Abstract
The effects of root chilling (2 °C; during 1, 5 h, 1, 2, 4 and 7 days) on the ultrastructure, functional activity of chloroplasts and cold tolerance of leaf cells of wheat (Triticum aestivum L.) were studied. Results indicated that the area of the chloroplasts increased and the number of grana in the chloroplast decreased already within first hours of the experiment. On the 2nd–7th day of the cold treatment, the length of photosynthetic membranes in the chloroplasts increased owing to the membranes of thylakoids in grana. The number of chloroplasts per cell was increased by the end of the experiment. Reduction of electron transport rate and intensification of non-photochemical quenching of chlorophyll fluorescence were observed in the first hours of root chilling. The growth of the leaves slowed in the first day of the treatment and resumed on the second day. Leaf area in the root-chilled plants by the end of the experiment exceeded the initial values by 60 %. The significant rise in cold tolerance of leaf cells was detected after 24 h of root chilling. After 48 h of the treatment, the cold tolerance reached a maximum, and did not change thereafter. It is assumed that most of the observed structural and functional changes are adaptive, and meant to support the photosynthetic function and promote the cold tolerance of the plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chloroplasts, which perform the photosynthetic and some other functions, are essential organelles in plant cells. Studies have demonstrated that they are quick to respond to changes in the environment, including exposure to low temperature (Kratsch and Wise 2000). This process involves structural and functional changes such as an increase in size and quantity of chloroplasts, formation of a great number of small grana in them, emergence of numerous plastoglobules, reduction of starch grains (Kratsch and Wise 2000; Kutik et al. 2004; Hola et al. 2008; Vella et al. 2012; Venzhik et al. 2013), as well as deceleration of photosynthesis and leaf growth, changes in chlorophyll and carotenoids content, intensification of the non-photochemical quenching of chlorophyll fluorescence, etc, (Hurry et al. 1995; Artuso et al. 2000; Yamasaki et al. 2002; Wang and Guo 2005; Ensminger et al. 2006; Garbero et al. 2012; Venzhik et al. 2011).
It quite often happens in the nature that only the roots are exposed to chilling, whereas the above-ground parts of the plant remain under physiologically normal temperature. However, the plant is a complete system in which all parts interact. On the one hand, chilling of roots causes water deficit (Aroca et al. 2001; Bloom et al. 2004; Lee et al. 2004) and degradation of mineral nutrition of plant (Bigot and Boucaud 2006; Luo et al. 2009). On the other hand, earlier studies have demonstrated (Titov et al. 2003; Talanova et al. 2010) that short-term chilling of winter wheat roots at hardening temperature initiates an increase of cold tolerance of leaf cells. Moreover, low temperature in root zone induces such biochemical and physiological changes in leaf cells as intensification of genes expression (Talanova et al. 2010), changes in the hormonal balance (Veselova et al. 2003, 2006) and inhibition of carbon dioxide assimilation (Al-Hamdani and Thomas 2000). Based on these and other data, many researchers have suggested that root cells may send the stress signal to the leaf (Jeschke et al. 1997; McCully 1999; Mishra et al. 2001; Fromm and Lauther 2007; Sicher et al. 2012). The nature of this signal is being studied now, but is not completely clear. Some scientists suggest that such signal may be bioelectric (Mishra et al. 2001; Fromm and Lauther 2007), hydraulic (Chazen and Neumann 1994; McCully 1999) or hormonal (Ternesi et al. 1994; Jeschke et al. 1997; Sicher et al. 2012). We hypothesize that the stress signal induces not only physiological but also ultrastructural changes in leaf during root chilling. Little is known about this, although the structural changes along with functional changes of chloroplasts play a significant role in the plant adaptation to cold temperatures (Kratsch and Wise 2000; Kutik et al. 2004; Hola et al. 2008; Vella et al. 2012; Venzhik et al. 2013).
Therefore, this work is aimed to study the effects of root chilling on the ultrastructure and functional activity of chloroplasts, as well as on the cold tolerance of cells in wheat leaves.
Materials and methods
Plant material and treatments
The research was carried out using the facilities of the Equipment Sharing Centre of the Institute of Biology, KarRC of RAS. Winter wheat (Triticum aestivum L. cv. Moskovskaya 39) seedlings were grown on Knop’s nutrient solution, pH 6.2–6.4, in the growth chamber for 7 day at air temperature of 22 °C, air relative humidity of 60–70 %, photosynthetic photon flux density (PPFD) of 180 μmol m−2 s−1 and 14-h photoperiod. After this roots of the seedlings were chilled for 7 day at 2 °C, which is an optimal temperature for cold hardening of wheat plants (Balagurova et al. 2001). Seedlings were placed for this in a specially designed device (Fig. 1) that allows to maintain different temperatures in the roots and aerial parts of the plants. A TZhR-02/-20 thermoelectric thermostat (Interm, Russia) was used for maintaining the required temperature around the root system (2 °C). It was installed in a growth chamber that maintained the required air temperature (22 °C). All measurements were taken from the first leaf of the seedlings in control variant (both roots and shoots at 22 °C) and during root chilling (roots at 2 °C, shoots at 22 °C) 1, 5 h, 1, 2, 4 and 7 days after the beginning of the process.
Ultrastructure study
Transmission electron microscopy was performed according to the standard procedure (Kutik et al. 2004; Garbero et al. 2012; Venzhik et al. 2013). Leaf samples (2 × 2 mm) were fixed in 3 % glutaraldehyde with phosphate buffer, pH 7.2. After postfixation with 2 % OsO4, the samples were dehydrated in graded series of ethanol, acetone and embedded in the epoxy resin Epon-812. Thin sections of leaf were cut in an ultramicrotome Ultracut (Reichert, Austria) and post-stained with uranyl acetate and lead citrate. Samples were photographed in a transmission electron microscope Hitachi 600 (Hitachi, Japan). The morphometric analysis was performed on cells of the 1st subepidermal mesophyll layer according to conventional techniques (Kutik et al. 2004; Venzhik et al. 2013).
Chlorophyll fluorescence parameters, pigment determination and growth parameters
Chlorophyll fluorescence parameters were measured with a MINI-PAM fluorimeter (Walz, Germany). Prior to the measurements, the leaves were dark adapted for 30 min. The electron transport rate (ETR) and the coefficient of non-photochemical quenching (qN) were calculated by formulas (Maxwell and Johnson 2000; Lichtenthaler et al. 2005). The chlorophyll (Chl) and carotenoids (Car) contents were determined after extraction of the pigments with ethanol using a spectrophotometer SF-2000 (Spectr, Russia) (Lichtenthaler and Wellburn 1983). Leaf area was calculated as a product of leaf length, leaf width and coefficient 0.67.
Determination of cold tolerance
The cold tolerance of the leaf cells was judged by the temperature (LT50) lethal to 50 % of the palisade cells in the leaf sections after 5 min of freezing in a TZhR–02/–20 thermoelectric microcooler (Interm, Russia), the temperature reduced gradually at regular intervals of 0.4 °C (Balagurova et al. 2001). Cell viability was assessed using a LOMO Micmed-2 light microscope (LOMO, Russia) by cytoplasm coagulation and destruction of chloroplasts.
Statistical analysis
All experiments were repeated at least three times. Approximately 25 chloroplasts (in each exposure) were examined by ultrastructural studies. The data are presented as mean ± standard error and were tested by paired Student’s t test.
Results
Effects of root chilling on ultrastructure of chloroplasts
In our study, the ultrastructure of chloroplasts in the leaves of wheat seedlings in the control (22 °C) was typical for this species: the chloroplasts were lens-shaped, with a well-developed thylakoid system in the fine-grained stroma (Fig. 2a). Root chilling (2 °C) induced multiple structural changes in leaf chloroplasts. After 1–5 h of root chilling, there appeared “distorted”, as well as “dilated” thylakoids––with enlarged lumen (intra-thylakoid space) (Fig. 2b). After 24 h of root chilling, the stroma in the chloroplasts looked more compact, and grana were hard to visualize (Fig. 2c). Note also that the chloroplasts contained invaginations (Fig. 2c) and had numerous protrusions (outgrowths) (Fig. 2d), which multiplied by the 4th–7th day of the experiment.
The morphometric treatment of the experimental samples confirmed the presence of considerable changes in the ultrastructure of the chloroplasts. Thus, after 1–5 h of root chilling (2 °C), the area of the chloroplasts increased owing to enlargement of the stroma area (Table 1). The rapid increase in size of plastids occurred during the first day of the experiment. Later on (on the 2nd–4th day of the cold treatment) this parameter steadied, and then it increased again on 7th day (Table 1). The number of chloroplasts per cell also increased by the end of the experiment (7th day) (Table 1). The number of grana in a chloroplast and the number of thylakoids per granum decreased already after 1 h of root chilling (Table 2). Further on (on the 2nd–7th day of the cold treatment), the length of photosynthetic membranes in the chloroplasts of leaf cells increased owing to the membranes of thylakoids in grana (Table 2). On the 4th–7th day of the cold impact, the number of thylakoids in grana increased (Table 2), i.e., large grana formed.
Effects of root chilling on functional activity of chloroplasts
Significant changes in the functional activity of the chloroplasts were detected during root chilling (2 °C). To wit, reduction of the ETR in the chloroplasts (Fig. 3a) and an increase in the coefficient of the non-photochemical quenching of chlorophyll fluorescence (Fig. 3b) were observed in the very first hours of the treatment. By the end of the experiment (on the 7th day), the ETR was nearly half that on the control (Fig. 3a), whereas the coefficient of non-photochemical quenching increased by more than 60 % (Fig. 3b). Chlorophyll content in wheat leaves remained constant throughout the experiment, and was lower than in the control (Fig. 4a). An increase in the carotenoid content was recorded already in the first hours of root chilling (Fig. 4b). After 24 h of exposure to cold, the carotenoid content in the leaves declined, and did not differ significantly from the control on the 7th day of the experiment (Fig. 4b).
Effects of root chilling on cold tolerance of leaf cells and growth of leaves
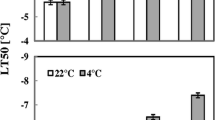
Concerning all the data, listed above, the information about the changes in the cold tolerance of leaf cells during root chilling is very important. We recorded a significant rise in this parameter after 24 h of root chilling (Fig. 5a). After 48 h of the treatment, the cold tolerance of the leaf cells reached a maximum, and did not change thereafter (4th–7th day) (Fig. 5a). At the same time, on the first day of the treatment, already the growth of the leaves slowed down (Fig. 5b). On the second day, the growth resumed, and by the end of the experiment (7th day) the leaf area in the root-chilled plants exceeded the initial values by 60 % (Fig. 5b).
Discussion
It follows from our data that the first ultrastructural changes in plastids appear very early after the roots had been exposed to cold for 1–5 h. Most of these changes are typical for the plants exposed to low air temperature (Kutik et al. 2004), and ensue from the physiological and biochemical processes in the plastids. Thus, the visually observed compaction of the stroma may be indicative of changes in its chemical composition, in the synthesis of proteins and sugars involved in cold hardening of the plants (Kratsch and Wise 2000; Cui et al. 2012). The “distorted” and “dilated” thylakoids probably appear due to modifications in the chemical composition of the stroma or to rapid transformations of the photosynthetic membranes themselves (Ma et al. 1990; Kratsch and Wise 2000; Vella et al. 2012).
Our data suggest that the size of chloroplasts increased under root chilling. This process may occur due to several factors. First, it can result from the change of the stroma area. This response is typical for plants exposed to low temperatures (Kutik et al. 2004; Vella et al. 2012). Some authors believe it to be an adaptation, because the stroma accumulates cryoprotectors and Calvin cycle enzymes (Ma et al. 1990; Kratsch and Wise 2000; Vella et al. 2012). On the other hand, enlarged stroma may also be the result of the water metabolism disturbance under root chilling (Bloom et al. 2004; Lee et al. 2004). Secondly, the increase in chloroplast’s size (especially on the 2nd–4th day of the treatment) may be related to the increased length of photosynthetic membranes and formation of large grana. It has been mentioned both in our previous studies (Venzhik et al. 2013) and by other authors (Kratsch and Wise 2000; Hola et al. 2008) that chloroplasts with small grana form in mesophyll cells in response to chilling. In our case, the peculiarity is that while the roots were cold-treated, the leaves of the wheat plants remained under physiologically normal temperature. We can assume that chloroplasts with a modified structure (large grana) formed through transduction of stress signal from the roots.
Furthermore, the increase in plastids size involves the formation of numerous protrusions and invaginations (most pronounced by the end of the treatment). They quite often appear in plants under the impact of various stress factors (Buchner et al. 2007). Protrusions and invaginations are believed to facilitate the exchange of metabolites between the cell organelles and the cytoplasm (Lütz and Engel 2007). The increase in the number of chloroplasts alongside with their enlargement observed by the 7th day of the treatment helps to maintain a certain rate of photosynthesis under stressful conditions (Aldridge et al. 2005).
It is quite obvious that at least a greater part, if not all, of the above structural changes are adaptive. The same thing we can say about functional changes in leaves observed during cooling roots. For instance, increased non-photochemical quenching of chlorophyll fluorescence is connected with the dissipation of excess light energy in the form of heat emission (Demmig-Adams and Adams 2006). This process prevents excessive cooling of photosystem II (Rapacz et al. 2004; Wang and Guo 2005). The reduced rate of electron transport, as well as fluctuations in pigments content may occur due to adaptive transformations of the membrane complex of the chloroplasts which react very responsively to a decrease in temperature (Kratsch and Wise 2000; Kutik et al. 2004).
As it is known, there is a close relationship in the response to unfavorable temperatures between the roots and above-ground parts of plant. For example, low temperature in root zone causes violations in absorption of mineral elements such as N (Macduff et al. 1987; Bigot and Boucaud 2006), P (Miyasaka and Grunes 1997; Luo et al. 2009), K (Siddiqi et al. 1984), Cu (Miyasaka and Grunes 1997), B (Ye et al. 2000) etc., Nevertheless, root chilling induced changes in water status (Aroca et al. 2001; Lee et al. 2004; Bloom et al. 2004) and growth of whole plant (Malone 1993). As already demonstrated, root chilling leads to effect on genes expression in leaf cells (Talanova et al. 2010), hormone level in leaves (Smith and Dale 1988; Veselova et al. 2003; 2006) and photosynthesis (Musser et al.1983; Al-Hamdani and Thomas 2000). Our studies have shown (Titov et al. 2003; Talanova et al. 2010) that cold tolerance of leaf cells rapidly and significantly increases under root chilling. This data suggest that root cells may send the signal to the leaf. According to research (Wildon et al. 1992; From and Bauer 1994; Herde et al. 1999), such signal may be bioelectrical in nature and quickly spread from root to the leaf tissue, causing changes in photosynthesis, phloem transport, protein synthesis. According to some scientists, such signal may have hormonal nature (Ternesi et al. 1994; Jeschke and Hartung 2000). We assume that the changes we found in the chloroplast ultrastructure may also be caused by the stress signal (it is probably fast bioelectrical signal). However, this is only an assumption that requires further investigation.
In summary, it is shown that most of changes in ultrastructure and functional activity of chloroplast that observed in our research are adaptive. Thus, the increase in size and number of chloroplasts, the intensification of non-photochemical quenching of chlorophyll fluorescence, the changes in pigment content are characteristics of plants exposed to chill and their adaptive nature known (Ma et al. 1990; Hurry et al. 1995; Kratsch and Wise 2000; Yamasaki et al. 2002; Vella et al. 2012). We compared the effects of root chilling and chilling of leaf and identified a number of differences in the effects on ultrastructure of chloroplast between the two types of temperature treatment. In particular, the chloroplasts with small grana formed in mesophyll cells in response to chilling of whole plants (Venzhik et al. 2013). On the contrary, the formation of large grana was observed in chloroplasts of plants with cooling roots. However, we identified common features in the ultrastructure of chloroplasts under the root chilling and cooling of whole plant. These include the formation of the “distorted” and “dilated” thylakoids and the increase in size of chloroplasts due to change of the stroma area. Therefore, this study demonstrates that root chilling induces the complex of changes in ultrastructure and functional activity of leaf chloroplasts contribute to the enhanced cold tolerance, which, in turn, is needed for the plants to keep functioning under the low-temperature stress.
Author contribution
Yu.V. Venzhik performed experiments and was responsible for data analysis and result interpretation. V.V. Talanova supervised all physiological aspects of investigation and involved significantly in paper preparing. E.A. Miroslavov supervised all aspects of the transmission electron microscopy. A.F. Titov, research team leader, designed and instructed the research work and involved significantly in paper preparing. All the authors were involved in the preparation and revision of the manuscript.
Abbreviations
- ETR:
-
Electron transport rate
- qN:
-
Coefficient of non-photochemical quenching
- Chl:
-
Chlorophyll
- Car:
-
Carotenoids
- LT50 :
-
Temperature lethal to 50 % of palisade parenchyma cells
References
Aldridge C, Maple J, Møller SG (2005) The molecular biology of plastid division in higher plants. J Exp Bot 56:1061–1077
Al-Hamdani SH, Thomas TS (2000) Influence of root chilling on winter and spring wheat growth and carbon dioxide assimilation. Acta Agric Scand 50:149–154
Aroca R, Tognoni F, Irigoyen JJ, Sanches-Dias M, Pardossi A (2001) Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiol Biochem 39:1067–1073
Artuso A, Guidi L, Soldatini GF, Pardossi A, Tognoni F (2000) The influence of chilling on photosynthesis and activities of some enzymes of sucrose metabolism in Lycopersicon esculentum Mill. Acta Physiol Plant 22:95–101
Balagurova NI, Akimova TV, Titov AF (2001) The effect of local cooling of cucumber and wheat seedlings on various kinds of stress resistance of their leaves and roots. Russ J Plant Physiol 48:95–99
Bigot J, Boucaud J (2006) Short-term responses of Brassica rapa plants to low temperature: effects of nitrate uptake and its translocation to the shoot. Physiol Plant 96:646–654
Bloom AJ, Zvieniecki MA, Passioura JB, Randal LB, Holbrook NM, Clair D (2004) Water relations under root chilling in a sensitive and tolerant tomato species. Plant Cell Environ 27:971–980
Buchner O, Holzinger A, Lütz C (2007) Effects of temperature and light on the formation of chloroplasts protrusions in leaf mesophyll cells of high alpine plants. Plant Cell Environ 30:1347–1356
Chazen O, Neumann PM (1994) Hydraulic signals from the roots and rapid cell-wall hardening in growing maize (Zea mays L.) leaves are primary response to polyethylene glycol-induced water deficit. Plant Physiol 104:1385–1392
Cui H, Ma W, Hu J, Li Y, Zheng Y (2012) Chilling tolerance evaluation and physiological and ultrastructural changes under chilling stress in tobacco. Afr J Agric Res 7:3349–3359
Demmig-Adams B, Adams WW (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172:11–21
Ensminger I, Busch F, Huner N (2006) Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol Plant 126:28–44
From J, Bauer T (1994) Action potentials in maize sieve tubes change phloem translocation. J Exp Bot 45:463–469
Fromm J, Lauther S (2007) Electrical signals and their physiological significance in plants. Plant Cell Environ 30:249–257
Garbero M, Andrade A, Reinoso H, Fernández B, Cuesta C, Granda V, Escudero C, Abdala G, Pedranzani H (2012) Differential effect of short-term cold stress on growth, anatomy, and hormone levels in cold-sensitive versus resistance cultivars of Digiteria eriantha. Acta Physiol Plant. doi:10.1007/s11738-012-1007-x
Herde O, Pena-Cortes H, Fuss H, Willmitzer L, Fisahn J (1999) Effects of mechanical wounding, current application and heat treatment on chlorophyll fluorescence and pigment composition in tomato plants. Physiol Plant 105:144–179
Hola D, Kutik J, Kocova M, Rothova O (2008) Low-temperature induced changes in the ultrastructure of maize mesophyll chloroplasts strongly depend on the chilling pattern/intensity and considerably differ among inbred and hybrid genotypes. Photosynthetica 46:329–338
Hurry VM, Strand A, Tobiason M, Gardestöm P, Öquist G (1995) Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. Plant Physiol 109:697–706
Jeschke W, Hartung W (2000) Root-shoot interactions in mineral nutrition. Plant Soil 226:57–69
Jeschke WD, Holodbrata M, Hartung W (1997) Growth of Zea mays L. plants with their seminal roots only. Effects of plant development, xylem transport, mineral nutrition and the flow and distribution of abscisic acid (ABA) as a possible shoot to root signal. J Exp Bot 48:1229–1239
Kratsch HA, Wise RR (2000) The ultrastructure of chilling stress. Plant Cell Environ 23:337–350
Kutik J, Hola D, Kocova M, Rothova O, Haise D, Wilhelmova N, Ticha I (2004) Ultrastructure and dimensions of chloroplasts in leaves of three maize (Zea mays L.) inbred lines and their F1 hybrids grown under moderate chilling stress. Photosynthetica 42:447–455
Lee SH, Singh AD, Chung GC, Ahn SJ, Noh EK, Stendie E (2004) Exposure of roots of cucumber (Cucumus sativus) to low temperature severely reduced root pressure, hydraulic conductivity and active transport of nutrients. Physiol Plant 120:413–422
Lichtenthaler HK, Wellburn AL (1983) Determination of total carotenoids and chlorophylls a and b of leaf exacts in different solvents. Biochem Soc Trans 11:591–593
Lichtenthaler HK, Buschmann C, Knapp M (2005) How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio Rfd of leaves with the PAM fluorimeter. Photosynthetica 43:379–393
Luo HY, Lee SK, He J (2009) Integrated effects of root-zone temperatures and phosphorus levels on aeroponically-grown lettuce (Lactuca sativa L.) in the tropics. Open Hortic J 2:6–12
Lütz C, Engel L (2007) Changes of chloroplasts ultrastructure in some light-alpine plants: adaptation to metabolic demands and climate. Protoplasma 231:183–192
Ma SF, Lin CY, Chen YM (1990) Comparative studies of chilling stress on alterations of chloroplast ultrastructure and protein synthesis in the leaves of chilling-sensitive (mungbean) and––insensitive (pea) seedlings. Bot Bull Acad Sin 31:263–272
Macduff JH, Hopper MJ, Wild A (1987) The effect of root temperature of growth and uptake of ammonium and nitrate by Brassica napus L. cv. Bien venu in flowing solution culture. II. Uptake from solution containing NH4NO3. J Exp Bot 38:53–66
Malone M (1993) Rapid inhibition of leaf growth by root chilling in wheat: kinetics and mechanisms. J Exp Bot 44:505–510
Maxwell K, Johnson G (2000) Chlorophyll fluorescence––a practical guide. J Exp Bot 51:659–668
McCully ME (1999) Root xylem embolism and refilling. Relation to water potentials of soil, roots, and leaves, and osmotic potentials of root xylem sap. Plant Physiol 119:1001–1008
Mishra NS, Mallick BN, Sopory SK (2001) Electrical signal from root to shoot in Sorghum bicolor: induction of leaf opening and evidence for fast extracellular propagation. Plant Sci 160:237–245
Miyasaka SC, Grunes DL (1997) Root zone temperature and calcium effects on phosphorus, sulfur, and micronutrients in winter wheat forage. Agron J 89:743–748
Musser RL, Thomas SA, Kramer PJ (1983) Short and long effects of root and shoot chilling of Ransome. Soybean Plant Physiol 73:778–783
Rapacz M, Gasior D, Zweirzykowski Z, Lesniewska-Bolianovska A, Humphreys MW, Gay AP (2004) Changes in cold tolerance and the mechanisms of acclimation of photosystem II to cold hardening generated by anther culture of Festuca pratensis × Lolium multifolium cultivars. New Phytol 162:105–114
Sicher RC, Timlin D, Bailey B (2012) Responses of growth and primary metabolism of water-stressed barley roots to rehydration. J Plant Physiol 169:686–695
Siddiqi MY, Memon AR, Glass ADM (1984) Regulation of K+ influx in barley: effects of low temperature. Plant Physiol 74:730–734
Smith PG, Dale JE (1988) The effects of root cooling and excision treatments on the growth of primary leaves of Phaseolus vulgaris L. Rapid and reversible increases in abscisic acid content. New Phytol 110:293–300
Talanova VV, Titov AF, Topchieva LV, Malysheva IE, Venzhik YV, Nazarkina EA (2010) Gene expression in wheat leaves under local exposure of roots to a low temperature. Doklady Biol Sci 435:438–440
Ternesi M, Andrade AP, Jorrin J, Benlloch M (1994) Root-shoot signaling in sunflower plants with confined root system. Plant Soil 166:31–36
Titov AF, Talanova VV, Akimova TV (2003) The effect of root treatment with various stress agents of plant cold- and heat-tolerance. Russ J Plant Physiol 50:94–99
Vella GF, Joss TV, Roberts TH (2012) Chilling-induced ultrastructural changes to mesophyll cells of Arabidopsis grown under short days are almost completely reversible by plant re-warming. Protoplasma 249:1137–1149
Venzhik YV, Titov AF, Talanova VV, Frolova SA, Talanov AV, Nazarkina EA (2011) Influence of lowered temperature on the resistance and functional activity of the photosynthetic apparatus of wheat plants. Biol Bull 38:132–137
Venzhik YV, Titov A, Talanova VV, Miroslavov EA, Koteeva NK (2013) Structural and functional reorganization of the photosynthetic apparatus in adaptation to cold of wheat plants. Cell Tissue Biol 7:168–176
Veselova S, Farhutdinov R, Mitrichenko A, Symonyan M, Hartung W (2003) The effect of root cooling on hormone content and root hydraulic conductivity of durum wheat seedlings (Triticum durum L.). Bulg J Plant Physiol, special Issue: 360–366
Veselova S, Farhutdinov R, Veselov DS, Kudoyarova GR (2006) Role of cytokinins in the regulation of stomatal conductance of wheat seedlings under conditions of rapidly changing local temperature. Russ J Plant Physiol 53:857–862
Wang G, Guo Z (2005) Effect of chilling stress on photosynthetic rate and chlorophyll fluorescence parameter in seedlings of two rice cultivars differing in cold tolerance. Rice Sci 12:187–191
Wildon DC, Thain JF, Minchin PEH, Gubb IR, Reilly AJ, Skipper YD, Doherty HM, O′Donnel PJ, Bowles DJ (1992) Electrical signaling and systemic proteinase inhibition in the wounded plant. Nature 360:62–65
Yamasaki T, Yamakawa T, Yamane Yo, Koike H, Satoh K, Katoh S (2002) Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in winter wheat. Plant Physiol 128:1087–1097
Ye Z, Bell RW, Dell B, Huang L (2000) Response of sunflower to boron supply at low root zone temperature. Comm Soil Plant Anal 31:2379–2392
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Feller.
Rights and permissions
About this article
Cite this article
Venzhik, Y.V., Titov, A.F., Talanova, V.V. et al. Ultrastructure and functional activity of chloroplasts in wheat leaves under root chilling. Acta Physiol Plant 36, 323–330 (2014). https://doi.org/10.1007/s11738-013-1413-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1413-8