
Lau NC, Lim LP, Weinstein EG, Bartel DP (2001) An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294(5543):858–862. doi:10.1126/science.1065062
Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, Lu XY, Cui X, Jin H, Zhu JK (2008) The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20(8):2238–2251. doi:10.1105/tpc.108.059444
Liu PP, Montgomery TA, Fahlgren N, Kasschau KD, Nonogaki H, Carrington JC (2007) Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J 52(1):133–146. doi:10.1111/j.1365-313X.2007.03218.x
Liu HH, Tian X, Li YJ, Wu CA, Zheng CC (2008) Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 14(5):836–843. doi:10.1261/rna.895308
Liu Q, Zhang YC, Wang CY, Luo YC, Huang QJ, Chen SY, Zhou H, Qu LH, Chen YQ (2009) Expression analysis of phytohormone-regulated microRNAs in rice, implying their regulation roles in plant hormone signaling. FEBS Lett 583(4):723–728. doi:10.1016/j.febslet.2009.01.020
Liuzheng Y, Chaohai L, Xiuping W (2008) Comparison of shade-tolerance among different maize (zea mays l.) inbred lines. Journal of Maize Sciences
Lu S, Sun YH, Shi R, Clark C, Li L, Chiang VL (2005) Novel and mechanical stress-responsive MicroRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell 17(8):2186–2203. doi:10.1105/tpc.105.033456
Lu S, Sun YH, Chiang VL (2008) Stress-responsive microRNAs in Populus. Plant J 55(1):131–151. doi:10.1111/j.1365-313X.2008.03497.x
Meyers BC, Axtell MJ, Bartel B, Bartel DP, Baulcombe D, Bowman JL, Cao X, Carrington JC, Chen X, Green PJ, Griffiths-Jones S, Jacobsen SE, Mallory AC, Martienssen RA, Poethig RS, Qi Y, Vaucheret H, Voinnet O, Watanabe Y, Weigel D, Zhu JK (2008) Criteria for annotation of plant MicroRNAs. Plant Cell 20(12):3186–3190. doi:10.1105/tpc.108.064311
Palatnik JF, Wollmann H, Schommer C, Schwab R, Boisbouvier J, Rodriguez R, Warthmann N, Allen E, Dezulian T, Huson D, Carrington JC, Weigel D (2007) Sequence and expression differences underlie functional specialization of Arabidopsis microRNAs miR159 and miR319. Dev Cell 13(1):115–125. doi:10.1016/j.devcel.2007.04.012
Reed AJ, Singletary GW, Schussler JR, Williamson DR, Christy AL (1988) Shading effects on dry matter and nitrogen partitioning, kernel number, and yield of maize. Crop Sci 28(5):819–825
Rodriguez RE, Mecchia MA, Debernardi JM, Schommer C, Weigel D, Palatnik JF (2010) Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development 137(1):103–112. doi:10.1242/dev.043067
Schmidt WH, Colville WL (1967) Yield and yield components of Zea mays L. as influenced by artificially induced shade. Crop Sci 7(2):137–140
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D (2005) Specific effects of microRNAs on the plant transcriptome. Dev Cell 8(4):517–527. doi:10.1016/j.devcel.2005.01.018
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16(8):2001–2019. doi:10.1105/tpc.104.022830
Sunkar R, Girke T, Zhu JK (2005) Identification and characterization of endogenous small interfering RNAs from rice. Nucleic Acids Res 33(14):4443–4454. doi:10.1093/nar/gki758
Sunkar R, Zhou X, Zheng Y, Zhang W, Zhu JK (2008) Identification of novel and candidate miRNAs in rice by high throughput sequencing. BMC Plant Biol 8:25. doi:10.1186/1471-2229-8-25
Tollenaar M, Daynard TB (1978) Relationship between assimilate source and reproductive sink in maize grown in a short-season environment. Agron J 70(2):219–223
Ulmasov T, Hagen G, Guilfoyle TJ (1997) ARF1, a transcription factor that binds to auxin response elements. Science 276(5320):1865–1868
Wu MF, Tian Q, Reed JW (2006) Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 133(21):4211–4218. doi:10.1242/dev.02602
Xie Z, Allen E, Fahlgren N, Calamar A, Givan SA, Carrington JC (2005) Expression of Arabidopsis MIRNA genes. Plant Physiol 138(4):2145–2154. doi:10.1104/pp.105.062943
Yuan L, Tang J, Wang X, Li C (2012) QTL analysis of shading sensitive related traits in maize under two shading treatments. PLoS One 7(6):e38696. doi:10.1371/journal.pone.0038696
Zhou X, Wang G, Zhang W (2007) UV-B responsive microRNA genes in Arabidopsis thaliana. Mol Syst Biol 3:103. doi:10.1038/msb4100143
Zhou X, Wang G, Sutoh K, Zhu JK, Zhang W (2008) Identification of cold-inducible microRNAs in plants by transcriptome analysis. Biochim Biophys Acta 1779(11):780–788. doi:10.1016/j.bbagrm.2008.04.005
Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L (2010) Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J Exp Bot 61(15):4157–4168. doi:10.1093/jxb/erq237
Acknowledgments
This work was supported by the Maize of China Agriculture Research System (nycytx-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P Sowinski.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, L., Tang, J., Liu, J. et al. Differential miRNA expression in maize ear subjected to shading tolerance. Acta Physiol Plant 38, 80 (2016). https://doi.org/10.1007/s11738-016-2094-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2094-x