Abstract
The objectives of this study were to compare freezing tolerance (FT) of 12 field-grown grapevine cultivars at four stages: Nov., Jan., Mar., and Apr., and to investigate the relationship between FT and soluble carbohydrates, proline and water content changes of buds during acclimation and deacclimation stages. After exposure to various freeze test temperatures bud LT50 values were estimated using electrolyte leakage and bud browning measurement. Bud FT of all cultivars studied increased with the overall trend of declining temperatures from Nov. through Jan., after which the buds began to deacclimate from Mar. and gradually lost FT in Apr.; overall LT50 means were −13.79, −21.74, −20.38, and −10.06, respectively. However, cultivars did not show alike acclimation and deacclimation rates and, for example, ‘Yaquti’, an early ripening cultivar, showed relatively higher FT in early autumn, but was one of the least hardy cultivars in later stages. In Jan. (full hardening), cultivars were classified as hardy (‘Red Sultana’, ‘Fakhri’, ‘Shahani’ and White Sultana), moderately hardy (‘Tabarzeh’, ‘Gaznei’, ‘Thompson Seedless’, Laal, ‘Sahebi’ and ‘Rishbaba’) and least hardy (‘Yaquti’ and ‘Ruby Seedless’). The pattern of soluble carbohydrates and proline changes were highly consistent with the LT50 profile. Bud water content, especially in early autumn, had a stronger correlation with FT, compared to the concentration of cytoplasmic osmoregulants. Likewise, soluble carbohydrates proved to be a superior indicator of FT, as compared to proline. Our results showed osmoregulants as well as bud water content are useful indices to screen FT in a range of grapevine germplasm resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Low temperature is one of the main environmental factors affecting crop productivity, quality and even survival (Alberdi and Corcuera 1991). Grapes are the world’s first and fourth largest fruit crop in terms of cultivated areas and fresh weight production, respectively. Grapevine grows on 215,000 ha in Iran, producing 2.15 million tons of fruit, ranking 8th and 10th in the world, respectively (Food and Agriculture Organization 2011). Despite the existence of hundreds cultivars of grapevine in Iran, only a few dozen cultivars account for the commercial production (Karimi and Ershadi 2015). Moreover, the majority of the commercial vineyards are located in cold climate regions where the winter minimal temperatures may plunge down −22 °C and exceptionally −30 °C for several hours, causing serious damages to vineyards. Under these harsh conditions, cold injury is expected in mid-winter, but it also often occurs in late fall or early spring. For example, when the temperature reached −21 °C for three consecutive days in 2007, a severe winter frost led to a 20 % loss in annual grape production, as compared to preceding years. For this reason, it would be useful to evaluate the freezing tolerance (FT) of a range of Iranian grapevine cultivars to assess their potential for breeding programs.
FT is dynamic, altering with developmental stage and variations in environmental temperature. It is also affected by cultivar and environment interactions, which can lead in differences in the level of FT across trees (Fennell 2004). The timing of cold acclimation and deacclimation and threshold of FT change with tree species, provenance and cultivars (Levitt 1980), and the expression of FT appears to be genetically controlled (Sutinen et al. 1992). Several changes occur during hardening cycle, including modifications in the cell wall and membrane lipid composition (Griffith and Brown 1982; Steponkus 1984), increases in soluble sugars and compatible solutes (Hamman et al. 1996; Jones et al. 1999; Valle 2002; John et al. 2010; Ghasemi et al. 2012; Zhang et al. 2012), altered gene expression and protein synthesis (Thomashow 1999; Salzman et al. 1996), changes in bud water content (Pogosyan et al. 1975; Salzman et al. 1996; Valle 2002) and hormone regulation (Koussa et al. 1998). Soluble carbohydrates accumulated in plants serve as cytoprotective compounds, which can prevent or slow ice crystal formation (Jones et al. 1999; Morin et al. 2007; Karimi and Ershadi 2015). Besides carbohydrates, trees accumulate proline with exposing to cold temperatures, which induces osmotic adjustment, maintains turgor in dehydrating cells, and allows plants to tolerate dehydrative stresses (Wang et al. 2003; Zhang et al. 2012; Ghasemi et al. 2012). The reduction in bud water content is effectively associated with increases in FT and is thought to contribute to an increased ability to super cool (Bourne and Moore 1991; Salzman et al. 1996; Valle 2002). Therefore, it is feasible to utilize alterations in these compatible materials in trees exposed to FT stress as indicators to assess FT in different grapevine cultivars.
Controlled laboratory freezing tests followed by accurate viability assays provide useful means for evaluation of new cultivars and rootstocks and help in identifying main ecological and cultural influences on established vines (Barka and Audran 1997; Fennell 2004; Jones et al. 1999). The truthfulness of FT estimation can be improved by combining results of two or more FT evaluation methods applied simultaneously (Sutinen et al. 1992). Among the methods of FT measurement, bud browning (BB) and electrolyte leakage (EL) tests are the most common (Linden 2002) and FT determined by these two methods correlate well (Sutinen et al. 1992). EL has been shown to be a good indicator of freeze-injured and -uninjured tissues during dormancy in woody plants, and its use in screening germplasm for breeding has been suggested (Barka and Audran 1997; Jones et al. 1999; Morin et al. 2007; Ghasemi et al. 2012; Zhang et al. 2012). Recording the level of browning or discoloration of plant tissues is a prevalent visual method for assessing injury level in laboratory tests of FT (Stergios and Howell 1973 ; Linden 2002). BB as a visual indication of freezing injury is the most common method of evaluating FT in grapevine which can be used reliably in both field and laboratory (Wolf and Cook 1992; Rekika et al. 2004). However, aside from bud browning assessment during dormancy period in winter, extremely finite data are obtainable on seasonal changes in FT of grapevine cultivars.
Screening of native commercial grapevine cultivars and understanding of the FT mechanisms are essential for matching the cultivars appropriately with growing sites to help in breeding and selecting cultivars with improved FT. Therefore, the aims of the present study were: (1) to screen different grapevine cultivars for FT by means of EL and BB measurement in four stages of hardening cycle, from mid-autumn to the early spring, (2) to compare seasonal changes in soluble carbohydrates, proline and bud water content of these cultivars during acclimation and deacclimation. Finally, the possible mechanisms for FT and its relationships with these physiological changes will be discussed.
Materials and methods
Plant materials
In this study, twelve different cultivars from 14 years old own-rooted vines grown at Malayer Grape Research Station (lat. 34°30′N, long. 48°85′E, alt. 1550 m), Iran were evaluated at four stages during autumn and winter 2011–12. The average annual temperature of Malayer is 12.2 °C. Although this region has relatively long and hot summers, but the winter temperatures may plunge down to −20 °C or even lower, causing serious damage to grapevine plants.
Cultivars included ten Iranian (‘Fakhri’, ‘Gaznei’, ‘Laal’, ‘Red Sultana’, ‘Rishbaba’, ‘Sahebi’, ‘Shahani’, ‘Tabarzeh’, ‘White Sultana’ and ‘Yaquti’) along with two non-Iranian (‘Ruby Seedless’ and ‘Thompson Seedless’) ones, currently commercially grown in Iran. Grapevines had been planted based on a complete randomized design with five replications; each replication contains two trees, for a total of ten trees per each cultivar.
Samples were collected from five randomly chosen vines for each cultivar (each vine as an experimental unit), and at least two representative cuttings (20–30 cm in length from intermediate sections of the canes) per each vine were collected at any sampling date. The cane sections were packed in polyethylene bags, and shipped on ice in Styrofoam boxes to the Laboratory.
Controlled freezing tests
Cane segments were rinsed under cold running demineralised water to remove surface contaminants. Samples were wrapped in moist paper towels to ensure ice nucleation, closed in polyethylene bags and were randomly assigned to predetermined test temperatures. Five replicates were assigned for each cultivar/test temperature. Freezing was accomplished in a programmable freezing chamber (Kimia Rahavard, Tehran, Iran) according to a stepwise lowering freezing program starting from the prevailing outdoor temperature. Cane segments were subjected to four different freeze test temperatures (−6, −10, −14 and −18 °C in Nov. and Apr., −16, −20, −24 and −28 °C in Jan. and Mar.). The control samples were kept in polyethylene bags at 4 °C. Freeze test temperatures were determined following preliminary tests on canes collected 2 days before each sampling date. The freezing rate was 2 °C h−1, and samples were held at each test temperature for 75 min, before removing from freezing chamber (Karimi and Ershadi 2015).
Electrolyte leakage assay
EL measurements began immediately, following thawing cane segments by cutting compound buds with approximately 2 mm of intact cane tissue surrounding and underlying the buds. Two buds (as a replicate) were placed into 70-mL test tubes containing 40 mL of demineralised water. Tubes were capped with aluminum foil and incubated at room temperature under constant shaking at 120 rpm. After 24 h of incubation, the electrical conductivity (EC1) of the bathing solutions was measured using a Cond-720 conductimeter (WTW GmbH, Weilheim, Germany). Samples were subsequently autoclaved at 120 °C for 20 min, allowed to cool to room temperature, and electrical conductivity was measured again (EC2). Electrical conductivity of bud samples remained at 4 °C for 24 h was considered as control (EC0). Relative electrolyte leakage (REL) was calculated using the formula: \( {\text{REL}} = \left[ {\left( {{\text{EC}}_{ 1} - {\text{EC}}_{ 0} } \right)/\left( {{\text{EC}}_{ 2} - {\text{EC}}_{ 0} } \right)} \right] \times 100 \).
Bud browning assay
After freezing treatments in Nov. and Apr., cane segments were maintained in polyethylene bags at room temperature for 1 week to allow oxidation of tissues before visual rating of freezing injury. Five freeze-thawed buds per each grapevine were cut at each test temperature. Compound buds were individually cross-sectioned by slicing horizontally through the middle part of the bud (roughly half the distance from the tip to the base) with a razor blade. These slices were examined under a stereomicroscope (Leica MS5; Wetzlar, Germany), and only the primary buds were checked for necrosis (Stergios and Howell 1977). The primary buds that appeared bright and green were considered alive, and those appearing dull, straw colored or black/brown were considered dead (Wolf and Cook 1992, Linden 2002). The proportion of injured buds at each test temperature was determined as the number of buds injured/total buds.
Soluble carbohydrates
Soluble carbohydrates were measured based on the anthrone method (Yemm and Willis 1954). Dormant buds from intermediate sections of the canes were oven-dried at 70 °C for 2–3 days, ground in a coffee grinder to pass 40-mesh and stored in airtight containers at room temperature, in the dark, until analysis. Soluble carbohydrates were extracted three times from 1 g of ground tissue with 5 mL of 80 % ethanol and centrifuged for 15 min at 3000 g n. One mL of 0.2 % anthrone reagent (2 g anthrone in 1 L of 72 % sulfuric acid) was added to 100 µL of the ethanolic extract. The reaction mixture was heated in a boiling water bath for 10 min and then rapidly cooled on ice. Absorbance of the extract was read at 620 nm using a Cary WinUV 100 spectrophotometer (Varian, Australia). The concentration of soluble carbohydrates was finally calculated using a calibration curve and exhibited as mg g−1 dry weight (DW).
Proline
Proline concentration was assessed following Bates et al. (1973) method. Bud samples from intermediate sections of the canes were ground in liquid nitrogen, and 0.5 g of ground tissue was homogenized in 10 mL of 3 % (w/v) aqueous sulfosalicylic acid. Homogenate was then filtered through a Whatman No. 1 filter paper. Two mL of filtered extract was taken for the analysis to which 2 mL ninhydrin and 2 mL glacial acetic acid were added. The reaction mixture was incubated in a boiling water bath for 1 h and the reaction was finished in an ice bath. Four mL of toluene was added to the mixture and the organic phase was extracted. Absorbance was spectrophotometrically measured at 520 nm, while toluene used as blank. Concentration of proline was calculated using a calibration curve and exhibited as µmol g−1 fresh weight (FW).
Water content
Canes were collected at four stages, from Nov. to Apr. Compound buds from intermediate sections of each cane were excised and weighed before and after placing in an oven for 3 days at 70 °C. Bud water content was expressed as percent of fresh weight using the following equation:
Statistical analyses
FT was expressed as LT50 (lethal temperature at which 50 % of the total ion leakage occurs; or, in the case of bud browning, the lethal temperature at which 50 % of the primary buds are dead) by fitting response curves with the following logistic sigmoid function:
where R = REL or BB percentage based on LT50 estimation method, x = treatment temperature, b = slope of the function at inflection point, and a, c and d determine the asymptotes of the function (Fiorino and Mancuso 2000). To evaluate differences among cultivars, results obtained using different methods of FT assessment (EL and BB assays) were subjected to analysis of variance using the GLM procedure of SAS (SAS Institute, Cary, NC) and means separated by Duncan’s multiple range tests at p ≤ 0.05. Correlation analysis between soluble carbohydrates, proline, water content, and LT50 values, estimated by EL and BB measurement, was performed using the CORR procedure of SAS.
Results
Freezing tolerance as estimated by EL measurement
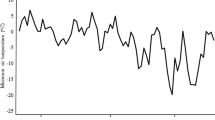
Vineyard daily low temperatures from Sept. 2011 through May 2012 are shown in Fig. 1. The first sub-zero temperature occurred on Nov. 8, and temperatures throughout Nov. and Dec. were considered conducive to good cold hardiness acquisition. Based on EL LT50 values, significant differences in FT were observed among cultivars at each sampling date (Table 1). In all cultivars, bud FT increased with the overall trend of declining temperatures from Nov. through Jan., after which the buds began to deacclimate from Mar. and gradually lost FT in Apr.; overall LT50 means were −13.79, −21.74, −20.38 and −10.06, respectively (Table 1). Based on buds EL LT50 values, a significant difference (p ≤ 0.001) was found in the FT of cultivars in Nov. (Table 1). ‘Red Sultana’, ‘Sahebi’, ‘Fakhri’, ‘White Sultana’, and ‘Shahani’ showed the highest FT, ‘Yaquti’, ‘Thompson Seedless’ and ‘Laal’ showed an intermediate hardiness, while ‘Rishbaba’ and ‘Ruby Seedless’ were the least hardy cultivars in this sampling stage.
Throughout the hardening cycle, from Nov. (early hardening) to Jan. (full hardening), FT of all cultivars remarkably enhanced (7.95 °C in average). However, cultivars did not show similar acclimation rates and, for example, bud FT in ‘Yaquti’ and ‘Rishbaba’ increased by 5.6 and 10.6 °C, respectively, during this period. A relatively lower variation of bud FT was shown in Jan., when ranged from −19.17 to −22.93 °C. In Jan., cultivars were classified as hardy (‘Red Sultana’, ‘Fakhri’, ‘Shahani’ and White Sultana), moderately hardy (‘Tabarzeh’, ‘Gaznei’, ‘Thompson Seedless’, Laal, ‘Sahebi’ and ‘Rishbaba’) and least hardy (‘Yaquti’ and ‘Ruby Seedless’). FT of all cultivars decreased slightly (1.36 °C in average) in Mar. ‘Thompson Seedless’, ‘Yaquti’, and’Ruby Seedless’, less hardy cultivars in this period, reached EL LT50 at ≥−18.58 °C, while the remaining cultivars had EL LT50 values lower than −20.27 °C. By mid-Apr., all cultivars showed a high deacclimation rate, and their cold hardiness substantially decreased (10.32 °C in average). However, cultivars did not show similar deacclimation rates and, for example, bud FT in ‘Rishbaba’ and ‘White Sultana’ decreased of about 13 and 8.7 °C, respectively, during the dehardening period. Based on the bud EL LT50 values, the highest FT in this stage was related to ‘Red Sultana’, ‘White Sultana’ and ‘Fakhri, while ‘Yaquti’, ‘Rishbaba’, ‘Ruby Seedless’ and ‘Thompson Seedless’ showed the lowest FT; other cultivars showed an intermediate FT. In general, a relatively high variation of bud FT was shown in Nov. and Apr., compared to the other sampling stages (Table 1).
Freezing tolerance as estimated by BB measurement
The FT of grapevine cultivars was also investigated with the BB assay in Nov. and Apr. All cultivars exhibited BB after a week following the artificial freezing tests and significant difference (p ≤ 0.001) was found among cultivars. In Nov., BB LT50 values were ≤−14 °C in ‘Red Sultana’, ‘White Sultana’ and ‘Fakhri’. ‘Thompson Seedless’, ‘Rishbaba’, ‘Ruby Seedless’ and ‘Laal’ showed 50 % BB at ≥−12.4 °C, whereas the remaining cultivars showed an intermediate FT (Table 2). A significant difference (p ≤ 0.001) in FT was observed among cultivars in Apr. (Table 2). BB LT50 in ‘Red Sultana’, ‘White Sultana’ and ‘Fakhri’ occurred at −11.2, −11 and −10.6 °C, respectively, but ‘Yaquti’, ‘Thompson Seedless’ and ‘Ruby Seedless’ indicated 50 % mortality at ≥−6.8. There was an overall agreement between results acquired from two procedures (Table 6). BB LT50 values were well-correlated with EL LT50 values in Nov. (r = 0.91, p ≤ 0.001, n = 50), and Apr. (r = 0.93, p ≤ 0.001, n = 60); however, FT estimated by EL measurement were higher than those estimated through BB in both stages (Tables 1 and 2).
Soluble carbohydrate
Soluble carbohydrates enhanced during cold acclimation from Nov. to Jan., then started to decrease in Mar. and was the lowest in Apr.; overall means were 46.47, 97.63, 59.95, and 23.33 mg g−1 DW, respectively (Table 3). In Nov., significant differences (p ≤ 0.001) existed in the concentration of soluble carbohydrates among cultivars. Maximum amounts were related to ‘Fakhri’ and ‘Red Sultana’, whereas, the least rate was observed in ‘Ruby Seedless’. From Nov. to Jan., concentration of soluble carbohydrates increased considerably in all cultivars except for ‘Yaquti’, this increase was concomitant with the LT50 decrease. At this stage, maximum amounts of soluble carbohydrates (116.7 mg g−1 DW) was detected in ‘Fakhri’, while the minimum amount (61.72 mg g−1 DW) was found in ‘Yaquti’. A slight reduction was found in soluble carbohydrates in Mar., compared to Jan, which was consistent with LT50 profile in this sampling stage. Soluble carbohydrates were noticeably decreased in Apr., and a significant difference was monitored among cultivars at this stage. High correlations were found between EL LT50 and BB LT50, and soluble carbohydrate concentration in all sampling stages (Table 6).
Proline
Proline concentrations showed a narrow range of variation among cultivars in Nov., ranging from 1.21 to 1.45 µmol g−1 FW (Table 4). A dramatic increase in proline was observed during the hardening period in all cultivars. The highest proline concentration in Jan. was found in ‘Red Sultana’ (7.25 µmol g−1 FW), followed by ‘Tabarzeh’, ‘Fakhri’, ‘Shahani’ and ‘White Sultana’. All cultivars showed a slight decrease in proline concentration in Mar., compared to Jan. (Table 4). In Apr., before bud break stage, amounts of bud proline decreased (1.48 µmol g−1 FW in average) in all cultivars and those with lower LT50 values had higher proline content. The highest amount of proline in Apr. was found in ‘Red Sultana’, and the lowest in both ‘Thomson Seedless’ and ‘Ruby Seedless’. There were high correlations between FT and proline concentrations of cultivars in Jan., Mar. and Apr., whereas no correlation was seen between proline and FT in Nov. (Table 6).
Water content
There were significant differences (p ≤ 0.01) in water content among cultivars in all sampling stages. From Nov. through Jan., marked bud dehydration took place in all cultivars; while bud water content enhanced with the onset of warm temperatures in Mar., and reached the highest amounts in Apr. (Table 5). In Nov., the highest bud water content was presented in ‘Ruby Seedless’, while the lowest amounts were observed in ‘Fakhri’, ‘Red Sultana’ and ‘White Sultana’. In Jan., bud water content decreased significantly in all cultivars (overall mean 42.60 % FW); however, cultivars did not show similar rates of reduction. Water content showed a general increase throughout the deacclimation period, the greatest increment was seen in Apr. At the same time, the lowest water content was found in ‘Red Sultana’ ‘Fakhri’ and ‘Shahani’, while the highest amounts were observed in ‘Yaquti’ and ‘Ruby Seedless’. Positive correlations were found between EL LT50 and BB LT50 values and bud water content in all stages; the highest correlations were seen in Nov. (Table 6).
Discussion
EL and BB measurement allowed us to discriminate grapevine cultivars, FT determined by these methods correlated well (r = 0.90). FT was developed quicker in ‘Fakhri’, ‘Red Sultana’, ‘Shahani’, ‘Sahebi’ and ‘White Sultana’, as compared to the other cultivars. These cultivars, except for ‘Sahebi’, were also the hardiest ones in Jan.; however, from Nov. through Jan., FT of these hardy cultivars enhanced by 7.01 °C on average. FT in ‘Rishbaba’ and ‘Ruby Seedless’, the least hardy cultivars in Nov., increased by 9.66 °C in Jan. (Table 1). According to Ghasemi et al. (2012), the late ripening of some fruit tree cultivars may cause a delay in leaf abscission and limit their cold acclimation in mid Nov. In contrast, ‘Yaquti’, the earliest ripening cultivar in this study (Tafazoli et al. 1994), showed an intermediate FT in the early stage of acclimation in Nov; however, it was among least hardy cultivars in later sampling stages. Grapevine cultivars which cannot complete morpho-physiological modifications involved in cold acclimation process earlier in autumn and, their bud and cane tissues mature late may experience chilling injury during exposing low temperature (Ahmedullah 1985; Karimi and Ershadi 2015).
EL LT50 of all cultivars considerably decreased in Jan., and a relatively lower variation of bud FT was found among cultivars. Some of the cell wall modifications such as lignification and suberization are crucial for plant cold acclimation development during the fall (Griffith and Brown 1982). The lower EL LT50 of mid-winter samples of grapevine cultivars might be due to the cold hardiness related alteration in cell wall structure which finally prevents electrolyte leakage from the cells of buds to the extracellular water. ‘Yaquti’ was one of the least cold tolerant cultivars from Jan. through Apr. ‘Yaquti’ is one of the leading cultivars in southern regions of Iran (lat. 30–31 N) with relatively mild winters. Generally, species/accessions from the north are more cold-hardy than those from the south, with accessions from similar latitudes usually being a little different in this regard (Zhang et al. 2012).
FT estimated based on EL LT50 in Mar. was slightly lower (1.36 °C in average), compared to Jan. (Table 1). As shown in Fig. 1, minimum daily temperatures remained relatively low since Jan. to Mar., and reached −5 °C on average in a period of 10 days prior to sampling date in mid-Mar. It is well established that preceding temperatures just prior to a freeze event intensely correlate with the FT of grape buds (Wolf and Cook 1992; Hubackova 1996). By mid-Apr., all cultivars showed a high deacclimation rate, and their FT substantially decreased. ‘Rishbaba’ and ‘White Sultana’ showed 13 and 8.7 °C decreases in FT in Apr., respectively. It seems that considerable variation in FT observed among cultivars in Apr. is partly due to their differences in deacclimation rate.
Soluble carbohydrates enhanced during cold acclimation from Nov. to Jan., then started to decrease in Mar., and was the lowest in Apr. This result is consistent with previous investigations on the seasonal changes of carbohydrates observed in other grape cultivars (Hamman et al. 1996; Jones et al. 1999). Correlation coefficients between FT and soluble carbohydrates in all stages were highly significant (Table 6), and the strongest correlations were observed in Jan., the full acclimation stage. The highest levels of soluble carbohydrates found in the cold hardy cultivars such as ‘Red Sultana’ and ‘Fakhri’ compared to the other cultivars, which confirms the involvement of carbohydrates in freezing protection and dormancy status in grapevine (Wample and Bary 1992; Hamman et al. 1996; Valle 2002). Under cold stress, polysaccharides are hydrolyzed to soluble sugars, which increase the cytoplasm osmotic potential and lower the freezing temperature (Zhang et al. 2012). Soluble carbohydrates can also function as protective substances and their concentration is positively correlated with cold hardiness (Jones et al. 1999; Zhang et al. 2012). Soluble carbohydrates decrease substantially in Apr. which may be due to its allocation to processes such as cell growth or conversion to starch during deacclimation stage (Morin et al. 2007).
There was a close relationship between proline content and FT of buds in Jan; however, this relationship was relatively weaker in Mar. and Apr. and non-significant in Nov. In higher plants, proline concentration is associated with serious abiotic stresses such as chilling stress (Taylor et al. 2004); it has also been shown that proline directly protect key cellular macromolecules, in particular, the lipid membranes and proteins such as enzymes (Verbruggen and Hermans 2008; Barka and Audran 1997; Zhang et al. 2012). Free proline can help maintain osmotic equilibrium between the symplast and apoplast and thus aid in resisting low temperature damage by maintaining the functional integrity of the cellular membranes (Dionne et al. 2001). Under low temperature stress, freezing-tolerant grape cultivars accumulate free proline, more than the less resistant ones (Dionne et al. 2001). Therefore, soluble carbohydrates and free proline contents may be considered as indicators for screening the FT of grape cultivars, especially in midwinter.
The close inverse relationship was seen between FT and bud water content during all stages (Table 6), and decreased water content was associated with increased bud hardiness confirming previous observations in grapevine (Salzman et al. 1996; Valle 2002; Kalberer et al. 2007). Our results showed that in Nov., bud water content had a stronger relationship with FT, as compared to osmoregulants. For example, ‘Rishbaba’ had fairly high soluble carbohydrate and proline contents but this cultivar, along with ‘Ruby Seedless’, had the highest bud water content and the least FT in this period. In contrast, ‘Shahani’, one of the hardiest cultivars in Nov., had relatively lower soluble carbohydrates, proline and bud water contents in this stage.
The loss of bud water content in Jan. is very often explained in terms of cold adaptation via concentration of the cell sap (Pogosyan et al. 1975). However, by spring, the dehydrative resistance decreased concomitantly with an increase in water content which reduced bud hardiness especially in cultivars with lower chilling and heat requirements such as ‘Yaquti’ and ‘Ruby Seedless’.
Cold hardiness in grapes involves a complex process of physiological changes in which molecular factors play a key role in this phenomenon. Several studies investigations have revealed the significance of C-repeat binding factors (CBFs) and dehydrins in the response of Vitis species to low temperature stress (Xiao et al. 2008; Goldsmith 2009; Tillett et al. 2012). Dehydrins are proteins believed to play a pivotal role in plant adaptation to abiotic stresses, such as drought and low temperature stresses, that lead to cellular dehydration (Fernandez-Caballero et al. 2012). Goldsmith (2009) found that grapevine tissues, which had the greatest capacity for midwinter freezing tolerance also showed the highest expression of a 39-kDa dehydrin. Four CBF genes have been already reported in Vitis species (Xiao et al. 2006, 2008). CBF1, CBF2 and CBF3 transcripts were detectable in different grapevine parts when exposed to low temperature, with higher levels in young tissues, compared to mature ones, while CBF4 expressed in both young and mature tissues. However, the cold induction of CBF genes lasts for a few hours to more than 1 day and it has not been certainly clarified whether CBF transcripts are effective enough in maintaining low temperature tolerance in over-wintering plants (Xiao et al. 2006). Furthermore, expression patterns of CBF genes did not seem to differ greatly between V. vinifera and it’s cold, hardy relative V. riparia (Xiao et al. 2008). However, CBF4-transformed ‘freedom’ grapevine showed improved freezing tolerance by 2 °C and reduced electrolyte leakage over control plants (Tillett et al. 2012). Attempts to clarify the molecular basis of FT in grapevines may lead to a better conception of the mechanism of FT.
In conclusion, the pattern of FT changes in different grapevine cultivars was consistent with bud tissue dehydration and accumulation of cryoprotectants, namely soluble carbohydrates and proline, during hardening and dehardening cycle. Thus, it is likely that the LT50 profile is a consequence of solute accumulation as well as bud dehydration. Soluble carbohydrates affirmed to be a preferable indicator of cold hardiness as compared to proline. Likewise, bud water content, especially in early autumn, had a stronger relationship with FT, compared to osmoregulants. According to our results, ‘Red Sultana’, ‘Fakhri’, ‘White Sultana’, and ‘Shahani’ can be considered to be the most frost-resistant among the twelve studied cultivars. These cultivars are excellent to be used in areas with high risk of frost incidence and could be considered in forthcoming breeding programs. On the contrary, ‘Ruby Seedless’ and ‘Yaquti’ has proven to be the most susceptible cultivars to low temperatures in autumn and winter. There was a relationship between the natural habitat of cultivars and their freezing tolerance. It should be noted that cultivars adapted to southern zones such as ‘Yaquti’ are more prone to freezing injury, compared to cultivar habituated in northern regions. Studying the expression pattern of CBF genes in these cultivars and their correlation with biochemical and physiological changes during the hardening cycle of grapevines is worthy to be considered in future researches.
Author contribution statement
Ahmad Ershadi was the project supervisor and author for correspondence. Rouhollah Karimi was responsible for collection of the plant material, and field and laboratory experiments. Karim Naderi Mahdei participated in editing the manuscript.
References
Ahmedullah M (1985) An analysis of winter injury to grapevines as a result of two severe winters in Washington. Fruit Var J 39:29–34
Alberdi M, Corcuera LJ (1991) Cold acclimation in plants. Phytochem 30:3177–3184
Barka EA, Audran JC (1997) Response of ‘Champenoise’ grapevine to low temperature: changes of shoot and bud proline concentrations in response to low temperatures and correlations with freezing tolerance. Hort Sci 72:557–582
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bourne TF, Moore JN (1991) Cold hardiness in grape cultivar development. Fruit Var J 45:26–28
Dionne J, Castonguay Y, Nadeau P, Desjardins Y (2001) Freezing tolerance and carbohydrate changes during cold acclimation of green-type annual bluegrass (Poa annua L.) ecotypes. Crop Sci 41:443–451
Fennell A (2004) Freezing tolerance and injury in grapevines. J Crop Improv 10:201–235
Fernandez-Caballero C, Rosales R, Romero I, Escribano MI, Merodio C (2012) Unraveling the roles of CBF1, CBF4 and dehydrin 1 genes in the response of table grapes to high CO2 levels and low temperature. J Plant Physiol 169:744–748
Fiorino P, Mancuso S (2000) Differential thermal analysis, supercooling and cell viability in organs of Olea europaea at subzero temperatures. Adv Hort Sci 14:23–27
Food and Agriculture Organization (2011) Statistical yearbook. FAOSTAT, New York
Ghasemi AA, Ershadi A, Fallahi E (2012) Evaluation of cold hardiness in seven Iranian commercial pomegranate (Punica granatum L.) cultivars. HortScience 47:1821–1825
Goldsmith LT (2009) Freezing tolerance and dehydrin protein expression in ‘Frontenac’ and ‘Seyval blanc’ grapevine bark and xylem cane tissues during acclimation, midwinter, and deacclimation. MSc Dissertations, Iowa State University, p 94
Griffith M, Brown GM (1982) Cell wall deposits in winter rye Secale cereale L. ‘Puma’ during cold acclimation. Bot Gaz 143:486–490
Hamman RA, Dami E, Walsh TM, Stushnoff C (1996) Seasonal carbohydrate changes and cold hardiness of Chardonnay and Riesling grapevines. Am J Enol Vitic 47:31–36
Hubackova M (1996) Dependence of Grapevine bud cold hardiness on fluctuations in winter temperatures. Amer J Enol Vitic 47:100–102
John C, Ferguson JM, Tarara LJ, Mills GG, Grove MK (2010) Dynamic thermal time model of cold hardiness for dormant grapevine buds. Ann Bot 49:4–12
Jones KS, Paroschy J, McKersie BD, Bowley SR (1999) Carbohydrate composition and freezing tolerance of canes and buds in Vitis vinifera. J Plant Physiol 155:101–106
Kalberer SR, Arora R, Leyva-Estrada N, Krebs S (2007) Cold hardiness of floral buds of deciduous azaleas: dehardening, rehardening, and endodormancy in late winter. J Amer Soc Hort Sci 132:73–79
Karimi R, Ershadi A (2015) Role of exogenous abscisic acid in adapting of ‘Sultana’ grapevine to low temperature stress. Acta Physiol Plant 37:15
Koussa T, Zaoui D, Broquedis M (1998) Relationship between the levels of abscisic acid in latent buds, in leaves and in internodes of Vitis vinifera L. cv. Merlot during the dormancy phase. J Int Sci Vigne Vin 32:203–210
Levitt J (1980) Responses of plants to environmental stress. Volume I. Chilling, freezing, and high temperature stresses. Academic, New York
Linden L (2002) Measuring cold hardiness in woody plants. Academic dissertation, University of Helsinki
Morin X, Ameglio T, Ahas R, Kurz-Besson C, Lanta V, Lebourgeois F, Miglietta F, Chuine I (2007) Variation in cold hardiness and carbohydrate concentration from dormancy induction to bud burst among provenances of three European oak species. Tree Physiol 27:817–825
Pogosyan KS, Sklyarova IA, Karapetyan ZG (1975) Relation between vine frost-resistance and tissue water content. Biol Zh Armenii 28:23–28
Rekika D, Cousineau J, Levasseur A, Richer C, Khanizadeh S, Fisher H (2004) The use of a freezing technique to determine the bud hardiness of twenty grape genotypes (Vitis sp.). Acta Hort 640:207–212
Salzman RA, Bressan RA, Hasegawa PM, Ashworth EN, Bordelon BP (1996) Programmed accumulation of LEA-like proteins during desiccation and cold acclimation of overwintering grape buds. Plant Cell Environ 19:713–720
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol 35:543–584
Stergios BG, Howell GS (1973) Evaluation of viability tests for cold stressed plants. J Amer Soc Hort Sci 98:325–330
Stergios BG, Howell GS (1977) Effect of site on cold acclimation and deacclimation of Concord grapevines. Amer J Enol Vitict 28:43–48
Sutinen ML, Palta JP, Reich PB (1992) Seasonal differences in freezing stress resistance of needles of Pinus nigra and Pinus resinosa: evaluation of the electrolyte leakage method. Tree Physiol 11:24l–254
Tafazoli E, Hekmati J, Firoozeh P (1994) Grapes. Shiraz University, Shiraz (in Persian)
Taylor CB, Thomas FM, Meyer G, Popp M (2004) Effects of defoliation on the frost hardiness and the concentrations of soluble sugars and cyclitols in the bark tissue of pedunculate oak (Quercus rubra L.). Ann Sci For 61:455–463
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Tillett RL, Wheathley MD et al (2012) The Vitis vinifera C-repeat binding protein 4 (VvCBF4) transcriptional factor enhances freezing tolerance in wine grape. Plant Physiol J 10:105–124
Valle RVB (2002) Mechanisms of frost adaptation and freeze damage in grapevine buds. PhD dissertation, Hohenheim University
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Wample RL, Bary A (1992) Harvest date as a factor in carbohydrate storage and cold hardiness of Carbernet Sauvignon grapevines. J Amer Soc Hort Sci 117:32–36
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wolf TK, Cook MK (1992) Seasonal deacclimation patterns of three grape cultivars at constant, warm temperature. Amer J Enol Vitic 43:171–179
Xiao H, Siddiqua M, Braybrook S, Nassuth A (2006) Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell Environ 29:1410–1421
Xiao H, Tattersall E, Siddiqua M, Cramer GR, Nassuth A (2008) CBF4 is a unique member of the CBF transcription factor family of Vitis vinifera and Vitis riparia. Plant Cell Environ 31:1–10
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514
Zhang J, Wu X, Niu R, Liu Y, Liu N, Xu W, Wang Y (2012) Cold-resistance evaluation in 25 wild grape species. Vitis 51:153–160
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Bavaresco.
Rights and permissions
About this article
Cite this article
Ershadi, A., Karimi, R. & Mahdei, K.N. Freezing tolerance and its relationship with soluble carbohydrates, proline and water content in 12 grapevine cultivars. Acta Physiol Plant 38, 2 (2016). https://doi.org/10.1007/s11738-015-2021-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-2021-6