Abstract
This study was aimed to investigate the effect of inoculation on three salt-tolerant, plant-growth-promoting rhizobacteria (PGPR) STR2 (Bacillus pumilus), STR8 (Halomonas desiderata) and STR36 (Exiguobacterium oxidotolerans), for their growth-promoting potential and efficacy in augmenting salt tolerance in Mentha arvensis, an essential oil-bearing crop and natural source of l-menthol, under varying levels of NaCl stress (0, 100, 300 and 500 mM) imposed through irrigating water. Increase in the levels of salt concentration led to a decrease in the growth, fresh weight, leaf–stem ratio, oil content and yield. However, the negative effects of salinity were observed to be convalesced in the PGPR inoculated plants. At salinity levels of 100 and 300 mM NaCl, H. desiderata inoculated plants recorded the highest herb yield, whereas at 500 mM NaCl, the plants inoculated with E. oxidotolerans yielded maximum herb. The oil content in non-inoculated, salt-stressed plants was observed to be 0.46, 0.42 and 0.35 % at 100, 300 and 500 mM NaCl, respectively, whereas the plants inoculated with H. desiderata recorded the oil content of 0.71 and 0.60 and 0.48 % at similar levels of NaCl stress, respectively. The halotolerant PGPR minimized the deleterious effects of salt toxicity producing at par or higher yields at lower and medium salinity levels (100, 300 mM NaCl) than the un-inoculated non-salt-stressed plants through improved foliar nutrient uptake and enhanced antioxidant machinery. Based on the results of the experiments reported herein, the use of salt-tolerant, plant-growth-promoting bacteria may provide an effective means of facilitating M. arvensis growth in salt-stressed environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cultivation of medicinal and aromatic plants has gained more importance and the trade in products of these plants is estimated to be over US $ 3,000 million per annum (Dagar and Singh 2007). Mentha arvensis L., commonly known as the Japanese mint or menthol mint is cultivated on a large scale in Argentina, Angola, Australia, Brazil, Bulgaria, China, Czechoslovakia, France, Hungary, India, Italy, Paraguay, Switzerland, Thailand and USA for its menthol rich essential oil used in pharmaceutical, food, flavour, cosmetic, beverage and allied industries. The annual world production of M. arvensis L. oil is 22,000 mt; India being the largest producer contributing around 16,000 mt of oil every year (Khanuja 2007; Patra et al. 2002).
Soil salinity is a serious problem and one of the major environmental factors limiting the growth and productivity of the plants (Parida and Das 2005). The amount of salt-affected land worldwide is estimated to be more than 800 million ha i.e., 6 % of the total global land mass (FAO 2008). Secondary salinisation of agricultural soils by irrigation is a serious land degradation problem in arid and semi-arid areas, where evaporation greatly exceeds precipitation and salts dissolved in the ground water reach and accumulate at the soil surface through capillary movement (Kohler et al. 2010). Soil salinization has led to increased concentration of dissolved salts in the soil profile to a level that impairs food production, environmental health and socio-economic wellbeing (Rengasamy 2006). Salinity has a direct effect on the physico-chemical and biological properties of soil leading to detrimental effects on growth as well as the productivity of the plants. The development of stress-tolerant crop varieties through genetic engineering and plant breeding is important but a long drawn process, whereas microbial inoculation to alleviate stresses in plants could be a more cost-effective and environmental friendly option, which could be obtained in a shorter time frame. Due to the ever-increasing population, the pressure on arable lands for cultivation of food crops has amplified; therefore, utilization of degraded wastelands including salt affected soils is a viable option for cultivation of medicinal and aromatic plants. With the increase in the environmental constraints such as salinity the cultivation of M. arvensis can be challenging.
The use of plant-growth-promoting rhizobacteria (PGPR) to promote plant growth under abiotic stress is a developing technology (Bacilio et al. 2004; Grover et al. 2011). PGPR comprise a diverse group of rhizosphere-colonizing bacteria which, when grown in association with a plant, stimulate growth of the host. PGPR can affect plant growth and development indirectly or directly (Vessey 2003). In indirect growth promotion, the bacteria decrease or eliminate certain deleterious effects of a pathogenic organism through various mechanisms, including induction of host resistance to the pathogen (Van Loon 2007). In direct promotion, the bacteria may provide the host plant with synthesized compounds which may facilitate uptake of nutrients; fix atmospheric nitrogen; solubilize minerals such as phosphorus; produce siderophores, which solubilize and sequester iron; synthesize phytohormones, including auxins, cytokinins, and gibberellins, which are useful at various stages of plant growth; or synthesize enzymes that modulate plant growth and development (Lucy et al. 2004; Gray and Smith 2005). With the objective of sustainable cultivation of M. arvensis, studies have demonstrated the potential of mycorrhizal fungi and bio-inoculants involving Azotobacter and mycorrhizal fungi as plant-growth-promoting micro-organisms (Gupta et al. 2002; Kashyap and Sharma 2005).
The high levels of salts particularly NaCl affect the growth and yield of plant (Coskun et al. 2013). The current study was designed to evaluate the potential of halotolerant, plant-growth-promoting rhizobacteria in ameliorating NaCl-induced salinity damage in M. arvensis. The main objective of the study was to establish their role in improving plant growth and yields under salt-stressed conditions and correlate this with the potential growth-promoting effects with overall physiology and changes in the activity of inherent antioxidant machinery of plants.
Materials and methods
Isolation of rhizobacteria
Rhizobacteria were isolated from the rhizosphere of naturally growing plants of the grass family (Poaceae) on the almost unproductive saline soils of Rae Bareilly, Uttar Pradesh, India. Ece, pH and Exchangeable Sodium Percentage (ESP) of the soil were 1.462 dS m−1, 10.71 and 50.154 %, respectively. The rhizospheric soil was serially diluted and plated on to nutrient agar (NA), King’s B agar and nitrogen free Jensen’s medium. The intrinsic resistance of all the rhizobacterial isolates against salinity was evaluated by observing the growth on nutrient agar medium amended with various concentrations of NaCl [5, 10, 12 and 20 % (w/v)]. The plates were incubated for 48 h at 28 ± 2 °C.
Functional traits of bacterial strains
The rhizobacteria were tested for presence or absence of various PGPR properties. The screening for phosphate solubilisation was done on Pikovskaya (PVK) medium (Pikovskaya 1948), respectively. IAA production and ACC deaminase activities were assessed using Salkowsky’s reagent in tryptophan amended medium (Piromyou et al. 2011) and DF minimal salts medium (Dworkin and Foster 1958) that contained ACC as the sole source of nitrogen (Shah et al. 1998), respectively. The presence of exo-polysaccharides (EPS) was confirmed following the method of Siddikee et al. (2011).
nif H amplification
Amplification of the nifH gene from the extracted DNA was performed using the primers Pol F (5′ TGCGAYCCSAARGCBGACTC-3′) and Pol R (5′-ATSGCCATCATYTCRCCGGA-3′). Amplification was performed in 50 μl final volume containing 1 μl genomic DNA (50 ng), 20 pmol each of forward and reverse primer, PolF and PolR, a 200 mM concentration of each of dNTPs (Sigma, USA), 10× Taq polymerase buffer and 2.5 U of Taq polymerase (Sigma, USA). PCR conditions consisted of initial denaturation step at 94 °C for 4 min, 30 amplification cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min and primer extension at 72 °C for 2 min; followed by a final extension at 72 °C for 5 min with thermocycler. Aliquots of the PCR products were analyzed in 1.5 % (w/v) agarose gels (Sigma, USA) by horizontal gel electrophoresis. DNAs were visualized by UV excitation after staining with ethidium bromide (Jha et al. 2009).
Plant material and salt treatments
The experiments were conducted following completely randomized block design in pots containing field soil; field soil supplemented with salt and soil supplemented with salt and inoculated with salt-tolerant PGPR. The experiments with five replicates were conducted in a glasshouse to evaluate the potential of halotolerant PGPR to mitigate salinity stress in M. arvensis. For obtaining plantlets of Mentha, one-inch long suckers were surface sterilized in 1 % HgCl2 and then planted in the earthen pots (20 cm high and 20 cm internal diameter). The potting mixture consisted of soil sterilized by autoclaving at 15 lbps/121 °C for 3 h. The soil used in this experiment was a sandy loam (Ustifluvent) with pH 7.16, EC 0.46 dS m−1, 4.64 g kg−1organic carbon, 127 kg ha−1 available N (alkaline permanganate extractable), 10.7 kg ha−1 available P (0.50 M NaHCO3 extractable), and 97.5 kg ha−1 available K (1 N NH4OAc extractable). Thirty-day-old plantlets were transplanted for use in experiments.
All the salinized pots were watered on a daily basis with sterile water for the first 2 weeks after sprouting of suckers and later supplemented with salt (NaCl) solution with concentration gradually increasing to 100, 300 and 500 mM NaCl solutions until plants were harvested. The NaCl concentrations were imposed incrementally by 50 mM every week until final concentrations were reached. The plants at zero salinity level were irrigated with water not supplemented with NaCl, i.e., non-saline water (Ec = 0.511 mS at 19.5 °C temperature).
Microbial inoculation
The bacterial inoculation to the plants was made prior to salt application. The inocula were prepared by growing rhizobacterial strains in 250 ml flasks containing nutrient broth (NB) with 5 % (w/v) NaCl till late exponential growth phase. The broth in each flask was inoculated with isolated rhizobacterial strain and incubated at 28 °C for 24 h in an orbital shaking incubator at 100 rpm. Optical density was measured to achieve uniform population of bacteria [~108 colony forming units (CFU) ml−1] in the broth prior to inoculation. The inoculums required of each culture were centrifuged at 8,000×g for 10 min. The pellets obtained were washed with sterile distilled water and then dissolved in 0.85 % saline solution. Bacterial suspension was adjusted to A600nm = 1.0. The transplants were dipped in their respective treatments for half an hour before planting and the used respective residual culture suspension (5 ml pot−1) was also poured in each treatment. Sterilized 0.85 % saline (no inoculum) was applied to plants with no rhizobacterial inoculation (control) (Bharti et al. 2013).
Experiment I: plant-growth-promoting potential of salt-tolerant PGPR
A preliminary pot experiment was conducted to determine the potential of the ten salt-tolerant rhizobacteria (STR2, STR7, STR8, STR9, STR11, STR12, STR14, STR20, STR24 and STR36) in ameliorating salinity stress in M. arvensis. Thirty-day-old transplants were planted (one per pot) with individual bacterial inoculums and subjected to 150 mM of NaCl via irrigation. The plants were harvested 60 days after transplantation. The pots subjected to salt stress without bacterial inoculation served as positive control and pots with no salinity stress and without bacterial culture acted as negative control. The three best performing, salt-tolerant, plant-growth-promoting rhizobacteria among the ten rhizobacteria inoculated were selected on the basis of their effect on growth parameters and their impact on oil content of the M. arvensis.
Experiment II: efficacy of the salt-tolerant PGPR at graded salinity levels
A second glass house experiment was conducted to determine the efficacy of the three halotolerant bacteria (STR2, STR8 and STR36) in mitigating salinity stress and enhancing productivity of M. arvensis plants under varying salinity levels, 0, 100, 300 and 500 mM NaCl.
Estimation of growth parameters
Plants were harvested 120 days after transplanting (Chand et al. 2004). Fresh shoot weight was weighed and leaf-stem ratio was calculated as [Weight of leaves (g)/Weight of stem (g)].
Essential oil extraction
For the estimation of essential oil content in fresh herb, total green shoot biomass was collected just before harvesting from each pot and was hydrodistilled in a Clevenger’s type hydro-distillation apparatus (Langenau 1948). To obtain the oil yield (ml/pot), fresh herb yield (g) obtained from the pot was multiplied with its corresponding oil content (% v/w) and further multiplied by 0.89, the specific gravity of the oil, to express this essential oil yield in g/pot (Singh et al. 2013).
Essential oil GC-FID analysis
The essential oil was analysed on an Agilent 4890D Gas Chromatograph fitted with a column (30 m × 0.25 mm, film thickness 0.25 μm, Supelco Wax-10). The column temperature was ranged from 40 to 120 °C at the rate of 3 °C min−1 with a hold time of 9 min; 120–140 °C at the rate of 2 °C min−1 with a hold time of 2 min; 140–250 °C at the rate of 5 °C min−1 with final hold time of 2 min using H2 as carrier gas at 20 psi constant column head pressure, a split ratio of 1:100, an injection size of 0.02 μl neat, and both injector and detector (FID) at temperatures were 250 °C. Identification was done using standards procured from Sigma-Aldrich.
Estimation of photosynthetic pigments concentration
Fresh leaf samples (0.1 g) were homogenised in 80 % acetone and filtered through muslin cloth. The final volume of the filtrate was maintained at 10 ml. The pigments were extracted and the absorbance was measured at 480, 510, 645 and 663 nm on a UV-Vis spectrophotometer (Specord 50). The quantification was done following the procedure of Zhang et al. (2009):
where, D663, D645, D480, D510 = absorbance at respective wavelengths (nm); v = final volume of the filtrate (10 ml) and \(w\) = fresh weight of the leaf (0.1 g).
Total proline content
The total proline in the leaves was measured using the modified procedure of Bates et al. (1973). 100 mg of plant leaves were homogenised in 1.5 ml of 3 % sulphosalicylic acid and residue was removed by centrifugation. 100 μl of extract was reacted with 2 ml glacial acetic acid and 2 ml acid ninhydrin for 1 h at 100 °C. The reaction was terminated in an ice-bath. The reaction mixture was extracted with 1 ml toluene. The chromophore containing toluene was warmed to room temperature and its absorbance was measured at 520 nm. The amount of proline was determined from a standard curve (Špoljarević et al. 2011).
Lipid peroxidation
Foliar lipid peroxidation was determined by estimating the amount of malondialdehyde (MDA) produced by the thiobarbituric acid reaction (Heath and Packer 1968). Leaves were homogenised in a mortar and pestle with ice-cold extraction buffer and centrifuged at 14,000 g for 30 min in a refrigerated centrifuge. The supernatant was used for the determination of MDA content. 4 ml of 0.5 % (w/v) thiobarbituric acid solution containing 20 % (w/v) trichloroacetic acid was added to a 1 ml aliquot of the supernatant. The mixture was heated at 95 °C for 30 min and then quickly cooled in an ice-bath. The absorbance was taken at 532 and 600 nm. After subtracting the non-specific absorbance (600 nm), the MDA concentration was determined by its molar extinction coefficient (155 mM−1 cm−1) and the results expressed as μM MDA g−1 FW.
Estimation of catalase activity
Catalase (EC 1.11.1.6) activity was estimated according to Bergmeyer (1970) which measures the initial rate of disappearance of H2O2 at 240 nm. 0.5 g tissue was ground in a cold mortar and pestle using liquid nitrogen and suspended in 1.5 ml of homogenization buffer solution (50 mM Tris–HCl; 0.1 mM EDTA; 0.2 % TritonX-100; 1 mM PMSF; 2 mM DTT). The suspension was centrifuged at 14,000 rpm for 30 min at 4 °C. The supernatant was taken for the enzyme assay.
Estimation of ascorbate peroxidase (APX) activity
APX (EC 1.11.1.11) activity was measured according to Nakano and Asada (1981). The assay depends on the decrease in absorbance at 290 nm with ascorbate oxidation. 0.5 g tissue, grinded in a cold mortar and pestle with liquid nitrogen and was suspended in 1.5 ml homogenization buffer (50 mM Na–P Buffer (pH 7.0); 2 % PVPP; 0.1 mM EDTA; 2 mM ascorbate. The suspension was centrifuged at 14,000 rpm for 30 min at 4 °C and the supernatant taken for the enzyme assay.
Foliar nutrient uptake (Na+, K+ and P)
Na+, K+ and P contents in the plant were assessed after wet digestion with HNO3 + H2O2 of air-dried ground plant samples. The K+ and P estimations were carried out as described by Singh et al. (2009). The uptake of phosphorus was determined by FIA (Flow Inject Analyzer Foss FIASTAR 5000) and potassium and sodium by flame photometer.
Molecular characterization of rhizobacteria
The three bacterial strains were characterized by 16S rRNA gene (rDNA) sequence analysis. Bacterial genomic DNA was isolated from overnight grown cells using standard procedures and 16S rDNA was sequenced (Awasthi et al. 2011). The quality and quantity of the isolated DNA was checked spectrophotometrically (Nanodrop ND1000) as well as through agarose gel electrophoresis. The universal primers (forward 5′-AGAGTTTGATCCTGGCTCAG-3′ and reverse 5′- ACGGCTACCTTGTTACGACTT-3′) were used for amplification of the 16S rRNA gene from the bacterial strain. Approximately 25 ng of bacterial genomic DNA and 5 pmol of each primer were used for amplification in a thermocycler programmed as 94 °C for 5 min; 34 cycles of 94 °C for 1 min, 57.4 °C for 1 min, 72 °C for 2 min; 72 °C for 10 min; 4 °C for an infinite period. The PCR product was purified with a PCR Cleanup Kit (Axygen) and directly sequenced using the forward universal primer and Big Dye® Terminator v3.1 cycle sequencing kit (Applied Biosystems, USA) on a 3130×l Genetic Analyzer (Applied Biosystems, USA) using the manufacturer’s protocol. Sequence analysis was carried out using the nucleotide BLAST (BLASTN) (NCBI).
Statistical analysis
The collected data were subjected to statistical analysis for analysis of variance method (ANOVA), suitable to completely randomized design (CRD) for pot experiment, with the help of software IBM SPSS PASW Statistics 18. Means and standard errors were calculated for five replicate values. Significant differences among treatments were based on ANOVA and means were calculated using Duncan’s multiple range test under a significance level of P ≤ 0.05. There were two trials conducted for each experiment. The experimental data of two trials had a similar variance value; hence the data were combined for further analysis. The results and discussion are based on the average of the two experiments. Pearson’s correlation coefficients were also computed on pooled data across salinity levels and bacterial treatments.
Results
Isolation of rhizobacteria and functional traits
Forty-five rhizobacteria were initially isolated and were screened for their plant-growth-promoting effect on Zea mays (data not shown) and the ten most promising strains were selected for further experiments. The ten rhizobacteria screened were tested for their plant-growth-promoting effects on M. arvensis in pot experiments. The isolates showed different PGP traits like P solubilisation, siderophore production, and ACC deaminase activity, exo-polysaccharide production and presence of nif H gene (Table 1).
Experiment I: plant-growth-promoting effects of salt-tolerant PGPR
The ten rhizobacteria were tested for their growth promoting and salinity amelioration effects on M. arvensis cv. Kosi. The salt-stressed plants inoculated with the salt-tolerant, plant-growth-promoting bacteria recorded improved plant growth in terms of fresh weight, leaf-stem ratio, oil yield and oil content in comparison to the un-inoculated, salt-stressed plants (Table 2). Of these ten bacteria, higher fresh weight, leaf-stem ratio and oil content, and yield were recorded in plants applied with STR2, STR8 and STR36. These three bacteria were used in the subsequent experiment.
Molecular characterization
On the basis of 16S rDNA homology the three bacteria were identified as: STR2, Bacillus pumilus (GenBank Accession No. JQ291796); STR8 Halomonas desiderata (GenBank Accession No. JQ436849) and STR36 Exiguobacterium oxidotolerans (GenBank Accession No. JQ804988).
Experiment II: efficiency of rhizobacteria at varying salt levels
Fresh weight and leaf-stem ratio
Addition of NaCl in the soil significantly decreased the herb yield and the leaf-stem ratio of un-inoculated plants (Table 3). However, inoculation with rhizobacteria significantly increased herb yield in all treatments compared to the un-inoculated plants. The plants inoculated with H. desiderata (STR8) recorded the maximum fresh weight followed by E. oxidotolerans (STR36) and B. pumilus (STR2) at levels of 100 mM NaCl stress. The fresh weight of the plants inoculated with B. pumilus, H. desiderata and E. oxidotolerans recorded increase of 34, 40 and 38 %, respectively, at 300 mM NaCl stress, whereas an increase of 23, 70 and 75 %, respectively, was noticed at 500 mM salt concentration in comparison to un-inoculated plants.
The plants not supplemented with NaCl recorded the highest leaf-stem ratio, with the maximum in the plants inoculated with H. desiderata followed by the plants inoculated with E. oxidotolerans and B. pumilus. The un-inoculated control plants exposed to 100 mM salt concentration showed a reduction of 20 % in fresh herb compared to the plants not subjected to any salt stress. H. desiderata inoculated plants recorded the maximum leaf-stem ratio among all the treatments at low and medium salinity levels as well as at zero level of salinity, whereas at 500 mM salinity level, the maximum leaf-stem ratio was observed in the E. oxidotolerans inoculated plants.
Oil yield and content
The essential oil yield among the non-salinized plants was higher than the salt-stressed plants among all the treatments (Table 3). A reduction of 31, 54 and 67 % in the oil yield of the untreated plants was observed at 100, 300 and 500 mM NaCl concentrations, respectively. The oil yield at 100 mM NaCl was reduced by 10, 17 and 23 % in plants inoculated with B. pumilus, H. desiderata and E. oxidotolerans, respectively. Likewise, the reduction at 300 mM salinity was 53 % in un-inoculated plants, 28 % in B. pumilus inoculated plants and 33 % in both H. desiderata and E. oxidotolerans treated plants. At 500 mM NaCl concentration, the oil yield in the B. pumilus, H. desiderata and E. oxidotolerans treated plants recorded a reduction of 44, 50, and 50 %, respectively, as compared to the loss of 67 % in the untreated plants.
The oil content in the non-inoculated control plants lowered to 0.46, 0.42 and 0.35 % at 100, 300 and 500 mM NaCl, respectively (Table 3). Among the PGPR inoculated plants at 0, 100 and 300 mM salinity, the highest oil content of 0.82, 0.71 and 0.60 %, respectively, was recorded in H. desiderata inoculated plants, whereas at 500 mM NaCl salinity, plants inoculated with B. pumilus recorded the highest oil content of 0.54 %.
Oil chemoarray
The gas chromatographic (GC) analysis of the M. arvensis essential oil enabled us to compare 11 major compounds, viz., α-pinene, β-pinene, sabinene, myrcene, limonene, 1,8-cineole, menthone, isomenthone, menthyl acetate, neomenthol and menthol (Table 4). The percentage concentration of menthol, the major characteristic constituent of the M. arvensis essential oil, varied with the treatments and salinity stress. The effect of salt stress on Mentha oil was more profound on its menthol content as it decreased with the increasing salinity irrespective of the microbiological application. Among all the non-stressed plants the maximum menthol content was found to be in the plants inoculated with B. pumilus followed by the plants inoculated with H. desiderata in comparison to the un-inoculated control. α-pinene and β-pinene, limonene and menthone concentration increased with increasing salt concentration irrespective of the bacterial treatment.
Photosynthetic pigments
The photosynthetic pigments recorded an overall reduction with increasing levels of salinity (Table 5). The chlorophyll a (chl a) levels in untreated control plants were found to decrease by 25.98, 49.51 and 70.80 % at 100, 300 and 500 mM salinity, respectively. Among the B. pumilus inoculated plants, the reduction at 100, 300 and 500 mM salinity levels was 15.04, 41.84 and 68.08 %, respectively, whereas, the corresponding reduction in plants inoculated with H. desiderata was 24.30, 36.58 and 62.68 %, and E. oxidotolerans was 18.43, 32.20 and 62.77 % with increasing salinity levels (Table 5).
Among the non-salinized plants, the chlorophyll b (chl b) content was higher in the rhizobacteria inoculated plants in comparison to the un-inoculated plants, with maximum being recorded in the plants treated with H. desiderata. Among the plants subjected to 100 mM NaCl solution, maximum chl b content was observed in the B. pumilus treated plants followed by H. desiderata and E. oxidotolerans when compared to the un-inoculated plants. The plants inoculated with H. desiderata recorded higher chl b content at both medium (300 mM) and high (500 mM) salinity levels among all the treated and untreated salt-stressed plants (Table 5).
The carotenoid levels in the non-salinized plants were higher in all the PGPR treated plants in comparison to the untreated plants. The non-inoculated plants recorded a higher degree of loss in the carotenoid content in comparison to the rhizobacteria inoculated plants at all salinity levels. The carotenoid levels were found to be highest in the plants inoculated with H. desiderata at all the three salinity levels as well among the non-salinized plants. The plants inoculated with B. pumilus showed higher carotenoid content in comparison to plants treated with E. oxidotolerans under non saline conditions. However, when exposed to salinity, not much difference was observed among the two rhizobacterial treatments (Table 5).
Foliar proline content
The foliar proline levels recorded a gradual increase with the increase in the salinity stress irrespective of the microbial treatments. However, the PGPR inoculated plants showed higher proline levels than the un-inoculated plants. The un-inoculated control recorded an increase of 17.34, 28.83 and 65. 71 % in proline content at 100, 300 and 500 mM of NaCl stress levels, respectively, in comparison to the non-salinised plants (Table 6). At 100 mM of salinity stress, the plants inoculated with B. pumilus, H. desiderata and E. oxidotolerans recorded an increase of 36.75, 50.99 and 57.42 %, respectively, in the proline content as compared to their non-salinized counterparts. Likewise at 300 mM of salt stress, the plants inoculated with B. pumilus, H. desiderata and E. oxidotolerans showed an increase of 68.82, 75.15 and 92.61 %, respectively, in the proline levels in comparison to the respective, non-salinized, PGPR-inoculated plants. At 500 mM, the highest increase in the proline concentration was observed in the plants inoculated with E. oxidotolerans (99.81 %) followed by H. desiderata (88.05 %) and B. pumilus (84.42 %) in comparison to non-salt-stressed plants (Table 6).
Lipid peroxidation
Foliar malondialdehyde (MDA) concentration increased at all salt concentrations indicating a concentration-dependent increase, although it was lower in the PGPR-treated plants in comparison to the untreated plants (Table 6). The plants raised without NaCl showed similar levels of MDA in all the treatments. At 500 mM salt concentration, the untreated control plants recorded twofold increased MDA levels in comparison to non-salinized untreated plants, whereas the PGPR-treated plants recorded 26.75, 33.88 and 28 % reduction in the MDA content when compared to the salt-stressed untreated plants. At low salinity levels, the plants inoculated with B. pumilus, H. desiderata and E. oxidotolerans recorded a decrease of 8.63, 7.65 and 16.56 %, respectively, in MDA levels in comparison to non-inoculated M. arvensis plants. Similarly, at medium salinity levels the MDA concentration reduced by 18.03, 11.65 and 13.63 % in plants inoculated with B. pumilus, H. desiderata and E. oxidotolerans compared to the un-inoculated plants (Table 6).
Catalase activity
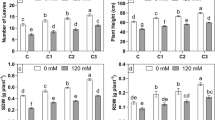
Significantly higher catalase activity was observed in the PGPR-inoculated plants at all salinity levels in comparison to the un-inoculated plants (Fig. 1). A gradual increase in the catalase activity was noticed with the increased salt stress levels. The un-inoculated plants recorded an increase of 46.82, 71.00 and 161.93 % in catalase activity at 100, 300 and 500 mM of salinity levels in comparison to non-salt-stressed plants. At 100 mM NaCl stress, among the PGPR treated plants H. desiderata inoculated plants recorded a higher catalase activity. At 300 mM NaCl salinity all the three PGPR recorded similar catalase activity, whereas at 500 mM NaCl, H. desiderata and E. oxidotolerans inoculated plants showed comparable levels of enzyme activity and B. pumilus inoculated plants recorded lower activity of catalase (Fig. 1).
The effect of the three salt-tolerant, plant-growth-promoting bacteria STR2 (Bacillus pumilus), STR8 (Halomonas desiderata) and STR36 (Exiguobacterium oxidotolerans) on the catalase content in the leaves of M. arvensis cv. Kosi exposed to 0, 100, 300 and 500 mM NaCl stress. Control indicates the plants with no bacterial treatment. Values are the means of five replicates. The bars indicate the standard error of mean. Different letters above the error bars show significant difference at P ≤ 0.05 (Duncan’s test)
Ascorbate peroxidase (APX) activity
The non-salinized plants recorded similar levels of ascorbate peroxidase activity irrespective of the microbial inoculations (Fig. 2). An increased enzyme activity was observed in all the plants exposed to salinity stress in comparison to non-salinized plants. However, the enzyme activity in PGPR inoculated plants was higher than the non-inoculated plants. The plants inoculated with H. desiderata and E. oxidotolerans recorded similar levels of enzyme activity at all salinity levels.
The effect of the three salt-tolerant, plant-growth-promoting bacteria STR2 (Bacillus pumilus), STR8 (Halomonas desiderata) and STR36 (Exiguobacterium oxidotolerans) on the ascorbate peroxidase content in the leaves of Mentha arvensis cv. Kosi exposed to 0, 100, 300 and 500 mM NaCl stress. Control indicates the plants with no bacterial treatment. Values are the means of five replicates. The bars indicate the standard error of mean. Different letters above the error bars show significant difference at P ≤ 0.05 (Duncan’s test)
Foliar nutrient uptake
The plants subjected to salinity stress demonstrated an exponential increase in the sodium levels in comparison to the non-salinized plants irrespective of the microbiological treatments (Table 7). At the low salinity level (100 mM NaCl) Na+ content of 1.02, 0.80 and 0.93 % was observed in the B. pumilus, H. desiderata and E. oxidotolerans inoculated plants, respectively, in comparison to 1.66 % Na+ content in un-inoculated control. The sodium ion content in plants exposed to 300 and 500 mM salinity was as high as 2.00 and 2.10 %, respectively, in the un-inoculated control whereas in B. pumilus inoculated plants it was found to be 1.45 and 1.86 %, respectively. The lowest Na+ content (0.93 %) among all the microbiological treatments at 300 mM NaCl of salt stress was found to be in H. desiderata inoculated plants, whereas E. oxidotolerans applied plants recorded a Na+ content of 1.84 %. The PGPR-inoculated plants did not have much significant difference in the Na+ content at high salinity level (500 mM NaCl). The negative correlation between the growth parameters and sodium ion content in plants (Table 8) indicates the damaging impact of the salt-stressed conditions on plant growth and survival.
Unlike the Na+ content, the K+ levels dropped in all the plants subjected to salinity. The un-inoculated plants recorded a decrease of 33, 44.5 and 57.26 % in the K+ levels at 100, 300 and 500 mM NaCl stress, respectively, in comparison to the plants not subjected to any salt stress. Similarly, the B. pumilus inoculated plants showed 36, 42.2 and 51 % decrease in 100, 300 and 500 mM NaCl treated plants, respectively. The H. desiderata and E. oxidotolerans inoculated plants showed ~15 % decrease in the K+ content when subjected to 100 mM NaCl solution in comparison to non-salinized PGPR treated plants. At 300 mM of salt stress H. desiderata and E. oxidotolerans inoculated plants recorded a decrease of 31.92 and 24.13 % in the K+ levels, respectively, whereas a decrease of 37.30 and 48.27 % was recorded in H. desiderata and E. oxidotolerans treated plants, respectively, in the foliar K+ levels at 500 mM of salt stress (Table 7).
The non-salinized plants treated with B. pumilus and E. oxidotolerans recorded 10.86 and 16.86 % increase in the foliar phosphorus content in comparison to the non-inoculated control. The un-inoculated plants subjected to 100 mM NaCl irrigating solution recorded a decrease of 30.12 % in P content whereas the plants inoculated with B. pumilus, H. desiderata and E. oxidotolerans showed a reduction of 30.43, 17 and 22.6 %, respectively, in comparison to their non-salinized control plants. The plants not applied with any microbial treatment showed a decrease of 43.37 and 56.62 % in the content of P at 300 and 500 mM of salinity, respectively, in comparison to the control plants not subjected to salt stress. The plants treated with B. pumilus, H. desiderata and E. oxidotolerans recorded a decrease of 36.95, 37.80 and 34.02 %, respectively, in the phosphorus content of Mentha leaves in comparison to their respective non-salinized control plants. Similarly, at 500 mM of salinity level a reduction of 42, 46.34 and 44.32 % was observed in the plants inoculated with B. pumilus, H. desiderata and E. oxidotolerans, respectively, when compared to PGPR-treated plants not subjected to any salt stress (Table 7).
Discussion
The increasing salinity levels adversely affected the growth and essential oil yield of the M. arvensis plants. However, when the plants were inoculated with the salt-tolerant PGPR, the extent of growth suppression decreased, which suggested participation of the rhizobacteria in alleviating some of the debilitating effects of salt stress.
Of the three PGPR strains applied to Mentha plants, two strains H. desiderata (STR 8) and E. oxidotolerans (STR 36) produced copious amounts of exo-polysaccharide (EPS) under salt stress. EPS forms an organo-mineral sheath around cells which leads to an increase in micro aggregates as an indirect additional effect increasing aggregate stability. EPS also possesses unique water holding and cementing properties, playing a vital role in the formation and stabilization of soil aggregates and regulation of nutrients and water flow across plant roots through biofilm formation (Roberson and Firestone 1992). The growth promoting and increased salt tolerance effects of the B. pumilus (STR2) inoculation could be attributed to its 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity as well as the siderophore producing capacities as observed under in vitro salt-stressed conditions. ACC deaminase activity of rhizobacteria has been reported to play a vital role in ameliorating abiotic stress conditions (Barnawal et al. 2012). Many reports suggest the role of Bacillus spp. and Exigubacterium spp. in promoting plant growth as well as ameliorating abiotic stress (Dastager et al. 2010; Selvakumar et al. 2010; Sgroy et al. 2009).
Salinity reduces plant productivity first by reducing plant growth during the phase of osmotic stress and subsequently by inducing leaf senescence during the phase of toxicity when excessive salt is accumulated in transpiring leaves (Munns 2002). The leaves being the source of the essential oil are thus the most economically viable parts of the M. arvensis and the oil yield is hence directly proportional to the number of leaves. The oil content in a plant is largely dependent on the physiological state of the plant (Maffei et al. 1986). Salinity reduced the oil yield in Mentha plants, presumably by imposing additional energy requirements on plant cells and less carbon available for growth leading to reduced synthesis of essential oil. Farooqi et al. (1999) reported that a number of environmental conditions/factors have high impact on the performance of Mentha spp. plants including essential oil content and composition. In the large-scale production of plants containing essential oil, even relatively small increases of the essential oil concentration in combination with an improved plant growth can result in an enhanced essential oil yield of economic interest. The present study correlates the PGPR-induced increased oil yield and content under various levels of salt stress to the improved productivity and herb yield, as suggested by highly significant positive correlation between oil yields, fresh shoot weight and leaf-stem ratio (Table 8). Gharib et al. (2008) reported that aqueous extracts of biofertilizers, composts and PGPRs increased the essential oil yield in Origanum majorana plants. An investigation has revealed that inoculation of Ocimum basilicum roots with plant-growth-promoting rhizobacteria (PGPR) improved growth and accumulation of essential oils (Ordookhani et al. 2011). Essential oil yield was also significantly increased relative to non-inoculated plants, without alteration of oil composition in P. fluorescens and Bradyrhizobium inoculated O. majorana (Banchio et al. 2008). Contrary to the results obtained in the present study, (Karray-Bouraouia et al. 2009) suggested that with the increase in salt stress, the oil content in Mentha pulegium increased owing to the higher trichome density.
The developmental arrangement and coexistence of new as well as mature glands lead to a heterogeneous pattern in oil content. Limonene and menthone are the major monoterpenes present in the youngest leaves and the proportion of limonene declines rapidly with development, whereas menthone increases its prominence and declines only at later stages as menthol becomes the dominant monoterpene constituent (Gershenzon et al. 2000). New glands are continually produced during leaf growth and that newly initiated glands do occur together with mature glands in growing regions, such that neighboring glands within the same leaf zone are often of different ages. Brun et al. (1991) and Voirin and Bayet (1996) observed relatively high menthone content in young leaves and high menthol content in older leaves with an increase in menthyl acetate content during leaf senescence. Significant differences in the menthone and menthol content of glands were shown to correlate with gland position along the leaf axis of expanding leaves. Thus, the higher menthol content in all the PGPR-treated plants in comparison to the un-inoculated plants at all the salinity levels could be attributed to the increased leaf-stem ratio and delayed senescence of mature leaves.
Plants, owing to their bioenergetic lifestyle, are at higher risk of reactive oxygen species (ROS)-induced oxidative damage (Gill and Tuteja 2010). Malondialdehyde (MDA) content, a product of lipid peroxidation, is an indicator of the degree of oxidative membrane damage owing to the salt stress. The decrease in MDA content in response to salinity recorded in the PGPR treated plants is in congruence with the earlier studies correlating management of lipid peroxidation to better stress tolerance mechanisms (Khan and Panda 2008). Proline can account for up to 20 % of the free amino acid pool after sodium chloride stress as observed in Arabidopsis (Kavi Kishor et al. 2005). Owing to the inherent plant salt tolerance and adaptation mechanisms the proline levels increased in all the salt-stressed plants irrespective of the microbial application, but the proline levels were much higher in the rhizobacteria-treated plants in comparison to the untreated plants. The proline biosynthesis mediates increased NADP+/NADPH ratio; this change in ratio affects carbon flux through oxidative pentose phosphate pathway (OPPP) (Hare and Cress 1997). This in turn provides precursors in the form of erythrose-4-phosphate to synthesize phenylpropanoids or secondary metabolites during stress conditions (Maggio et al. 2002). Earlier a positive correlation has been observed between stress pressure and proline accumulation (Claussen 2005). Yoshiba et al. (1997) reported that the PGPR inoculation improved the proline content which may be due to the upregulation of proline biosynthesis pathway to keep proline in high levels, which help in maintaining cell water status thus helping plants to cope up with the salinity stress.
The catalase enzyme is associated with the decomposition of hydrogen peroxide into H2O and O2 and is crucial for ROS detoxification (Scandalios et al. 1997). APX is involved in scavenging of H2O2 in water–water and ASH-GSH cycles and utilizes ASH as the electron donor. In the present study the catalase activity, ascorbate peroxidase activity as well as the proline levels increased with the increasing levels of salinity in both the PGPR inoculated and the un-inoculated plants. Thus, increased stress tolerance in PGPR inoculated plants could be correlated with the higher proline levels and antioxidant enzyme activity. Chlorophyll concentration in stressed tissue can be construed as an index of tissue tolerance to NaCl. High carotenoids content is closely associated with antioxidant enzyme systems of the plants (Sairam et al. 1997). In accordance with the earlier studies the plants recorded increasing decrease in the chlorophyll content with increasing the salinity levels whereas the carotenoid content ascended with ascending levels of stress. These evidences support the model that PGPR application promotes plant growth and ameliorates stress by inducing plant physiological protection against oxidative damage.
Potassium has been reported to be one of the most growth limiting factors in M. pulegium under saline conditions (Oueslati et al. 2010). The decrease in the content of potassium and phosphorus in the salt-stressed Mentha as well as increased absorption of Na+ in plants resulted in poor plant growth and oil yield regardless of the PGPR inoculation. However, the microbial treatments reduced the Na+ uptake and increased the K+ uptake compared with control plants under salt stress, thus increasing the K+/Na+ ratio. A favourable K+/Na+ ratio in the cytosol are essential for maintaining plant cell function (Syed 1999). Since, the K+/Na+ ratio was higher in H. desiderata and E. oxidotolerans treated plants in comparison to the B. pumilus plants, the exo-polysaccharides produced by H. desiderata and E. oxidotolerans could play a role in the restricted sodium uptake. Rhizobacteria have been reported to down regulate hkt1 (High Affinity K Transporter 1) expression in roots but upregulate it in shoots, orchestrating lower Na+ levels and recirculation of Na+ in the whole plant under salt conditions (Zhang et al. 2008).
Phosphate nutrition of plants is a limiting factor for growth under salinity; higher salinity inhibiting phosphate uptake in plants (Pick et al. 1990; Martinez and Lauchli 1994). Inoculation of PGPR, with the ability to dissolve phosphorus, enhance the quantity of effective phosphorus and increase crop yields under salt stress (Yao 2004; Prasad et al. 2012). These evidences support the fact that inoculation of E. oxidotolerans (STR36) promoted plant growth and stress tolerance via increase in the phosphate availability to the Mentha plants.
The escalating degradation of soil and ensuing salinity levels have been a major constraint in crop productivity. Countless physical and chemical means of ameliorating these salt-stressed soils have been exploited which are cost intensive. Thus, evolving efficient, low cost, easily adaptable methods for the management of salt-stressed soil is a major challenge. The study on the role of microbes in management of saline soil is gaining momentum owing to their cost effective and environment friendly approach. The results of the present study endorse the idea of managing salt-stressed soil for improved crop productivity through the application of salt-tolerant, plant-growth-promoting rhizobacteria.
Author contributions
N. Bharti and A. Kalra designed the experiments and wrote the manuscript. N. Bharti carried out the experiments. D. Barnawal helped in interpretation of the results and drafting the manuscript. A. Awasthi helped with the molecular characterization of rhizobacteria. A. Yadav analysed the essential oil samples. All authors have read and approved the final manuscript.
References
Awasthi A, Bharti N, Nair P, Singh R, Shukla AK, Gupta MM, Darokar MP, Kalra A (2011) Synergistic effect of Glomus mosseae and nitrogen fixing Bacillus subtilis strain Daz26 on artemisinin content in Artemisia annua L. Appl Soil Ecol 49:125–130
Bacilio M, Rodriguez H, Moreno M, Hernandez JP (2004) Mitigation of salt stress in wheat seedling by a gfp-tagged Azospirillum lipoferum. Biol Fertil Soils 40:188–193
Banchio E, Bogino PC, Zygadlo J, Giordano W (2008) Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem Sys Ecol 36:766–771
Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2012) 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol Biochem 58:227–235
Bates LS, Waldren RD, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bergmeyer N (1970) Methoden der enzymatischen analyse. Akademie Verlag, Berlin, pp 636–647
Bharti N, Yadav D, Barnawal D, Maji D, Kalra A (2013) Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World J Microbiol Biotechnol 29(2):379–387
Brun N, Colson M, Perrin A, Voirin B (1991) Chemical and morphological studies of the effects of aging on monoterpene composition in Mentha × piperita leaves. Can J Bot 69:2271–2278
Chand S, Patra NK, Anwar M, Patra DD (2004) Agronomy and uses of menthol mint (M. arvensis)––Indian perspective. Proc Ind Natl Sci Acad B70(3):269–297
Claussen W (2005) Proline as a measure of stress in tomato plants. Plant Sci 168:241–248
Coskun D, Britto DT, Jean Y-K, Kabir I, Tolay I et al (2013) K+ efflux and retention in response to NaCl stress do not predict salt tolerance in contrasting genotypes of rice (Oryza sativa L.). PLoS One 8(2):e57767. doi:10.1371/journal.pone.0057767
Dagar JC, Singh G (2007) Biodiversity of saline and waterlogged environments: documentation, utilization and management. NBA Scientific Bulletin Number: 9. National Biodiversity Authority, Chennai p78
Dastager SG, Kumaran DC, Pandey A (2010) Characterization of plant growth-promoting rhizobacterium Exiguobacterium NII-0906 for its growth promotion of cowpea (Vigna unguiculata). Biologia 65(2):197–203
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
FAO (2008) FAO land and plant nutrition management service. http://www.fao.org/ag/agl/agll/spush
Farooqi AHA, Sangwan NS, Sangwan RS (1999) Effect of different photoperiodic regimes on growth, flowering and essential oil in Mentha species. Plant Grow Regul 29:181–187
Gershenzon J, McConkey ME, Croteau RB (2000) Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol 122:205–213
Gharib FA, Moussa LA, Massoud ON (2008) Effect of compost and bio-fertilizers on growth, yield and essential oil of sweet marjoram (Majorana hortensis) plant. Int J Agri Biol 10:381–387
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol Biochem 37:395–412
Grover M, Ali Sk Z, Sandhya V, Rasul A, Venkateswarlu B (2011) Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J Microbiol Biotechnol 27:1231–1240
Gupta ML, Prasad A, Ram M, Kumar S (2002) Effect of the vesicular-arbuscular mycorrhizal fungus Glomus fasciculatum on the essential oil yield related characters and nutrient acquisition in the crops of different cultivars of menthol mint (M. arvensis) under field conditions. Biores Technol 81:77–79
Hare PD, Cress WA (1997) Metabolic implications of stress induced proline accumulation in plants. Plant Grow Regul 21:79–102
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jha B, Thakur MC, Gontia I, Albrecht V, Stoffels M, Schmid M, Hartmann A (2009) Isolation, partial identification and application of diazotrophic rhizobacteria from traditional Indian rice cultivars. Eur J Soil Biol 45:62–72
Karray-Bouraouia N, Rabhib M, Neffatic M, Barbara Baldand B, Ranierie A, Marzoukc B, Lachaâla M, Smaouib A (2009) Salt effect on yield and composition of shoot essential oil and trichome morphology and density on leaves of M. pulegium. Ind Crops Prod 30:338–343
Kashyap S, Sharma S (2005) Role of bioinoculants and auxin in development of salt tolerant Mentha arvensis. Hort Sci (Prague) 32:31–41
Kavi Kishor PB, Sangam S, Amrutha RN, Sri Laxmi P, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sc 88(3):10
Khan MH, Panda SK (2008) Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant 30:81–89
Khanuja SPS (2007) Aroma vision 2020: technology path of cultivating today for a fragrant tomorrow. National convention and seminar on business enabling of aromatic plants and products, Dehradun, pp 21–22
Kohler J, Caravaca F, Roldán A (2010) An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa. Soil Biol Biochem 42:429–434
Langenau EE (1948) The examination and analysis of essential oil synthetics and isolates. In: Guenther E (ed) The essential oils vol I. N.J.D. Von Nostrand Co.Inc., Princeton, pp 229–367
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86:1–25
Maffei M, Gallino Sacco T (1986) Glandular trichomes and essential oils of developing leaves in Mentha viridis lavanduliodora. Planta Med 52:187–193
Maggio A, Miyazaki S, Veronese P, Fujita T, Ibeas JI, Damsz B, Narasimhan ML, Hasegawa PM, Joly RJ, Bressanet RA (2002) Does proline accumulation play an active role in stress-induced growth reduction? Plant J 31:699–712
Martinez V, Lauchli A (1994) Salt-induced inhibition of phosphate uptake in plants of cotton (Gossypium hirsutum L.). New Phytol 125:609–614
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ordookhani K, Sharafzadeh S, Zare M (2011) Influence of PGPR on growth, essential oil and nutrients uptake of sweet basil. Adv Env Biol 5(4):672–677
Oueslati S, Karray-Bouraoui N, da Attia H, Rabhi M, Ksouri R, Lachaal M (2010) Physiological and antioxidant responses of M. pulegium (Pennyroyal) to salt stress. Acta Plant Physiol 32:289–296
Parida SK, Das AB (2005) Salt tolerance and salinity effects on plants. Ecotoxicol Environ Saf 60:324–349
Patra NK, Kumar B, Shukla K, Ram P, Srivastava HK (2002) Problems and issues of agrotechnology transfer in menthol mint: a case study with variety kosi. In: Proceedings of First National Interactive Meet on Medicinal and Aromatic Plants CIMAP, pp 440–443
Pick U, Bental M, Chitlaru E, Weiss M (1990) Polyphosphate hydrolysis––a protective mechanism against alkaline stress? FEBS Lett 274:15–18
Pikovskaya RI (1948) Mobilization of phosphorous in soil in connection with the vital activity of some microbial species. Microbiologiya 17:362–370
Piromyou P, Buranabanyat B, Tantasawat P, Tittabutr P, Boonkerd N, Teaumroong N (2011) Effect of plant growth promoting rhizobacteria (PGPR) inoculation on microbial community structure in rhizosphere of forage corn cultivated in Thailand. Euro J Soil Biol 47:44–54
Prasad A, Kumar S, Pandey A, Chand S (2012) Microbial and chemical sources of phosphorus supply modulate the yield and chemical composition of essential oil of rose-scented geranium (Pelargonium species) in sodic soils. Biol Fertil Soils 48:117–122
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57(5):1017–1023
Roberson E, Firestone MK (1992) Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol 58:1284–1291
Sairam RK, Shukla DS, Saxena DC (1997) Stress induced injury and antioxidant enzymes in relation to drought tolerance in wheat genotypes. Biol Plant 40:357–364
Scandalios JG, Guan L, Polidords AN (1997) Catalases in plants: gene structure, proprieties, and expression. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defences. Cold Spring Harbor, New York, pp 343–406
Selvakumar G, Kundu S, Joshi P, Nazim S, Gupta AD, Gupta HS (2010) Growth promotion of wheat seedlings by Exiguobacterium acetylicum 1P (MTCC 8707) a cold tolerant bacterial strain from the Uttarakhand Himalayas. Ind J Microbiol 50(1):50–56
Sgroy V, Cassán F, Masciarelli O, Florencia M, Papa D, Lagares A, Luna V (2009) Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl Microbiol Biotechnol 85(2):371–381
Shah S, Li J, Moffatt BA, Glick BR (1998) Isolation and characterization of ACC deaminase genes from two different plant growth-promoting rhizobacteria. Can J Microbiol 44:833–843
Siddikee MA, Glick BR, Chauhan PS, Yim WJ, Sa T (2011) Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol Biochem 49:427–434
Singh R, Parameswaran TN, Rao EVSP, Puttanna K, Kalra A, Srinivas KVNS, Bagyaraj DJ, Divya S (2009) Effect of arbuscular mycorrhizal fungi and Pseudomonas fluorescens on root-rot and wilt, growth and yield of Coleus forskohlii. Biocontrol Sci Technol 19:835–841
Singh R, Soni SK, Patel RK, Kalra A (2013) Technology for improving essential oil yield of Ocimum basilicum L. (sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind Crop Prod 45:335–342
Špoljarević M, Agić D, Lisjak M, Gumze A, Wilson ID, Hancock JT, Teklić T (2011) The relationship of proline content and metabolism on the productivity of maize plants. Plant Sign Behav 6(2):251–257
Syed MA (1999) Nutrient uptake by plants under stress conditions. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 295–296
Van Loon LC (2007) Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Voirin B, Bayet C (1996) Developmental changes in the monoterpene composition of Mentha × piperita leaves from individual peltate trichomes. Phytochemistry 43:573–580
Yao T (2004) Associative nitrogen-fixing bacteria in the rhizosphere of Avena sativa in an alpine region III. Phosphate-solubilizing power and auxin production. Acta Prataculturae Sinica 13:85–90
Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 38:1095–1102
Zhang H, Kim MS, Sun Y, Dowd SE, Shi H, Paré PW (2008) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant Microbe Inter 21:737–744
Zhang J, Han C, Liu Z (2009) Absorption spectrum estimating rice chlorophyll concentration: preliminary investigations. J Plant Breed Crop Sc 1(5):223–229
Acknowledgments
The authors wish to thank the Director, CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, India, for providing necessary facilities and encouragement during the course of investigation and the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing the financial support in form of SRF to NB, DB and AA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Kuzniak-Gebarowska.
Rights and permissions
About this article
Cite this article
Bharti, N., Barnawal, D., Awasthi, A. et al. Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in Mentha arvensis . Acta Physiol Plant 36, 45–60 (2014). https://doi.org/10.1007/s11738-013-1385-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1385-8