Abstract
The present study aimed to evaluate the quality of cell suspension of Coffea arabica cv. Catiguá at four different ages by morphological and gene expression analysis. For this purpose, cell suspension samples were collected at 30, 45, 60 and 75 days. Morphological analysis revealed the presence of embryogenic regions in all samples, and after 60 days, non-embryogenic regions were also identified. The genic analyses revealed that as the cells were transferred to a new medium a change in both their physical and chemical conditions was noticed that caused stress. The decrease in SERK and BBM genes expression after 75th day may also be due to the non-embryogenic regions, characterized by large, elongated and vacuolated cells that were observed in the periphery of embryogenic regions, starting from 60 days of culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coffea arabica L. originates from Ethiopia. Due to its high phenotypical plasticity this species can be satisfactorily grown in several agroforestry systems. It was the first Coffea species to be described, and nowadays it stands for 70 % of all the coffee yielded and traded worldwide (Morais et al. 2003; Morais and Melo 2011). Catiguá MG2 cultivar outstands the arabica species. Due to its rust resistance (Hemileia vastatrix), small trees and specially the high cup quality it has been recommended for special coffee production. Such characteristics are appreciated of by special coffee consumers, also known as gourmet (Oliveira and Pereira 2014).
The use of somatic embryogenesis for micropropagation is thought to be a potential alternative for large-scale clonal propagation of long cycle species as it has advantages such as: highest multiplication rate, production increase by keeping the plant material in liquid medium or cryopreserved, thus eliminating researchers depending on specific periods of propagating material availability (Ferreira et al. 2005).
During the indirect embryogenesis the initial cells undergo a calli formation intermediate phase prior to the embryo formation. Those calli provide the material for the embryogenic cell suspensions (ECS) development consisting of cell aggregates dispersed in liquid medium under constant shaking (Gatica-Arias et al. 2008). As calli are naturally heterogeneous it is crucial that their most homogeneous regions with embryogenic characteristics be identified so that high-quality ECS can be obtained.
For maintenance of the nutritional balance of embryogenic cells, there is a need for periodic renewal of the culture medium. However, little is known about the exact timing of maintenance of the ECS and how this affects the embryogenic quality.
During the transition of somatic cells to the embryogenic state, the cells must dedifferentiate and activate the cell division cycle, either through physiological and metabolic changes and/or changes in gene expression patterns. Understanding of the molecular mechanisms that promote gene expression during somatic embryogenesis has advanced in recent years. Among several genes that have already been characterized, emphasis has been given to the expression of SERK (Somatic embryogenesis receptor-like kinase) and BBM (Baby Boom) genes (Schmidt et al. 1997; Boutilier et al. 2002; Schellenbaum et al. 2008).
The SERK gene belongs to the receptors kinase superfamily (RLK—Receptor-like kinases) and their products are involved in signal transduction pathways and expressed specifically in embryogenic cells (Schmidt et al. 1997; Savona et al. 2011). The BBM gene encodes a plant-specific AP2/ERF transcription factor that is related to the embryogenic process, to cell proliferation in meristematic regions and to embryo development. The AP2 transcription factor is related to embryogenic processes, whereas ERF relates to responses to biotic and abiotic stresses (Boutilier et al. 2002; Heidmann et al. 2011).
The present study aimed to evaluate the quality of a cell suspension of C. arabica cv. Catiguá at four different ages by morphological and gene expression analysis. It is expected that through SERK and BBM expression and morphological analysis, it will be possible to characterize the age of ECS that has greater embryogenic potential.
Materials and methods
Induction of cell suspension
Embryogenic calli of C. arabica cv. Catiguá MG2 were used, obtained according to the protocol of Samson et al. (2006). Calli with embryogenic features were transferred to 10-ml flasks containing 3 ml of multiplication medium (5 µM 2,4-d; 4,92 µM IBA; 9,84 µM 2-iP; 500 mg l−1 of citric acid; 20 g l−1 sucrose). As the ECS multiplied, they were transferred to larger volume flasks (25, 50 and 125 ml). The suspension was kept in the dark at room temperature, under 100 rpm orbital agitation. The culture medium was renewed by 90 % every 15 days. The renewal of the culture medium occurred on the same day of collection of cell suspensions.
Morphological characterization
Cell suspension samples of 50 ml were collected at 30, 45, 60 and 75 days of culture for photonics analysis. The samples were fixed in FAA 50 % (formaldehyde, acetic acid and alcohol) fixation solution for 48 h at room temperature and dehydrated in an ethanol series of 70, 80, 90 and 100 % for 1 h in each series, with one repetition at 100 % ethanol. Subsequently, the samples were infiltrated for 24 h with a 1:1 Historesin Leica® epoxy resin (Leica Mycrosistems Nußloch GmbH, Heidelberg, Germany) and ethanol solution, and then 24 h in pure resin. After infiltration, the samples were embedded in resin and polymerizer (15:1) and placed in an incubator at 37 °C for 3 days. Slides of 3 µm were made in microtome (EasyPath EP-31-20091), stained with 0.05 % toluidine blue solution and weak Lugol solution, and viewed under a light microscope (Axio Scope, ZEISS Germany).
Molecular characterization
Analysis of SERK and BBM expression by qPCR
Total RNA was extracted from four ECS samples corresponding to the time of collection using the NucleoSpin® kit (Macherey–Nagel, Germany), according to the manufacturer’s protocol. The RNA was quantified by NanoDrop 1000 (Thermo Fisher Scientific, Wilmington, USA) spectrophotometer (260 nm) and the integrity of RNA samples was verified by electrophoresis gel at 1 % agarose, 1 × TAE buffer, and stained with ethidium bromide. To eliminate genomic DNA the samples were treated with DNase Turbo Kit (Ambion, Waltham, MA, USA), according to the manufacturer’s protocol. DNA contamination was verified by PCR amplification of the housekeeping gene actin and visualized on a 1 % agarose gel, 1X TAE buffer, and stained with ethidium bromide. The cDNA was obtained from total RNA extracted from the ECS using the High-Capacity Kit (Applied Biosystems, Waltham, MA, USA), according to the manufacturer’s protocol. The amount of total RNA was standardized to obtain a pool of RNA at a concentration of 1 μg.
The generated cDNAs were used for quantitative analyses by real-time PCR (qPCR) [ABI PRISM 7500 Real-Time PCR (Applied Biosystems)] using the SYBR® Green (Applied Biosystem) kit, according to the manufacturer’s protocol. The PCRs containing 10 ng of cDNA template and 10 ng of primers for the gene BBM (forward primer CGAAACTTGTCACGATCCACAT and reverse primer ACCCTTGGGAAGGACTTGCT) (Silva 2011) and for the gene SERK (forward primer AAATGACCGGAGAGACCGGAAGTT and reverse primer CTGCCCAGCACTGCTCAA) (Silva et al. 2013) occurred for 5 min at 50 °C, 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 1 min at 60 °C, and termination for 15 s at 95 °C. The genes 14-3-3 and GAPDH were used as endogenous control to normalize the data (Barsalobres-Cavallari et al. 2009). The gene expression data, resulting from the amplification reactions, were normalized using the formula [ΔCT = mean CT (target gene) − mean CT (endogenous control)]. The ΔΔCT value [ΔΔCT = ΔCT (target) − ΔCT (calibrator)] was calculated for each target gene. Gene expression values were relatively quantified by the formula (RQ = 2−ΔΔCT).
Results
The ECS used in this work are part of the in vitro development material in the Central Laboratory of Molecular Biology of the Federal University of Lavras. The potential of embryogenic suspensions in terms of somatic embryo regeneration capacity is being analyzed systematically (data not shown). These data indicate that the ECS multiply faster than mature.
The effective parameter of the embryogenic capacity of a suspension cell is the frequency of regeneration of somatic embryos. With the preliminary data obtained (data not shown) and with future data regeneration experiments, we expect that high-quality suspensions could be obtained as reference, and thus the BBM and SERK relative quantification will be an evaluation parameter of the development of suspension cells very important for biotechnological purposes.
Morphological characterization
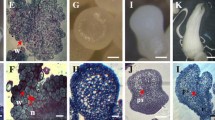
The morphological analysis of the ECS of C. arabica cv. Catiguá showed that all samples (collected at 30, 45, 60 and 75 days) had regions with embryogenic aspects (Fig. 1). These regions have features similar to that of actively dividing meristematic cells, such as the presence of small isodiametric cells that form cellular clusters. These cells have dense cytoplasm, large nuclei undergoing cell division, with evident nucleoli (Fig. 2) and small vacuoles (Guerra et al. 1999; Carvalho et al. 2001; Quiroz-Figueroa et al. 2002; Stein et al. 2010; Ribas et al. 2011; Savona et al. 2011). Another important aspect is the presence of a large number of starch grains, which affords these cells a high metabolic activity (Fig. 3). The highest starch granules intensity was observed at the beginning of the culture (at 30 days), with the concentration levels decreasing as the ECS were cultured for prolonged periods.
Starting from 60 days of culture, non-embryogenic regions, characterized by large, elongated and vacuolated cells, were observed in the periphery of embryogenic clusters (Fig. 4).
Molecular characterization
The primers had efficiencies exceeding 90 %, which allowed carrying forward the analysis of gene expression. SERK and BBM expression in ECS cultures of C. arabica varied among the four sampling times (30, 45, 60 and 75 days), showing similar behavior for both genes, with higher expression at 30 and 60 days and subsequent reductions at 45 and 75 days. It was observed that BBM expression was higher than SERK during the first 60 days of culture, at which point the expression of both genes decreased and SERK expression was maintained slightly above that of BBM at 75 days (Fig. 5).
Discussion
Morphological characterization
Little information is available in the literature in regards to ideal growing conditions, particularly with respect to the maximum time of cultivation in which the cell suspensions still maintain their embryogenic potential, as well as the frequency and volume required for renewal of the culture medium. Embryogenic cells, when exposed to ideal growth conditions, maintain continuous cell divisions that result in the formation of embryogenic cell masses called proembryogenic masses (PEMs) (George et al. 2008; Savona et al. 2011).
Toluidine blue is a dye membrane, cell and nuclear, staining of deep blue color, giving also a blue background color. Lugol is a dye used as a test for the detection of starch, staining the nuclei of brown.
In the present study, through morphological and starch content analysis, it was observed that the period of maintenance of ECS of C. arabica affects their quality. Within the first stages of cultivation (30 and 45 days), only cells with embryogenic features were observed, characterized, according to the literature, as small, isodiametric cells with prominent nuclei and nucleoli and high starch content. At subsequent stages (60 and 75 days), elongated and vacuolated cells, characteristic of non-embryogenic cells, were identified in the periphery of the embryogenic masses.
The presence of embryogenic and non-embryogenic regions in the same tissue was also observed in studies aimed at the induction of somatic embryos in C. arabica cv. Caturra (Quiroz-Figueroa et al. 2002). Morphological and histological analysis of embryogenic calli from another Coffea arabica cultivar (cv. Caturra) also showed heterogeneity of the material, with simultaneous presence of embryogenic and non-embryogenic regions in the same callus (Ribas et al. 2011).
The prescribed time to perform maintenance of the ECS as well as the media renewal volume can interfere with the quality of the cell suspension. In the present study, a biweekly renovation of 90 % of the culture medium was adopted. In another study with the same cultivar, conducted by our research group, renewal of 60 % of the culture medium every 10 days was not sufficient to prevent the formation of non-embryogenic regions (Silva 2011). This result evidences the need for further studies in this field.
Molecular characterization
The peak expression recovery of both genes (45 to 60 days) might be due to increased nutritional stress, which was more intense at 60 days. From the moment that the new medium was provided to the cells, an instantaneous change in their physical and chemical conditions caused significant stress. It should be noted that stressful conditions are, in themselves, sufficient to induce somatic embryogenesis, since BBM encodes a plant-specific AP2/ERF transcription factor. ERF proteins are related to responses against biotic and abiotic stresses (Boutilier et al. 2002; Heidmann et al. 2011; Silva 2011).
The BBM expression level was high at 30 days when fresh medium was provided to the cells. Consequently, more auxin was offered, thus decreasing SERK expression, highlighting the possibility that another stress factor to cells may be the presence of growth regulators. Thus, the generated stress causes a metabolic state that induces the expression of BBM after 45 days. With increased AP2/ERF protein, SERK expression is also induced and with increased LRR kinase and activation of the signal transduction mechanism (which occurs at the moment fresh culture medium is added), a new metabolic stress state is formed and leads to a decrease in BBM expression, inducing also the expression of SERK at 60 days.
The decline in the expression of both genes at 75 days, possibly occurred due to cell death. This can be verified by cell morphology, characterized by the presence of non-embryogenic cells that are elongated and vacuolated in the vicinity of the cell clusters (Fig. 4). The low frequency of starch grains (Fig. 3c, d) in the cells indicates low energy activity, no longer viable to reach the embryogenic state (Moura et al. 2008; Stein et al. 2010; Steinmacher et al. 2011).
Even in decline, SERK was slightly more expressed than BBM at 75 days SERK expression is up-regulated by auxin (usually 2,4-d) or other growth regulator (Santos and Aragão 2009; Savona et al. 2011). This expression possibly occurred due to the presence of some live cells that still maintained their embryogenic potential (Fig. 4). Furthermore, SERK is expressed in all stages of somatic embryogenesis as well as in non-embryogenic tissues (Mahdavi-Darvari et al. 2015).
Given the above, the renewal of 90 % of the medium leads to cellular stress, which is directly related to the expression of SERK and BBM genes, inhibiting their normal expression. The presence of growth regulators in the culture medium probably also influenced the decline in SERK expression. The biweekly medium renewal schedule exposes the cells to low nutrient depletion for long period of times, which can also cause cellular stress. Furthermore, with the delay in the renewal of the medium, the cells decrease their rate of multiplication and old and/or dead cells are mixed with new cells.
Conclusion
We identify a transient relationship between SERK and BBM induction during growth of suspension cells, first at 30 days when there was a higher expression of BBM than SERK, and after 60 days a higher expression of SERK compared to BBM. The BBM gene works as an inducer of SERK expression, as BBM encodes a transcription factor AP2/ERF which induces expression of genes associated with embryogenesis and is associated with the proliferation and cellular organization into growth regions, as well as SERK gene.
The renewal of 90 % of the medium caused a level of stress to cells, with direct relation in the genes expression, not allowing their natural expression. The presence of growth regulators in the culture medium possibly also influenced in the SERK reduction. The tissue culture explant technique leads to a significant level of stress, once they are removed from their original environment and placed on a medium containing non-physiological concentrations of growth regulators, salts and organic components. The result of this stress depends on two main factors: the stress level and the physiological state of cells. If the stress level exceeds cell tolerance, the cells die. In contrast, low levels of stress are capable of activating mechanisms that will induce cells to new conditions. The medium renewal time can be every 10 days, once that renewal is made every 15 days the cells stay longer in presence of low depletion of nutrients, which can also lead the cells to stress.
The somatic embryogenesis induction in different plant species is performed under ideal conditions, which are usually discovered by testing that evaluate the effects of different factors on the development of the explant morphogenetic responses such as growth regulators, osmotic balance, pH and nutrient concentrations. Thus, it is possible to determine which is the best factor combination and establish more efficiently a regeneration protocol, noting that the induction frequency of embryos depends not only on the cultivation conditions, but also on genotype (cultivar/species), explant type, development stage and levels of endogenous hormone.
With the regeneration experiments data in progress, with the data of this study, it is expected that the SERK and BBM gene quantification and histological images, constitute an effective parameter for evaluating the development and quality of suspension cells which have high biotechnological value.
Author contribution statement
Luana Ferreira Torres: performing experiments, data taking, manuscript writing and data analysis. Luciano Vilela Paiva: manuscript writing, data analysis and helping in preforming experiments. Kalynka Gabriella do Livramento: manuscript writing and helping in performing experiments. Luciana Lima Freire: helping in performing experiments. Leandro Eugenio Cardamone Diniz: manuscript writing, data analysis and helping in performing experiments.
Abbreviations
- MG2:
-
Minas gerais 2
- ECS:
-
Embryogenic cell suspensions
- SERK :
-
Somatic embryogenesis receptor-like kinase
- BBM :
-
Baby Boom
- 2,4-d :
-
2,4-Dichlorophenoxyacetic acid
- IBA:
-
Indole-3-butyric acid
- 2-iP:
-
2-Isopentenyladenine
- qPCR:
-
Real-time quantitative PCR
- PCR:
-
Polymerase chain reaction
- RNA:
-
Ribonucleic acid
- TAE:
-
Tris–acetate–EDTA
- DNAse:
-
Deoxyribonuclease
- cDNA:
-
Complementary DNA
- ml:
-
Milliliters
- μg:
-
Microgram
- ng:
-
Nanogram
- µM:
-
Micromolar
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
References
Barsalobres-Cavallari CF, Severino FE, Maluf MP, Maia IG (2009) Identification of suitable internal control genes for expression studies in Coffea arabica under different experimental conditions. BMC Mol Biol 10:1–11. doi:10.1186/1471-2199-10-1
Boutilier K, Offringa R, Sharma V, Kieft H, Ouellet T, Zhang L, Hattori J, Liu C, Van Lammeren A, Miki B, Custers J, Campagne M (2002) Ecotopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749. doi:10.1105/tpc.001941
Carvalho JMFC, González-Benito E, Perez C (2001) Análise histológica de calogênese e embriogênese das cultivares de algodão cnpa precoce 2 e coker 312. Rev Fibros 5:235–239
Ferreira MGR, Carvalho CHS, Carneiro AA, Damião-Filho CF (2005) Indução de embriogênese somática em cupuaçu (Theobroma grandiflorum Schum). Revista Brasileira de Fruticultura Jaboticabal 3:500–503
Gatica-Arias AM, Arrieta-Espinoza G, Esquivel AME (2008) Plant regeneration via indirect somatic embryogenesis and optimisation of genetic transformation in Coffea arabica L. cvs. Caturra and Catuaí. Electron J Biotechnol 11:1–12. doi: 10.2225/vol11-issue1-fulltext-9
George EF, Hall MA, Klerk GJ (2008) Plant propagation by tissue culture, 3rd edn. The Background, Dordrecht, p 501
Guerra PG, Torres AC, Teixeira JB (1999) Embriogênese somática e sementes sintéticas. In: Torres AC, Caldas LS, Buso LA (eds) Cultura de tecidos e transformação genética de plantas, vol 2. Embrapa, Brasilia, pp 533–568
Heidmann I, Lange B, Lambalk J, Angenent GC, Boutilier K (2011) Efficient sweet pepper transformation mediated by the BABY BOOM transcription factor. Plant Cell Rep 30:1107–1115. doi:10.1007/s00299-011-1018-x
Mahdavi-Darvari F, Noor NM, Ismanizan I (2015) Epigenetic regulation and gene markers as signals of early somatic embryogenesis. Plant Cell Tissue Organ Cult 120:407–422. doi:10.1007/s11240-014-0615-0
Morais TP, Melo B (2011) Biotecnologia aplicada ao melhoramento genético do cafeeiro. Ciência Rural 5:753–760
Morais H, Marur CJ, Caramori PH, Ribeiro AMA, Gomes JC (2003) Características fisiológicas e de crescimento de cafeeiros sombreados com guandu e cultivados a pleno sol. Pesquisa Agropecuária Brasileira 10:1131–1137
Moura EF, Ventrella MC, Motoike SY, Sá AQJR, Carvalho M, Manfio CE (2008) Histological study of somatic embryogenesis induction on zygotic embryos of macaw palm (Acrocomia aculeata (Jacq.) Lodd. ex Martius). Plant Cell Tissue Organ Cult 95:175–184. doi:10.1007/s11240-008-9430-9
Oliveira AC, Pereira AA (2014) CaféPoint. Disponível em: http://www.cafepoint.com.br/radares-tecnicos/variedades-de-cafe/cultivares-de-cafe-arabica-desenvolvidas-pela-epamig-47444n.aspx. Accessed em 17 May 2014
Quiroz-Figueroa FR, Fuentes-Cerda CFJ, Rojas-Herrera R, Loyola-Vargas VM (2002) Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea Arabica. Plant Cell Rep 20:1141–1149. doi:10.1007/s00299-002-0464-x
Ribas AF, Dechamp E, Champion A, Bertrand B, Combes MC, Verdeil J, Lapeyre F, Lashermes P, Etienne H (2011) Agrobacterium-mediated genetic transformation of Coffea arabica (L.) is greatly enhanced by using established embryogenic callus cultures. BMC Plant Biol 11:92. doi:10.1186/1471-2229-11-92
Samson NP, Campa C, Le Gal L, Noirot M, Thomas G, Lokeswari TS, Kochko AD (2006) Effect of primary culture medium composition on high frequency somatic embryogenesis in different Coffea species. Plant Cell Tissue Organ Cult 86:37–45. doi:10.1007/s11240-006-9094-2
Santos MO, Aragão FJL (2009) Role of SERK genes in plant environmental response. Plant Signal Behav 4:1111–1113. doi:10.4161/psb.4.12.9900
Savona M, Mattioli R, Nigro S, Falasca G, Della Rovere F, Costantino P, De Vries S, Ruffoni B, Trovato M, Altamura MM (2011) Two SERK genes are markers of pluripotency in Cyclamen persicum Mill. J Exp Bot 63:471–488. doi:10.1093/jxb/err295
Schellenbaum P, Jacques A, Maillot P (2008) Characterization of VvSERK1, VvSERK2, VvSERK3, and VvLIL genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.). Plant Cell Rep 27:1799–1809. doi:10.1007/s00299-008-0588-8
Schmidt ED, Guzzo F, Toonen MA, De Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Silva AT (2011) Análise da expressão dos genes baby boom (BBM) e somatic embryogenesis receptor kinase (SERK) envolvidos na embriogênese somática do cafeeiro. 2011. 132 f. Dissertação (Mestrado em Biotecnologia Vegetal). Universidade Federal de Lavras, Lavras
Silva AT, Barduche D, Livramento KG, Ligterink W, Paiva LV (2013) Characterization of a putative Serk-like ortholog in embryogenic cell suspension cultures of Coffea arabica L. Plant Mol Biol Rep 32:176–184. doi:10.1007/s11105-013-0632-x
Stein VC et al (2010) Ultrastructural calli analysis of Inga vera Willd. Rev Árvore 34:789–796
Steinmacher DA, Guerra MP, Saare-Surminski K, Lieberei R (2011) A temporary immersion system improves in vitro regeneration of peach palm through secondary somatic embryogenesis. Ann Bot 108:1463–1475. doi:10.1093/aob/mcr033
Acknowledgments
The authors would like to thank Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Wang.
Rights and permissions
About this article
Cite this article
Torres, L.F., Diniz, L.E.C., Do Livramento, K.G. et al. Gene expression and morphological characterization of cell suspensions of Coffea arabica L. cv. Catiguá MG2 in different cultivation stages. Acta Physiol Plant 37, 175 (2015). https://doi.org/10.1007/s11738-015-1924-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1924-6