Abstract
The influences of silicon (Si) on parameters, such as plant growth, pigment contents, photosynthesis, chlorophyll fluorescence, soluble sugar and starch concentration, and some cell ultra-structures, were investigated in grapevines under salt stress. Compared with the control, the treatment with 100 mM NaCl dramatically inhibited the growth of grapevines and greatly decreased the content of pigments. Silicon treatment in the absence of salt had negative effects in most observed parameters. However, the addition of Si under salt stress improved all growth parameters and increased the pigments and photosynthetic rates compared with the NaCl treatment. Furthermore, investigation of chlorophyll fluorescence, soluble sugars, starch concentration and cell ultra-structure indicated that photosynthesis in the NaCl treatment decreased. The supplement of silicon mitigated the inhibited photosynthesis caused by NaCl, and increased the maximum yield and potential photochemical efficiency of the photochemical reactions in photosystem II. On the other hand, the addition of exogenous Si and NaCl also increased the concentration of soluble sugars and starch, and influenced ultra-structural changes. It is possible that silicon might play an important role in protecting photosynthetic machinery from damage and improving the salt-tolerance of the grape by increasing the concentration of soluble sugars and starch.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinity is one of the most important environmental factors for limiting plant growth and productivity in many regions in the world (Perez-Alfocea et al. 1996) and approximately one-third of the world’s cultivated land is influenced by salts (Parida and Das 2005). Salinity suppresses the growth of plant by osmotic stress, nutritional imbalance, and specific ion toxicity (Cornillon and Palloix 1997; Parida and Das 2005). Salinity in soil is being gradually exacerbated by agronomic measures such as fertilization and irrigation, particularly in arid and semi-arid regions. Grape (Vitis vinifera L.) is an important commercially grown fruit crop (Cramer et al. 2007) that can be cultivated under varied agro-ecological conditions, from tropical to temperate regions, and from cool-humid to hot-arid conditions. However, grapevines are considered to be a moderately salt-sensitive fruit crop (Downton et al. 1990; McEAlexander and Obbink 1971).
Some agronomic technologies such as reclamation, irrigation and drainage can minimize salinity, but these projects are very costly. An alternative strategy is to supplement exogenous silicon (Si) to overcome the negative influences of salts on plant growth and yield (Tuna et al. 2008). Although silicon was considered a nonessential nutrient by plant physiologists for a long time, the essentiality of Si has recently been recognized (Liang et al. 2007; Ma et al. 2001). Silicon has been shown to be effective in mitigating salinity for example, in rice (Oryza sativa L.; Amirjani 2011; Gong et al. 2006), maize (Zea mays L.; Cengiz et al. 2006), barley (Hordeum vulgare L.; Liang 1999), wheat (Triticum aestivum L.; Tuna et al. 2008), tomato (Lycopersicon esculentum M.; Romero-Aranda et al. 2006), and cucumber (Cucumis sativus. L; Zhu et al. 2004).
Generally, effects of salinity on grapevines include reduced rates of CO2 fixation, lower dry matter accumulation in organs, decreasing bunch number, smaller berry size, poorer yield, and an overall reduced growth (Downton et al. 1990; Walker et al. 2008). Previous research has shown that Si alleviated plant growth inhibition due to salinity (Liang et al. 2007). Until now, there have been no reports about the influences of Si on photosynthetic characteristics of grapevines under salt stress. In the current work we investigated the influences of exogenous Si on chlorophyll and gas exchange parameters, the concentration of starch and soluble sugars, as well as the ultra-structure in chloroplasts.
Materials and methods
Plant materials and growth conditions
“Cabernet Sauvignon” grapevines (Vitis vinifera L. cv. Cabernet Sauvignon) were grown from 1 year old rooted cuttings, then transferred into plastic pots (45 cm × 32 cm × 13 cm) containing a mixture of sand and perlite (1:1, v/v) in April and kept in greenhouse condition to accelerate their growth. The vines were watered daily using half-strength Hoagland nutrient solution (Zheng et al. 2008) with microtubes for 60 days before treatment began.
The experiment was conducted at the Hebei Normal University of Science and Technology in Qinhuangdao, China during 2013–2014. Salinity and silicon treatments were performed on by the addition of sodium chloride (NaCl) and potassium silicate (K2SiO3·9H2O) when the shoots were 25–30 cm in length and the experiment lasted 10 days. The K introduced from K2SiO3 was subtracted from KNO3, and the loss of nitrate in the resulting solution was supplemented with dilute nitric acid. In order to avoid shock, the level of salinity was progressively increased by 25 mM per day until the final concentration was reached. The experimental design consisted of: (1) the control (CK), with no NaCl nor Si added; (2) NaCl, with 100 mM NaCl added; (3) Si, with 2.0 mM K2SiO3·9H2O added; (4) NaSi, with 100 mM NaCl and 2.0 mM K2SiO3·9H2O added. There were six replicates of each treatment, and each treatment contained one plant.
Three plants of six replicates per treatment were used for chlorophyll fluorescence, pigment contents, starch and sugar content, and chloroplast ultra-structure determination after the treatment lasted 5–10 days. Another three plants were used for gas exchange parameters every 2 days and when harvested at the end of the experiment, were divided into leaf, stem and root portions. Samples were dried to constant weight in a forced-air oven at 70 °C. All colorimetric measurements were determined using a spectrophotometer (Shimadzu UV/VIS 1201).
Determination of plant growth
Plant growth was quantified as the daily height increment, the expansion rate of leaf area, and the dry weight (DW) of roots, stems and leaves. The determination of leaf areas were made with an automatic area meter (YMJ-B, Zhejiang Top Instrument Co., Ltd., China). The weight of each plant was analyzed using oven-dried materials.
Determination of chlorophyll concentration
Fifteen leaf discs (diameter 0.9 cm) from three plants in each treatment were weighed and extracted with 20 mL of 95 % ethanol in the dark for 2 days (48 h) at room temperature. The concentrations of chlorophyll a (Chl a) and chlorophyll b (Chl b) were estimated as described by Wellburn (1994) using a spectrophotometer (Shimadzu UV/VIS 1201).
Determination of gas exchange parameters
Measurements of leaf gas exchange parameters (net photosynthesis rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (Gs), and transpiration rate (Tr)) were simultaneously made with a photosynthetic system GFS-3000 (Walz, Effeltrich, Germany). The determination of ambient conditions of photosynthesis was as follows: photosynthetically active radiation (PAR) 800 μmol m−2 s−1, relative humidity around 50 %, and chamber temperature at 30 °C. Carbon dioxide concentration was maintained at 400 μmol L−1 with a LI-6400-01 CO2 injector. Measurements were repeated three times from three different individuals in each treatment.
Determination of chlorophyll fluorescence parameters
The measurement of leaf Chl fluorescence was made using an IMAGING-PAM chlorophyll fluorometer (Walz, Effeltrich, Germany). Prior to the analysis, the leaves were dark-adapted for 20 min in order to obtain the maximum fluorescence (F m), the minimum Chl fluorescence (F 0), and the variable fluorescence (F v = F m – F0). After the dark period, all centers of photosystem II were “open”, i.e., their electron acceptors were oxidized. In order to measure actinic and saturating light pulses, the IMAGING-PAM chlorophyll fluorometer was employed with an array of blue light-emitting diodes. Images of chlorophyll fluorescence were captured using a video camera. On every image, the data from the chosen fluorescence parameters were averaged. Measurement of chlorophyll fluorescence and photosynthetic parameters were made sequentially on the same three leaves.
Starch and sugar estimation
Concentrations of soluble sugar and starch were determined using the method of Outlaw and Manchester (1979). About 1 g of leaf tissue was extracted in 80 % ethanol and put in a water bath at 80 °C for 30 min twice. The homogenate was centrifuged at 3000×g for five min. The supernatant was utilized for measuring soluble sugar. The precipitate was kept for starch analysis. The precipitate was resuspended in 3 mL of H2O and boiled for 15 min, and then starch was dissolved in 2 mL of perchloric acid and 3 mL of H2O. The supernatant was used for starch determination after centrifuging at 20 °C for ten min at 3500×g.
Determination of cell ultra-structure
Cell ultra-structure was analyzed by TEM using the method of Xu et al. (2008). Small leaf samples (approximately 2 mm × 1 mm) were obtained from the middle of healthy mature leaves, which then were fixed in 3.5 % glutaraldehyde for a day (24 h) and washed using 0.1 M PBS (pH 7.0). The specimens were post fixed in 1 % osmic acid for 4 h at 4 °C. The specimens were progressively dehydrated by gradient concentrations of ethanol from 30 to 100 %, embedded into beam capsules, filled with spur resin, and they were polymerized at 60 °C. Finally, thin sections were cut using an LKB Ultra-microtome (Bromma, Sweden). Specimens were viewed with a transmission electron microscope (TEM; JEM-1200EX; JEOL Ltd., Tokyo, Japan) at 80 kV.
Sodium (Na), chloride (Cl) and silicon content determination
The dried tissues were finely milled to powder for ion analysis. Sodium in grapevine leaves was determined using a flame photometer (FP6410, INESA) according to Ashraf and colleagues (2010). Chloride was estimated by silver nitrate (AgNO3) titration according to Xu et al. (2008). The concentration of silicon was measured by a molybdenum blue method with a spectrophotometer at 600 nm (Ma et al. 2001).
Statistical analyses
The Statistic Analysis System (SPSS for Windows Version 17.0, SPSS Inc.,) software was employed for analysis of variance (ANOVA), followed by Duncan’s Test (P < 0.05) for average value separation. Data presented here are the mean values of three individuals.
Results
Plant growth
As shown in Table 1, compared to the control, both the plant height and the leaf-area expansion (LAE) rates of grapevine plants were significantly inhibited by 100 mM NaCl. The reductions of the height growth rates and the LAE rates were 51 and 65 %, respectively. In contrast, exogenous Si significantly improved their levels under 100 mM NaCl. However, application of exogenous Si had no significant effects on height growth rates and LAE rates of grapevine plants in the absence of NaCl. Treatment with 100 mM NaCl dramatically decreased the dry weights of leaves, stems and roots. The addition of Si slightly increased the dry weights of plants when treated with 100 mM NaCl.
Pigments
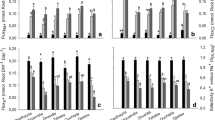
In general, the chlorophyll contents of leaves decreased under salt stress. In comparison with the control, the contents of Chl b and Chl a + b were not significantly reduced in the NaCl treatment at 5 days after treatment (P < 0.05; Fig. 1). Silicon had no significant effect on Chl a, Chl b and Chl a + b contents regardless of salt treatment. Compared with the control, Chl a and Chl a + b contents of all treatments showed a reduction at 10 days of treatment. The addition of exogenous Si alleviated the decrease of Chl a and Chl a + b content caused when grapevine plants were treated with NaCl alone. The rate of chlorophyll a/b was higher in the control treatment than in other treatments at both 5 and 10 days after the treatment began.
Changes of chlorophyll a content a, chlorophyll b content b, chlorophyll a + b content c and chlorophyll a/b d of grapevine leaves in the control (CK), NaCl, Si, and NaSi treatments. Data are the average values of three replications and vertical bars indicate standard errors. Different letters indicate a significant difference (P < 0.05) according to the Duncan test on the same treatment day
Gas exchange parameters
As shown in Fig. 2, 100 mM NaCl significantly (P < 0.05) inhibited Pn, Gs, Tr, and Ci after 4 days of NaCl treatment compared to the control. The addition of Si alleviated the inhibition of Pn, Tr, and Gs caused by NaCl stress. The application of exogenous Si slightly increased Pn, Gs, and Tr in the absence of NaCl; however, this trend changed after 6 days of treatment with NaCl.
Changes of Pn a, Tr b, Gs c, and Ci d of the grape leaves in the control (CK), NaCl, Si, and NaSi treatments. Data are the average values of three replications and vertical bars indicate standard errors. Different letters indicate a significant difference (P < 0.05) according to the Duncan test on the same treatment day
Compared with the control, the reduction of Pn, Gs, and Tr was sharper after 6 days under salt stress, but Ci significantly (P < 0.05) increased. As shown in Fig. 2a–c, exogenous Si decreased Pn, Tr, and Gs without salt stress, compared to the control. Silicon alone had a negative effect on Pn, Gs, and Tr after 6 days of treatment; however, this difference was not significant. The application of Si markedly alleviated the reduction of Pn, Tr, and Gs, and the increase of Ci caused by NaCl.
Chlorophyll fluorescence parameters
As shown in Fig. 3, the maximum yield of PSII photochemical reactions (F v/F m) decreased under 100 mM NaCl stress, especially at 10 days after the beginning of the treatment. In contrast, the introduction of Si had no significant (P < 0.05) effects on F v/F m of grapevine leaves at 5 days regardless of salt. F v/F m under NaCl stress showed the deduction after 10 days regardless of if the plants were treated with silicon.
Changes of F v/F m a and F v/F 0 b of the grape leaves in the control (CK), NaCl, Si, and NaSi treatments. Data are the average values of three replications and vertical bars indicate standard errors. Different letter indicate a significant difference (P < 0.05) according to the Duncan test on the same treatment day
The potential photochemical efficiency of PS II (F v/F 0) significantly (P < 0.05) decreased in 100 mM NaCl stress at 10 days after initial treatment. The addition of Si caused no obvious changes on F v/F 0 in the absence of salt. The addition of Si had no significant (P < 0.05) effect on F v/F 0 in the presence of NaCl salt at 5 days of treatment. However, F v/F 0 values were higher in plants treated with NaSi than NaCl alone after 10 days of treatment. The addition of silicon alleviated the deduction of F v/F 0 values caused by NaCl stress.
Soluble sugars and starch contents
Compared with the control group (Fig. 4), the amount of soluble sugars and starch in other treatments increased significantly (P < 0.05) at 10 days. There was no significant difference in the amount of soluble sugars present in NaSi-treated plants and Na-treated plants after 10 days. Compared with Na-treated plants, the amounts of starch in NaSi-treated and Si-treated plants were significantly increased (P < 0.05) after 5 and 10 days. The addition of Si considerably increased starch content and this influence was more obvious with the increasing duration of the treatment. The highest starch content was observed in the NaSi-treatment after 10 days of treatment. The starch within plants is mainly derived from soluble sugars and it was therefore speculated that Si might promote the conversion of soluble sugars into starch when plants are subjected to NaCl stress.
Changes of the soluble sugar a and starch content b of the grape leaves in the control (CK), NaCl, Si, and NaSi treatments. Data are the average values of three replications and vertical bars indicate standard errors. Different letter indicate a significant difference (P < 0.05) according to the Duncan test on the same treatment day
Chloroplast ultra-structure
Some changes to the cell ultra-structure were observed (Fig. 5). In comparison with control cells (Fig. 5Aa), a greater number of osmiophilic globules (lipid droplets) per cell, larger chloroplasts, and a dilation of thylakoid membranes were observed in spongy and palisade tissue of grapevine leaves under 100 mM salt stress. The increase of osmiophilic globules in cells was a distinctive feature in chloroplast ultra-structure under salt stress (Parida and Das 2005). The appearance of ‘huge’ starch grains in chloroplasts in spongy and palisade tissue of grape leaves were a characteristic of chloroplast ultra-structure in the presence of Si regardless of treatment with NaCl (Fig. 5Cc, Dd).
Transmission electron microscopy (TEM) micrographic images of the palisade (spongy) tissues of grapevine leaves in the control (CK), NaCl, Si, and NaSi treatments (8000 X). Aa control cells, Bb cells treated with 100 mM NaCl; Cc cells treated with Si, and Dd cells treated with NaSi (bar 2 μm). Chl chloroplast, S starch grain, V vacuole, P plastoglobules
Sodium, chloride and silicon concentrations
As shown in Table 2, the salinity treatment markedly increased sodium and chloride concentration in the leaves. The addition of exogenous silicon significantly decreased sodium concentration in leaves in the salt treatment. When the salt treatment was performed together with the addition of exogenous silicon, the grape leaves contained a high concentration of chloride compared with Na-treated plants. As expected, the application of exogenous Si in solution increased the Si concentration in leaves compared to the control group. Silicon concentration was lower in leaves and stems than in roots.
Discussion
High soil salinity is the result of the presence of abundant salts in soil. Generally, high Na+ and Cl− are the main ions that induce plant damage (Downton et al. 1990). High salt concentration in nutrient medium caused stunted growth in plants (Parida and Das 2005). High salt concentrations affected the many physiological processes, for example photosynthesis, protein synthesis, and lipid metabolism (Parida and Das 2005). In our work, grapevine growth was significantly decreased by the application of 100 mM NaCl (Table 1). Similar observations have been observed in many plants (Cheeseman 1988; Parida and Das 2005).
In the present work, the application of NaCl to the solution in which grapevines were planted caused significant reductions in leaf-area expansion rates, height growth rates, dry weight of plant, and in pigment contents, as well as markedly increasing sodium and chloride concentration in the leaves. Leaf-area expansion rates of grapevines in salt stress were in agreement with the study conducted by Seemann and Critchley (1985). Moreover, data for the height growth rates was in accordance with the work of Fisarakis et al. (2001). Reductions in biomass under salinity stress have been reported in many plants, including rice (Amirjani 2011), wheat (Tuna et al. 2008), tomato (Romero-Aranda et al. 2006), and grapevine (Fisarakis et al. 2001; McEAlexander and Obbink 1971). Present values for chlorophyll concentrations were in accordance with studies on rice and barley, which indicated the negative influence of NaCl on the Chl content of leaves (Liang et al. 2007).
Inhibition of salt on plant growth may either be due to reduction of osmotic potential in soil or accumulation of excessive ions in plant tissues (Parida and Das 2005). In addition, it was reported that exogenous Si is effective in relieving salinity in different plant species (Liang et al. 1996; Yeo et al. 1999; Zhu et al. 2004). In this work, supplementary silicon under NaCl stress caused significant increases in the leaf-area expansion rate, dry weight of leaves, stems, and roots, chlorophyll contents, and the reduction of Na+ and Cl− contents in leaves of grapevines. These results were in accordance with other studies (Liang et al. 2007; Ma et al. 2001) and indicated that exogenous Si alleviated salt stress in grapevines by reducing the accumulation of Na+ and Cl−, degradation of pigments, and stimulating plant growth.
Photosynthetic activity and Chl fluorescence kinetics were closely related to the photosynthetic pigment contents (Al-aghabary et al. 2004; Parida and Das 2005). The photosynthetic capacities and the growth rate of grapevines significantly decreased due to the reduction of pigment concentrations caused by growth in a high salt environment (Downton et al. 1990). In this experiment, Pn, Gs, and Tr in grape leaves significantly decreased in a high salt environment (Fig. 2). Reduction of the photosynthesis rate was not simultaneously accompanied by lower CO2 concentrations in grape leaves grown in a salt environment for 10 days. The increase of Pn in leaves was apparent with the supplement of Si under salt stress, but the Ci levels of grape leaves in plants treated with NaSi were not higher than the NaCl treatments. The inhibition of photosynthesis caused by the supplement of silicon under NaCl stress in grapevines might be considered a result of non-stomatal restriction (Caemmerer and Farquhar 1981). The non-stomatal restriction could arise from the inhibition of the electron transport chain, activity of key enzymes in the Calvin cycle, as well as serious damage to the mesophyll cells (Krause and Weis 1984). The addition of Si increased the photosynthetic ability of grapevines grown in a high salt environment, which likely links to protective mechanisms that avoid the damage of the photosynthetic apparatus. It was reported that the mitigating effects of Si on barley (Hordeum vulgare L.) under salt stress greatly depended on silicon’s improvement of the ultra-structure of chloroplasts (Liang et al. 2007). Similarly, the role that Si plays in the protection the chloroplast might aid in the increase of salt tolerance in grape plants.
In order to rapidly estimate the quantum efficiency of photosystem II in plant leaves, Chl fluorescence measurements were previously employed (Krause and Weis 1984), which is related to CO2 assimilation (Loreto and Velikova 2001). Chlorophyll fluorescence can be used as an indicator of salt stress in plants, because it can give information about the extent of damage to the photosynthetic machinery (Seemann and Critchley 1985). In this study, chlorophyll fluorescence kinetics showed that F v/F 0 and F v/F m were markedly decreased in leaves of salt-stressed grapevines (Fig. 3) and similar reports have been found in some plants under salt stress, for example in rice (Yeo et al. 1999) and barley (Liang et al. 1996). The ratio of F v/F m shows the primary photochemical efficiency of photosystem II, and the reduction of F v/F m is a signal of photoinhibition (Krause and Weis 1984). The ratio of F v/F 0 is sensitive to environmental changes that have an effect on the efficiency of PSII to capture energy (Liang et al. 2007; Parida and Das 2005). In this work, the decrease of F v/F 0 in leaves of grapevines grown in a high salt environment might be caused by the changes of chlorophyll biosynthetic pathways. On the contrary, compared to control, the application of Si increased F v/F m and F v/F 0 in leaves of grapevines under salt stress. This indicates that salt-treated grapevine plants could keep higher photosynthetic activities in the reaction center when Si was applied.
Generally, the accumulation of carbohydrates such as sugars and starch increased under salinity (Parida et al. 2002). Sugar and starch play a role in maintaining osmotic balance and functions as carbon storage and free radical scavengers. Increased accumulations of soluble sugar and starch have been reported in some plant species exposed to salt (Parida and Das 2005). In this work, the levels of sugar and starch in leaves of grapevines were increased by salt stress treatments. The content of soluble sugar in grape leaves increased in the treatment with NaSi, but was lower than in the NaCl treatment. The increase of starch content was prominently influenced by the addition of silicon and NaCl. Therefore, silicon might promote the transformation of sugars into starch and reduce the accumulation of sugars in grape leaves. Under NaCl stress the accumulation of sugars along with other compatible solutes contributed to an osmotic adjustment that allowed plants to maximize sufficient storage reserves to support basal metabolism (Hurry et al. 1995). The transformation of sugars into starch by Si might help plants to avoid metabolic alterations by reducing feedback inhibition caused by excess amounts of sucrose in cytoplasm.
An increased accumulation of starch has been found in some plant species exposed to salinity (Parida and Das 2005). It has been reported that starch may not play a critical role in the mechanism of salt-tolerance (Krapp and Stitt 1995). The increase of starch content in grapevines treated with Si, regardless of salt treatment, could be attributed to the accumulation of Si in grape leaves, which might affect the transportation of organic matter. The accumulation of Si in cells of grape leaves was concentrated through water loss (transpiration) as explained by Ma and Yamaji (2006). Further investigation of starch granules with an electron microscope showed that the size of the starch granules were enlarged in grapevine leaves after treatment with Si for 10 days (Fig. 5). Therefore, the higher accumulation of starch grains in chloroplasts could be due to the application of Si. Consequently, a slight reduction of photosynthesis, F v/F m, and F v/F 0 at 10 days of treatment with Si could contribute to the destruction of thylakoid membranes caused by excess amounts of starch granules, which might have brought about the reduction of the largest number of PSII photochemical reactions and highest photosynthetic rates.
Author contribution statement
L. Qin and W. H. Kang were responsible for the formulation of research theme and writing of article. Y. L. Qi and Z. W. Zhang experimented pigments, photosynthesis and chlorophyll fluorescence; N. Wang did soluble sugar and starch analysis and cell ultra-structure; W. H. Kang analyzed the data. L. Qin, Y. L. Qi, Z. W. Zhang, N. Wang and W. H. Kang contributed equally to the write up of the manuscript.
References
Al-aghabary K, ZhuJun Z, QinHua S (2004) Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr 27:2101–2115
Amirjani MR (2011) Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int J Bot 7:73–81
Caemmerer S, Farquhar G (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Cengiz K, Levent T, David H (2006) Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J Plant Nutr 29:1469–1480
Cheeseman JM (1988) Mechanisms of salinity tolerance in plants. Plant Physiol 87:547–558
Cornillon P, Palloix A (1997) Influence of sodium chloride on the growth and mineral nutrition of pepper cultivars. J Plant Nutr 20:1085–1094
Cramer GR, Ergül A, Grimplet J, Tillett RL, Tattersall EAR, Bohlman MC, Vincent D, Sonderegger J, Evans J, Osborne C (2007) Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomics 7:111–134
Downton W, Loveys B, Grant W (1990) Salinity effects on the stomatal behaviour of grapevine. N Phytol 116:499–503
Fisarakis I, Chartzoulakis K, Stavrakas D (2001) Response of Sultana vines (V. vinifera L.) on six rootstocks to NaCl salinity exposure and recovery. Agric Water Manag 51:13–27
Gong H, Randall D, Flowers T (2006) Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ 29:1970–1979
Hurry VM, Strand A, Tobiaeson M, Gardestrom P, Oquist G (1995) Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. Plant Physiol 109:697–706
Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195:313–323
Krause GH, Weis E (1984) Chlorophyll fluorescence as a tool in plant physiology. Photosynth Res 5:139–157
Liang Y (1999) Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 209:217–224
Liang Y, Shen Q, Shen Z, Ma T (1996) Effects of silicon on salinity tolerance of two barley cultivars. J Plant Nutr 19:173–183
Liang Y, Sun W, Zhu YG, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1799
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397
Ma J, Miyake Y, Takahashi E (2001) Silicon as a beneficial element for crop plants. Stud Plant Sci 8:17–39
McEAlexander D, Obbink J (1971) Effect of chloride in solution culture on growth and chloride uptake of Sultana and Salt Creek grape vines. Aust J Exp Agric Anim Husb 11:357–361
Outlaw WH Jr, Manchester J (1979) Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol 64:79–89
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Parida A, Das AB, Das P (2002) NaCl stress causes changes in photosynthetic pigments, proteins, and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J Plant Biol 45:28–36
Perez-Alfocea F, Balibrea M, Cruz AS, Estan M (1996) Agronomical and physiological characterization of salinity tolerance in a commercial tomato hybrid. Plant Soil 180:251–257
Romero-Aranda MR, Jurado O, Cuartero J (2006) Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J Plant Physiol 163:847–855
Seemann JR, Critchley C (1985) Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 164:151–162
Tuna AL, Kaya C, Higgs D, Murillo-Amador B, Aydemir S, Girgin AR (2008) Silicon improves salinity tolerance in wheat plants. Environ Exp Bot 62:10–16
Walker RR, Read PE, Walker RR, Blackmore DH (2008) Rootstock and salinity effects on rates of berry maturation, ion accumulation and colour development in Shiraz grapes. Aust J Grape Wine Res 6:227–239
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Xu PL, Guo YK, Bai JG, Shang L, Wang XJ (2008) Effects of long term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol Plant 132:467–478
Yeo A, Flowers S, Rao G, Welfare K, Senanayake N, Flowers T (1999) Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ 22:559–565
Zheng Y, Jia A, Ning T, Xu J, Li Z, Jiang G (2008) Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J Plant Physiol 165:1455–1465
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533
Acknowledgments
This work was supported by the Nature Science Foundation of Hebei Provincial of China (Grant No. C 2010001529; C 2014407013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L Bavaresco.
Rights and permissions
About this article
Cite this article
Qin, L., Kang, Wh., Qi, Yl. et al. The influence of silicon application on growth and photosynthesis response of salt stressed grapevines (Vitis vinifera L.). Acta Physiol Plant 38, 68 (2016). https://doi.org/10.1007/s11738-016-2087-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2087-9