Abstract
Stress caused by soil salinity and soil drought limits cotton productivity in China. To determine the tolerance levels of cotton, we assessed the effects of soil salinity and soil drought on the biochemical characteristics of the roots of two cotton cultivars (CCRI-44, salt-tolerant; Sumian 12, salt-sensitive). Specifically, we analyzed root biomass, fatty acid composition, antioxidative enzyme activity, lipid peroxidation, H+-ATPase and Ca2+-ATPase activities. The cotton root biomass of the two cultivars declined significantly under conditions of soil salinity, soil drought, and the two stressors combined. The antioxidant enzyme activity of the roots also decreased markedly, which caused lipid peroxidation to increase, and changed the composition of the fatty acid membrane. H+-ATPase, Ca2+-ATPase and antioxidant enzyme activity decreased more under the two stressors combined. However, H2O2 content and O2 − generation increased under the two stressors combined, compared to each stressor separately. Overall, the combination of soil salinity and drought has a greater inhibitory effect and more harmful impact on root growth than each stressor separately. The higher tolerance of CCRI-44 to soil salinity and drought stress than Sumian 12 might be explained by differences in cotton root antioxidative enzyme activity. The lipid peroxidation levels of cotton roots might represent an important biochemical trait for stress tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton responds to change in its growth environment because of its indeterminate growth habit (Reddy et al. 1997), and it has been reported to be both salt- and drought-tolerant (Thomas 1980). With population growth and increasing food demand in China, large areas of fertile land previously cultivated with cotton are now planted with food crops, such as rice and maize (Zhao and Tisdell 2009). As a result, cotton is primarily planted on non-irrigated and saline-alkali soils, particularly coastal saline soils. However, cotton growth in coastal salt marshes and other saline-alkali soils tends to be negatively affected by both high soil salt content and limited water availability. High soil salinity content and soil drought are common abiotic stressors that adversely affect rice crop growth and yield formation (Xiong et al. 2002). Therefore, it is necessary to study the physiological response of cotton to soil salinity, soil drought, and the two stressors combined to develop appropriate strategies for cotton production under adverse environmental conditions.
A number of existing studies have described the effect of soil salinity and soil drought on cotton growth, along with the resistance mechanisms of cotton to these stress factors (Ashraf and Ahmad 2000; Garratt et al. 2002; Meloni et al. 2003b; Vulkan-Levy et al. 1998). Soil salinity inhibits cotton growth, with declining growth decline often being associated with decreased photosynthetic capacity (Meloni et al. 2003a). Soil drought is also assumed to cause a decrease in photosynthesis and growth, and is associated with changes in carbon and nitrogen metabolism (Lawlor and Cornic 2002).
The root is the only organ of the plant that penetrates the soil and is, therefore, the only organ that is in direct contact with adverse stressors. Hence, it is expected that the root is the first organ to be subject to salinity stress. The root growth of cotton seedlings is noticeably inhibited by high concentrations of sodium chloride (NaCl) solution (Zhong and Lāuchli 1993). Salinity generally reduces the root growth of crops; yet, the root growth of some cultivars is actually enhanced by mild salinity stimulation. However, such effect mainly involves induced oxidative stress through the overproduction of reactive oxygen species (ROS), such as superoxide anion radicals (O ·−2 ), hydroxyl radicals (OH·), and hydrogen peroxide (H2O2) (Ashraf 2009). These ROS are extremely cytotoxic, and seriously disrupt normal metabolism through oxidative damage to lipids, nucleic acids, and proteins (Herbette et al. 2002). As a consequence, a series of cellular degeneration processes are triggered, including the peroxidation of membrane lipids and programmed cell death (Gechev et al. 2002).
To avoid damage caused by ROS compounds, plants have evolved molecular defense systems that both limit the formation of ROS and promote their removal (Alscher et al. 2002). Plant enzymatic defenses include superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), which minimize cellular damage caused by ROS (Khatun et al. 2008). SOD is a major scavenger of superoxides, transforming dismutase superoxide into hydrogen peroxide and molecular oxygen. Hydrogen peroxide, which is produced by dismutase superoxide, is scavenged by CAT and a variety of POD with the concomitant oxidation of co-substrates, such as phenolic compounds and antioxidant molecules (Mckersie and Leshem 1994). Antioxidant enzyme activity decreases with increasing ROS accumulation in plants subject to high soil salinity or soil drought conditions (Mittler 2002).
Oxygenated fatty acid (FA) derivatives called oxylipins are central to the regulation of plant development processes and defense responses. LOX is a dioxide synthase that uses C18:2 and C18:3 FAs as substrates to catalyze the degradation of lipid membranes, causing the structure and function of the membrane to change (Isamah 2004; Sofo et al. 2004). LOX catalyzes the dioxygenation of polyunsaturated FAs to produce hydroperoxy FAs, which are highly reactive compounds that are toxic to plant cells. These FAs are rapidly degraded into metabolites for the production of jasmonate, conjugate dienoic acid, and volatile aldehyde, such as malondialdehyde (MDA) (Bird and Draper 1984; Siedow 1991). Greater amounts of MDA (as the decomposition product of polyunsaturated fatty acids of biomembranes) accumulate under salt stress (Gosset et al. 1994). A significant increase of LOX activity and MDA content has also been observed during progressively greater increments of soil salinity stress in functional cotton leaves (Zhang et al. 2013).
Plasma membrane (PM) stability is widely used to differentiate salt-tolerant cultivars from salt-sensitive cultivars of some crop plants. For instance, in some cases, higher membrane stability is correlated with abiotic stress tolerance (Hernandez et al. 2000; Luna et al. 2000; Meloni et al. 2003b). Plant PMs contain an H+-ATPase that is pivotal to nutrient uptake (Fricker and Willmer 1990). The enzyme generates an electrochemical potential gradient across the PM for active transport. The transportation of inorganic nutrients to the root xylem and organic nutrients to the leaf phloem is dependent on the activity of this enzyme (Bowling 1981). The presence of H+-ATPase in the functional PM of root endodermal cells and xylem parenchyma cells indicates that the control of ion uptake across the PM is regulated by this enzyme (Cooke and Burden 1990).
Several preceding studies have analyzed the functional properties, biosynthesis, and genetics of the leaf/environment interface of cotton (Meloni et al. 2003b; Norbert and Tchiadje 2007). However, limited information is available about the plant/soil interface formed by cotton roots. Thus, here, we aimed to evaluate the effect of long-term soil salinity (ST), soil drought (DT), and a combination of the two stressors (SD) on the antioxidative enzyme activity and lipid peroxidation of the roots of two cotton cultivars (genotypes) with different levels of drought and salinity stress. The acquisition of such information is anticipated to contribute toward improving our understanding about the physiological and biochemical mechanisms of drought and salinity tolerance.
Materials and methods
Plant materials and treatments
Pot experiments were conducted in the summer of 2008 in a greenhouse at the Pailou Experimental Station of Nanjing Agricultural University, which is located in Nanjing (32°02′N and 118°50′E), Jiangsu Province, China. The minimum and maximum air temperatures were 19 and 34 °C, respectively, while the relative humidity ranged from 40 to 68 %. The daily average value of solar irradiance was 6 MJ m−2.
Two cotton (Gossypium hirsutum L.) cultivars with different levels of salt tolerance were used in this study, namely, CCRI-44 (salt-tolerant) and Sumian 12 (salt-sensitive) (Zhang et al. 2011). Both cultivars are widely grown in the Yangtze River Valley region of China. Cotton seeds were sown in a nursery bed on 25 April 2008. Healthy and uniform seedlings with three true leaves were transplanted into plastic pots 50 cm in height, 33 cm in width, and filled with 30 kg of air-dried soil. Yellow brown soil collected from 0 to 30 cm topsoil layer of the experimental station was passed through a 2-mm sieve and packed into the pots. Selected physical and chemical properties of the soil are presented in Table 1.
Four treatments were performed for each cotton cultivar: (1) control (CK), in which the relative water content of 0–20 cm depth soil was monitored every 2 days using a portable time-domain reflectometry (TDR) soil moisture device (TRIME-EZ, IMKO, Germany), with the pot being constantly watered to maintain the relative water content of the soil in the range of 70–80 %; (2) soil drought (DT), soil water content was monitored every 2 days using a portable TDR soil moisture device, and the pot was constantly watered to maintain the relative water content of the soil in the range of 50–60 %; (3) soil salinity (ST): seven different salts (sodium carbonate, sodium bicarbonate, sodium chloride, calcium chloride, magnesium chloride, magnesium sulfate, and sodium sulfate) were individually mixed into CK soil at an even molar ratio, to obtain a salt/dry soil ratio of 0.60 % (w/w, dry soil weight base; the resulting ion concentrations in the treated soil were similar to those observed in local coastal saline soil); and (4) the combined action of soil salinity and soil drought (SD). CK and ST were implemented during the transplanting of seedlings, whereas DT and SD were imposed 20 days after transplanting. Before placing soil to the pots, 4.5 g N, 0.36 g P2O5, and 0.9 g K2O were added to the soil. An additional 4.5 g N was used to top-dress each pot at the early flowering stage (35 days after cotton seedling transplantation) for all treatments. The experiment was arranged using a completely random design. Each treatment had 20 replicates, with a single plant in 1 pot representing 1 replicate.
Measurement of root dry weight
Three plants from each treatment were cut at the cotyledonary node during the budding stage, the flowering and boll-forming stage, and the boll-opening stage. The root was separated from the soil by washing. White young root samples of 0.5 g weight were immediately used to assay root activity using the triphenyl-tetrazolium chloride (TTC) method (Li 2000). Meanwhile, 7.0 g of white young roots were immediately frozen in liquid N2 and stored at −80 °C for enzyme activity analysis. The remaining root was dried to a constant weight in an oven at 80 °C and used to determine dry weight.
Determination of H2O2 content, O2 − generation rate, and antioxidant enzyme activity
The O2 − generation rate was determined by following the methods of Yang et al. (2008). In brief, the roots (0.2 g) were homogenized with 1.8 mL 50 mM potassium phosphate (pH 7.8), and centrifuged at 5,000×g for 10 min. The incubation mixture, containing 0.5 mL potassium phosphate (pH 7.8) and 1 mL of 1 mM hydroxylamine hydrochloride, was incubated at 25 °C for 20 min and then added to 1 mL of 17 mM sulfanilamide and 1 mL of 7 mM α-naphthylamine. The mixture was incubated at 25 °C for 20 min. The O2 − generation rate was then determined by the absorbance at 530 nm.
The H2O2 content was assayed using a modified method of Ferguson et al. (1983). In brief, the frozen root (1 g) was homogenized in 5 mL cold acetone after the extract was centrifuged (5,930×g) at 4 °C for 15 min. The supernatant was collected and 0.5 mL of titanium tetrachloride (TiCl4) reagent (20 % in concentrated HCl) was added. While mixing, 3.5 mL NH4OH (25 %) was added in a drop-wise fashion. The sample was centrifuged (5,930×g) at 4 °C for 5 min. The supernatant was discarded. The precipitate was dissolved in acetone until the solution became colorless. The washed precipitate was dissolved in 20 mL of 1 M H2SO4, and filtered prior to measuring absorbance at 415 nm against a blank, which had been subjected to the same procedure but contained no plant material. Standards in the range of 0.75–5.35 mM H2O2 were treated with TiCl4 and subjected to the same procedure.
Superoxide dismutase (EC 1.15.1.1) activity was determined based on the method of Foster and Hess (1980). In brief, the root (100 mg) was homogenized in 5 mL of extraction buffer (0.1 M phosphate buffer of pH 7.5, containing 0.5 mM ethylenediaminetetraacetic acid [EDTA]). The enzyme extract was centrifuged at 10,000×g for 10 min at 4 °C. After centrifugation, the supernatant was collected and used as the enzyme source. The reaction mixture (3.0 mL) contained 0.1 mL of 1.5 M sodium carbonate, 0.2 mL of 200 mM methionine, 0.1 mL of 2.25 mM NBT, 0.1 mL of 3 mM EDTA, 1.5 mL of 100 mM potassium phosphate buffer, 0.7 mL of distilled water, and 0.2 mL of enzyme extract. The reaction was started by adding 0.1 mL of riboflavin (60 μM) and placing the tubes below the light source of two 15-W florescent lamps for 15 min. Illuminated and non-illuminated reactions without enzymes were used for calibration. Absorbance was recorded at 560 nm by using a spectrophotometer. One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of the basic rate of the reaction. Catalase (EC 1.11.1.6) activity was measured following the method of Beers and Sizer (1952), with minor modifications. A quantity of 100 mg of root tissue was homogenized in 5 mL of 0.1 M phosphate buffer (pH 6.4) using a chilled pestle and mortar. The extract was centrifuged at 10,000×g for 20 min at 4 °C. The supernatant was collected and used in the enzyme assay. The reaction mixture contained 2.6 mL of 0.1 M phosphate buffer (pH 6.4), 0.2 mL of enzyme extract, and 0.1 mL of 10 mM H2O2, to produce a final volume of 3.0 mL. The reaction mixture was mixed rapidly at room temperature. Changes in absorbance at 230 nm at an interval of 15 s were recorded for 2 min on an UV visible spectrophotometer. One unit of catalase activity was defined as the amount of enzyme catalyzing the decomposition of 1 nmol of H2O2 per min (extinction coefficient 36 mM cm−1).
Peroxidase (EC 1.11.1.7) activity was estimated following the protocol of Tan et al. (2008). The enzyme was extracted by homogenizing 100 mg of root tissue in 5.0 mL of 0.1 M phosphate buffer (pH 6.4). The crude extract was centrifuged at 10,000×g for 20 min at 4 °C. The supernatant was collected. The reaction mixture was prepared by adding 4.5 mL of 0.1 M phosphate buffer (pH 6.4), 0.2 mL of pyragallol (50 μM), 0.1 mL of 50 μM H2O2, and 0.2 mL of the supernatant. The mixture was incubated at 25 °C for 5 min. Then, 0.5 mL of 5.9 % H2SO4 was added to terminate the reaction. Absorbance was recorded at 420 nm. One unit of enzyme activity was defined as that amount of enzyme that formed 1 mmol of purpurogallin per minute under assay conditions.
Determination of lipoxygenase activity and MDA content
LOX was spectrophotometrically assayed at 234 nm following the method of You et al. (2009). In brief, frozen root segments (0.5 g) were crushed into fine power using a pestle and mortar in an ice bath. About 5.0 mL of 0.1 M phosphate buffer containing 0.5 mmol L−1 PMSF and 0.6 mmol L−1 EDTA (pH 7.0) was used as the extraction buffer. The homogenate was centrifuged at 15,000×g for 15 min at 4 °C, and then the supernatant was used for the measurement. LOX activity was analyzed in 2.8 mL of 0.1 M phosphate buffer containing 0.1 mL of 100 mmol L−1 sodium linoleate. The increase in absorbance at 234 nm was recorded after adding 0.2 mL of the enzyme extract.
Frozen root segments (0.5 g) were crushed into fine powder using a pestle and mortar in an ice bath. A total of 5.0 mL of 0.05 M phosphate butter (pH 7.8) with 1 % polyvinylpyrrolidone (PVP) was used as the extraction butter. The homogenate was centrifuged at 15,000×g for 15 min at 4 °C, and then the liquid was used to measure MDA content. The MDA content was measured by following the method of Zhang (1992).
Lipid extraction and analysis
Lipid was extracted from tonoplast vesicles, following the method of Yu and Su (1996). The fatty acids of the total lipid membrane extract were analyzed using the method described by Diao et al. (1997), with some modifications. In brief, the fatty acid methyl esters were analyzed using a gas chromatograph (GC-7AC, Shimadzu, Japan), equipped with a 2.1 mm × 3.2-mm silica capillary column, which was packed with 10 % Silar-10CP on Chromosorb W AW DMCS (80–100), and a flame ionization detector (FID). The working conditions were as follows: temperature of the column (175 °C); temperature at injection and detection (240 °C); flow rate of nitrogen (50 mL/min); air pressure (0.5 kg/cm2); and hydrogen pressure (0.6 kg/cm2). The methyl esters of the fatty acid samples were identified based on their retention time compared to that of standards. The amount of fatty acid methyl esters was determined using areas under chromatographic peaks, which were calculated with a data processor (C-RIB, Shimadzu, Japan). The double bond index (DBI) was used to measure the degree of FA unsaturation.
Preparation of plasma membrane vesicles and ATPase activity assay
Plasma membrane vesicles were isolated, as described by Qiu (1999), with modifications. Roots (100 mg) were cut into pieces and homogenized in an isolation medium (1:3, w/v) containing 0.25 M sucrose, 1 mM ATP, 10 % glycerol, 0.5 % bovine serum albumin, 3 mM EDTA, 1 mM DTT (dithiothreitol), 1 mM PMSF (phenylmethylsulfonyl fluoride), 15 mM mercaptoethanol, and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-Tris (pH 7.6). The homogenate was filtered through two layers of cheesecloth and centrifuged at 13,000×g for 15 min. The supernatant was then centrifuged for 30 min at 80,000×g. The crude microsomes were collected and suspended in a buffer solution containing 0.25 M sucrose, 0.1 mM DTT, and 5 mM K2PO4 at pH 7.8. The suspension was then added to the phase mixture to produce a phase system with 6.2 % PEG 3350, 6.2 % dextran T-500, 5 mM K2PO4, and 0.25 M sucrose at pH 7.8. The final upper phases were collected and diluted with a suspension buffer of 0.25 M sucrose, 1 mM dithiothreitol, and 2 mM Tris-MES (2-(N-morpholino)ethanesulfonic acid; pH 7.2). The suspension was centrifuged for 30 min at 100,000×g, after which the pellet was collected. All of the above steps were carried out at 4 °C. The purity of the PM was estimated based on the method described by Widell and Larsson (1990). Vanadate inhibition of H+-ATPase activity was 72 %. The inhibition rates of this enzyme by nitrate, azide, and molybdate were less than 2.1, 2.7, and 5.0 %, respectively.
H+-ATPase activity was determined by measuring the release of Pi from ATP, based on the methods of Qiu (1999), with minor modifications. The assay medium used for the present study contained 3 mM ATP, 3 mM MgSO4, 50 mM KCl, 1 mM NaN3, 50 mM NaNO3, 0.1 mM Na2MoO4, 0.02 % Triton X-100, 25 mM Tris-MES (pH 6.5), and 20 μg PM protein. Ca2+-ATPase activity was measured on the basis of the methods of He et al. (2001). Activity was expressed in μmol L−1 pi mg−1 pro min−1.
Data analysis
Data were subjected to an analysis of variance using the SPSS (SPSS Software Inc., USA) statistic package version 17.0. Difference between mean values greater than the LSD (P = 0.05) was determined as significant. Figures were illustrated using OrginPro 7.5 (OrginLab Inc. USA).
Results
Root dry weight
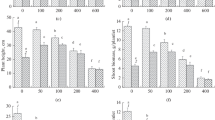
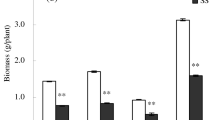
Although root dry weight decreased significantly under soil salinity, soil drought, and their combined action, a marked reduction of root dry weight was observed under the combined action of soil salinity and soil drought (Fig. 1). The root dry weight of the CK treatment was significantly higher than that of ST, DT, and SD. For example, during the flowering and boll-forming stage, the root dry weight of CK was higher by 30, 54, and 84 % for CCRI-44, and by 49, 82, and 98 % for Sumian 12, compared to the ST, DT, and SD treatments, respectively. This result shows the salt-tolerant cultivar, CCRI-44, grew better than the salt-sensitive cultivar, Sumian 12, when subjected to ST, DT, and SD.
Effects of soil salinity and soil drought on the dry weight of cotton root. Bars are ± one standard error (SE). When no bar is visible, the SE is smaller than the symbol. Values followed by the different letters within the same cultivar are significantly different at P = 0.05 probability level. Each data represents the mean of three replications. CK control, DT soil drought, ST soil salinity, SD combined action of soil salinity and soil drought, BS budding stage, FBS flowering and boll-forming stage, BOS boll-opening stage
Antioxidant enzyme activity, H2O2 content, and O2 − generation rate
As shown in Fig. 2a–c, no significant difference was found for CK, ST, DT, and SD treatments with respect to SOD, CAT, and POD activity in the cotton root at the budding stage. However, the SOD and POD activity of the two cultivars at the flowering and boll-forming stage and boll-opening stage decreased under ST, DT, and SD treatments, with a marked reduction under SD. SD proved to be a better inhibitor of enzyme activity in both cultivars compared to ST or DT treatments, particularly at the flowering and boll-forming stage and boll-opening stage.
Effects of soil drought and soil salinity on the activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and hydrogen peroxide (H2O2) content, and O2 − generation rate in cotton root. Bars are ± one standard error (SE). When no bar is visible, the SE is smaller than the symbol. Values followed by the different letters within the same cultivar are significantly different at P = 0.05 probability level. Each data represents the mean of three replications. CK control, DT soil drought, ST soil salinity, SD combined action of soil salinity and soil drought, BS budding stage, FBS flowering and boll-forming stage, BOS boll-opening stage
Both H2O2 content and the O2 − generation rate in cotton root were much higher under ST, DT, and SD compared to CK at different growth stages (Fig. 2d, e). SD had maximal values for H2O2 content and O2 − generation rate. Changes in the O2 − generation rate and H2O2 content were more noticeable for the salt-sensitive cultivar, Sumian 12, compared to the salt-tolerant cultivar, CCRI-44, at the flowering and boll-forming stage and boll-opening stage. Thus, under ST, DT, and SD, severe lipid peroxidation occurred in cotton roots as a result of enhanced H2O2 production and O2 − generation rates, which caused a significant decrease in SOD, CAT, and POD activity.
MDA content and LOX activity
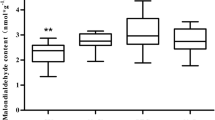
As shown in Fig. 3, the MDA content of cotton root significantly increased in the ST, DT, and SD treatments compared to the CK treatment at the budding stage, flowering and boll-forming stage, and boll-opening stage. Furthermore, a marked increase was observed in MDA content under the SD treatment. This result demonstrated serious effect of the combined action of soil salinity and soil drought on cotton roots.
Effects of soil salinity and soil drought on malondialdehyde (MDA) content and LOX activity in cotton root. Bars are ± one standard error (SE). When no bar is visible, the SE is smaller than the symbol. Values followed by the different letters within the same cultivar are significantly different at P = 0.05 probability level. Each data represents the mean of three replications. CK control, DT soil drought, ST soil salinity, SD combined action of soil salinity and soil drought, BS budding stage, FBS flowering and boll-forming stage, BOS boll-opening stage
Compared to the CK treatment, the LOX activity of cotton root increased under the ST, DT, and SD treatments (Fig. 3). A significant difference in LOX activity was observed between DT and ST at the boll-opening stage, whereas no statistically significant difference was observed among these two treatments at the budding stage or the flowering and boll-forming stage. However, SD had a more pronounced effect than the DT and ST treatments, and induced the most significant increase in LOX activity at all growth stages of cotton. The LOX activity and MDA content of cotton roots for salt-tolerant CCRI-44 under soil salinity, soil drought, and their combined action were significantly lower compared to the salt-sensitive Sumian 12.
PM H+-ATPase and Ca2+-ATPase activity
As shown in Fig. 4, the activity of PM H+-ATPase and Ca2+-ATPase in cotton roots was significantly affected by soil salinity and soil drought. Unlike root dry weight, the H+-ATPase and Ca2+-ATPase activity of cotton roots was more sensitive to soil drought compared to soil salinity. At the budding stage, the H+-ATPase and Ca2+-ATPase activity of the cotton roots had no significant difference for any of the treatments (ST, DT, or SD). In contrast, a significant decrease was recorded at the flowering and boll-forming stage and boll-opening stage, with a smaller reduction under DT compared to ST. Moreover, soil drought significantly exacerbated the adverse effect of salinity on H+-ATPase and Ca2+-ATPase activity. Thus, SD caused the most significant reduction in enzyme activity at the budding stage, the flowering and boll-forming stage, and the boll-opening stage. H+-ATPase and Ca2+-ATPase activity under soil salinity and soil drought conditions was reduced more for Sumian 12 compared to CCRI-44 for all treatments (ST, DT, SD) compared to the control (CK). These results indicate that Sumian 12 is more sensitive to soil salinity and soil drought compared to CCRI-44.
Effects of soil salinity and soil drought on the activities of plasma membrane H+-ATPase and Ca2+-ATPase in cotton root. Bars are ± one standard error (SE). When no bar is visible, the SE is smaller than the symbol. Values followed by the different letters within the same cultivar are significantly different at P = 0.05 probability level. Each data represents the mean of three replications. CK control, DT soil drought, ST soil salinity, SD combined action of soil salinity and soil drought; BS budding stage, FBS flowering and boll-forming stage, BOS boll-opening stage
FA composition
As shown in Table 2, FAs included palmitic acid (16:0), stearic acid (18:0), linoleic acid (18:2), and linolenic acid (18:3). These four types of FAs accounted for more than 95 % of total FA content in cotton roots. Soil salinity, soil drought, and their combined action changed the FA composition of PM, causing a particular decrease in the percentage of C18:2 and C18:3. This decrease was paralleled by an increase in the percentage of C16:0 and C18:0. Meanwhile, minor unsaturated oleic acid (C18:1) content slightly increased, which caused the double bond index (DBI) to decrease. ST and SD caused a similar decrease in DBI, with the additive effects of the combined action of soil salinity and soil drought being clearly shown. The FA composition of the salt-tolerant cultivar CCRI-44 was stable, with a higher proportion of unsaturated FAs and a lower proportion of saturated FAs compared to the salt-sensitive cultivar Sumian 12.
Discussion
Salinity and drought are two environmental constraints that often occur simultaneously in arid region. The ability of crops to overcome multiple stressors simultaneously is very important for plant growth and survival in stressful environments (Lichtenthaler 1996). The root is the first organ to come into contact with such stressors. As a result, the root is considered to be the main organ responsible for crop tolerance to drought and salinity, with it actively excluding excess ions (Chen and Plant 1999). However, root growth is known to be inhibited by drought (Amelia et al. 2011; Carmi et al. 1993) and salinity (Gapińska et al. 2008; Reinhardt and Rost 1995), separately. Yet, in the current study, both soil salinity and soil drought, either alone or in combination, reduced root dry weight. Ultimately, SD had a higher inhibitory effect on root growth compared to soil salinity and soil drought, which is similar to the results obtained in a study using cowpeas (Costa et al. 2007). In addition, the root dry weight of CCRI-44 (salt-tolerant) was higher than that of Sumian 12 (salt-sensitive) under DT, ST, and SD. This result indicates that the salt-tolerant cultivar is more tolerant to soil salinity, soil drought, and a combination of the two compared to the salt-sensitive cultivar.
Environmental stress, including high soil salinity and soil drought, causes oxidative stress via the production and accumulation of ROS. However, ROS is generated by LOX metabolites via the simulation of NADPH oxidase, with a potential signaling connection between LOX metabolites and NADPH oxidase possibly existing (Akter et al. 2010). These oxyradicals initiate lipid peroxidation, in which FA radicals (L, LO, and LOO) are produced. FA radicals are not very stable molecules; hence, FA radicals readily react with molecular oxygen, to become transformed into a variety of lipid peroxides, which ultimately break into MDA, along with other aldehyde and short-chain ketones, carboxylic acid, and hydrocarbons (Somerville and Browse 1991). These series of reactions cause the FA content to decrease and the MDA content to increase. This result supports the observed reduction in unsaturated FAs under soil salinity, soil drought, and their combined action in this study. LOX catalyzes unsaturated FA peroxidation, producing MDA and other products. Heidari and Tafazoli (2005) concluded that LOX activity increased in the three rootstocks leaves as salinity increased. In this study, LOX activity in cotton roots also increased under soil salinity, soil drought, and their combined action. MDA production increased with increasing LOX activity, indicating that LOX catalyzed FA peroxidation under soil salinity and soil drought conditions, similar to the findings of Kumar et al. (2011).
H2O2 is a ROS that may be adequately controlled by antioxidant and antioxidant enzymes under normal conditions. However, the overproduction of ROS under stressed conditions risks serious cellular damage (Zhou et al. 2008). In addition, free radical-induced peroxidation in the lipid membrane is both a reflection and a measure of stress-induced damage at the cellular level (Demiral and Turkan 2005). Lower levels of H2O2 were observed in the salt-tolerant cultivar CCRI-44 compared to the salt-sensitive cultivar Sumian 12 under ST, DT, and SD conditions. These results indicate that the capacity for protection from oxidative damage by soil salinity or soil drought stress is enhanced in CCRI-44. Similar results were reported for Alfalfas, whereby drought (Naya et al. 2007) and heavy metals (Zhou et al. 2007) caused the stress-tolerant cultivars of these species to exhibit a higher tolerance to oxidative damage under stress.
To cope with oxidative damage under extremely adverse conditions, crops have developed an antioxidant defense system that includes the antioxidant enzyme of SOD, POD, and CAT (Mittler 2002). The activity level of antioxidant enzymes is higher in tolerant versus sensitive species under various forms of environmental stress (İsmail et al. 2005). In the present study, higher SOD activity was observed in salt-tolerant CCRI-44 compared to salt-sensitive Sumian 12 under soil salinity, soil drought, and their combined action. This result indicates that the H2O2 and O2 − scavenging ability of the salt-tolerant cultivar was better compared to the salt-sensitive cultivar. This result also supports reports of increased SOD activity in salt-tolerant pea (Hernandez and Almansa 2002) and sugar beet (Bor et al. 2003) cultivars under salt stress, in addition to the drought-tolerant common bean cultivar (İsmail et al. 2005).
CAT and POD are also the most important enzymes involved in the regulation of H2O2 at an intracellular level. In this study, CAT, and POD activity significantly decreased under DT, ST, and SD conditions. The activities of CAT and POD under ST and DT were higher than those of SD. This result indicates cotton roots subject to ST and DT conditions have better O2 − radical scavenging ability compared to those subject to SD. Salt-tolerant plants exhibit higher CAT and POD activity, which helps to protect the plants against oxidative stress. This activity has not been observed in salt-sensitive plants (Scalet et al. 1995). In this study, CAT and POD activity in the cotton roots of CCRI-44 were higher compared to Sumian 12 under soil salinity, soil drought, and their combined action. Furthermore, the elevated activity of POD and CAT in CCRI-44 subject to the three stress conditions (ST, DT, and SD) might reflect an increase in ROS-scavenging capacity and a decrease in damage to the lipids of the plasma membrane. The evidence obtained in this study also indicated the important role of antioxidant enzymes in the establishment of cotton seedlings under soil salinity and soil drought conditions. The levels of various low-molecular antioxidants need to be determined in future studies to help further develop our understanding about the different levels of stress tolerance in the two cultivars.
Several publications have demonstrated that alterations in the ROS-scavenging system may cause considerable modifications to the oxidative stress tolerance of plants, which alters their tolerance to abiotic stressors (Pastori and Foyer 2002). The increased activity of the antioxidant enzyme was more marked in the salt-tolerant cultivar compared to the salt-sensitive cultivar. In addition, the antioxidant enzyme activity of SOD, CAT, and POD was positively correlated with the degree of salt tolerance of the two canola cultivars (differing in salt tolerance), and was considered to be a reliable criterion for elucidating salt tolerance (Ashraf and Ali 2008). Thus, the potential to engineer plants that over-express the antioxidant gene provides the opportunity to develop plants with enhanced tolerance to stress. Such modifications might be crucial for selecting/breeding stress-resistant cotton cultivars able to grow in nutrient-poor soils.
PM H+-ATPase establishes the transmembrane proton-driving force, and is important for maintaining cytoplasm pH and the selective absorption of K+, as well as the rejection and excretion of Na+ (Sondergaard et al. 2004). PM Ca2+-ATPase directly uses ATP to promote Ca2+-ATP transportation, to maintain low Ca2+ concentrations in the cytoplasm (Toyoshima 2009). In this study, H+-ATPase and Ca2+-ATPase activity decreased under soil salinity, soil drought, and their combined action. The largest reduction was observed under SD, showing the additive effect of soil salinity and soil drought. However, the decline in H+-ATPase activity would destroy the electrochemical potential gradient across the PM, which would reduce the ability of the plant to maintain the acidic cell wall environment, as suggested by Mansour et al. (2000) for wheat. In parallel, the K+ absorption capacity, Na+ rejection capacity, and Na+ excretion would be reduced, causing a decrease in K+/Na+ levels in the cytoplasm and damaging the normal physiological functioning of cell (Somerville and Browse 1991). PM Ca2+-ATPase decreases with reduced H+-ATP activity as the balance between Ca2+ and H+ in the cytoplasm is lost; consequently, the distress signal produced by cell might be interrupted, leading to the formation of physiological metabolic disorders (He et al. 2001). In this study, PM H+-ATPase and Ca2+-ATPase activity was higher in the cotton roots of CCRI-44 (salt-tolerant) compared to those of Sumian 12 (salt-sensitive). This result indicated that CCRI-44 is better able to regulate ion selective absorption and to facilitate the stability of PM and the protein membrane compared to Sumian 12. Under soil salinity, soil drought, and their combined action, the DBI of CCRI-44 was relatively stable and consistent with the other types of ATPase activity, because relatively high DBI ensured the membrane contained fluids, which strengthened the regulation of membrane competence and compatibility.
Conclusion
Soil salinity, soil drought, and their combined action caused a marked decline in root dry matter for the two cultivars; however, the combination of soil salinity and drought had a larger inhibitory effect and was more harmful to root growth compared to the individual effect of soil drought or soil salinity. The obtained results showed that differences in SOD, POD, and CAT activity, along with MDA content, of the two cultivars might be responsible for the different mechanisms underlying oxidative stress injury and subsequent tolerance to soil salinity and soil drought. Therefore, these differences might explain the greater tolerance of CCRI-44 to soil salinity, soil drought, and their combined action compared to Sumian 12. The lipid peroxidation level in cotton roots might be an important biochemical trait for stress tolerance in cotton plants. Furthermore, the results of this study indicate that the salt-tolerant cultivar CCRI-44 is more stable in PM associated with H+-ATPase and Ca2+-ATPase activity compared to Sumian 12 under soil salinity, soil drought, and their combined action. This study contributes novel information on improving our understanding about the vegetative growth behavior of cotton plants subjected to high salinity and drought conditions and might be useful for enhancing the sustainability of agricultural practices in saline-alkali soils.
Author contribution
Lei Zhang conceived, designed and performed the experiments; analyzed the data and wrote the paper. Binglin Chen conceived and designed the experiments; analyzed the data. Guowei Zhang performed the experiments; analyzed the data. Jianliang Li performed the experiments. Youhua Wang analyzed the data. Yali Meng analyzed the data. Zhiguo Zhou conceived, designed and performed the experiments; analyzed the data and wrote the paper.
References
Akter N, Sobahan MA, Hossain MA, Uraji M, Nakamura Y, Mori IC, Murata Y (2010) The involvement of intracellular glutathione in methyl jasmonate signaling in Arabidopsis guard cells. Biosci Biotechnol Biochem 74:2504–2506
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Amelia H, Veeresh RPG, Rolando OT, Kenneth LM, Rachid S (2011) Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the OryzaSNP panel in rained lowland fields. Field Crop Res 120:205–214
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93
Ashraf M, Ahmad S (2000) Influence of sodium chloride on ion accumulation, yield components and fibre characteristics in salt-tolerant and salt-sensitive lines of cotton (Gossypium hirsutum L.). Field Crop Res 66:115–127
Ashraf M, Ali Q (2008) Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ Exp Bot 63:266–273
Beers RF, Sizer IW (1952) A spectrophotometric method of measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bird RP, Draper HH (1984) Comparative studies on different methods of malonaldehyde determination. Methods Enzymol 105:299–305
Bor M, Ozdemir F, Turkan I (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves if sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci 164:77–84
Bowling DJF (1981) Release of ions to the xylem in roots. Physiol Plantarum 53:392–397
Carmi A, Plaut Z, Sinai M (1993) Cotton root growth as affected by changes in soil water distribution and their impact on plant tolerance to drought. Irrigation Sci 13:177–182
Chen CCS, Plant AL (1999) Salt-induced protein synthesis in tomato roots: the role of ABA. J Exp Bot 50:667–687
Cooke DT, Burden RS (1990) Lipid modulation of plasma membrane-bound ATPases. Physiol Plantarum 78:153–159
Costa JH, Jolivet Y, Hasenfratz-Sauder M-P, Orellano EG, da Guia Silva Lima M, Dizengremel P, Fernandes de Melo D (2007) Alternative oxidase regulation in roots of Vigna unguiculata cultivars differing in drought/salt tolerance. J Plant Physiol 164:718–727
Demiral T, Turkan I (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247–257
Diao F, Zhang W, Liu Y (1997) Changes in composition and function thylakoid membrane isolated from barely seeding leaves under salt stress. Acta Phytophylacica Sinica 23:105–110
Ferguson IB, Watkins CB, Harman JEC (1983) Inhibition by calcium of senescence of detached cucumber cotyledons. Plant Physiol 71:182–186
Foster JG, Hess JL (1980) Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487
Fricker MD, Willmer CM (1990) Some properties of proton pumping ATPases at the plasma membrane and tonoplast of guard cells. Biochemie Physiologie der Pflanzen 186:301–308
Gapińska M, Skłodowska M, Gabara B (2008) Effect of short- and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol Plant 30:11–18
Garratt LC, Janagoudar BS, Lowe KC, Anthony P, Power JB, Davey MR (2002) Salinity tolerance and antioxidant status in cotton cultures. Free Radic Bio Med 33:502–511
Gechev T, Gadjev I, Breusegem FV, Inze D, Dukiandjiev S, Yoneva V, Minkov I (2002) Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cell Mol Life Sci 59:708–714
Gosset DR, Millhollon EP, Lucas MC (1994) Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci 34:706–714
He LF, Liu YL, Shen ZG, Wang AQ (2001) Effects of aluminum on ATPase activity and lipid composition of plasma membranes of wheat roots. Scientia agricultura Sinica 34:526–531
Heidari M, Tafazoli E (2005) Effect of sodium chloride on lipoxygenase activity, hydrogen peroxide content and lipid peroxidation rate in the seedlings of three rootstocks. JWSS Isfahan University Technol 9:41–50
Herbette S, Lenne C, Leblanc N, Julien JL, Drevet JR, Roeckel-Drevet P (2002) Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur J Biochem 269:2414–2420
Hernandez JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Plant Physiol 115:251–257
Hernandez JA, Jimenez A, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ 23:853–862
Isamah GK (2004) ATPase, peroxidase and lipoxygenase activity during post-harvest deterioration of cassava (Manihot esculenta Crantz) root tubers. Int Biodeter Biodegr 54:319–323
İsmail T, Bor M, özdemir F, Koca H (2005) Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231
Khatun S, Ali MB, Hahn EJ, Paek Y (2008) Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. Environ Exp Bot 64:278–295
Kumar M, Gupta V, Trivedi N, Kumari P, Bijo AJ, Reddy CRK, Jha B (2011) Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales Rhodophyta). Environ Exp Bot 72:194–201
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:561–579
Li HS (2000) Principles and techniques of plant physiological experiment. High Education Press, Beijing, pp 119–120 (in Chinese)
Lichtenthaler HK (1996) Vegetation stress: an introduction to the stress concept in plants. J Plant Physiol 48:4–14
Luna C, Seffino LG, Arias C, Taleisnik E (2000) Oxidative stress indicators as selection tools for salt tolerance in Chloris gayana. Plant Breed 119:341–345
Mansour MMF, Hasselt PRv, Kuiper PJC (2000) NaCl effects on root plasma membrane ATPase of salt tolerant wheat. Biol Plantarum 43:61–66
Mckersie BD, Leshem YY (1994) Stress and stress coping in cultivated plants. Kluwer Academic Publishes, Dordrecht
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003a) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 48:69–76
Meloni DA, Oliva MA, Martinez V, Cambraia J (2003b) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Naya L, Ladrera R, Ramos J, Gonzalez EM, Arrese-Igor C, Minchin FR, Becana M (2007) The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiol 144:1101–1114
Norbert F, Tchiadje T (2007) Strategies to reduce the impact of salt on crops (rice, cotton and chili) production: a case study of the tsunami-affected area of India. Desalination 206:524–530
Pastori GM, Foyer CH (2002) Common components, networks, and pathways of cross-tolerance to stress. The central role of ‘redox’ and abscisic acid-mediated controls. Plant Physiol 129:460–468
Qiu QS (1999) Characterization of p-nitrophenyl phosphate hydrolysis by plasma membrane H+-ATPase from soybean hypocotyls. J Plant Physiol 154:628–633
Reddy KR, Hodges HF, Mckinion JM (1997) Crop modeling and applications: a cotton example. Adv Agron 59:225–290
Reinhardt DH, Rost TL (1995) On the correlation of primary root growth and treachery element size and distance from the tip in cotton seedlings grown under salinity. Environ Exp Bot 35:575–588
Scalet M, Federice R, Guido MC, Manes F (1995) Peroxidase activity and polyamine changes in response to ozone and simulated acid rain in Aleppo pine needles. Environ Exp Bot 35:417–425
Siedow JN (1991) Plant lipoxygenase: structure and function. Annu Rev Plant Biol 42:145–188
Sofo A, Dichio B, Xiloyannis C, Masia A (2004) Lipoxygenase activity and proline accumulation in leaves and roots of olive trees in response to drought stress. Physiol Plantarum 121:58–65
Somerville C, Browse J (1991) Plant lipids: metabolism, mutants and membrane. Science 252:80–87
Sondergaard TE, Schulz A, Palmgren MG (2004) Energization of transport processes in plants. Roles of the plasma membrane H+-ATPase. Plant Physiol 136:2475–2484
Tan W, Liu J, Dai T, Jing Q, Cao W, Jiang D (2008) Alterations in photosynthesis and antioxidant enzyme activity in winter wheat subjected to post-anthesis waterlogging. Photosynthetica 46:21–27
Thomas JR (1980) Osmotic and specific salt effects on growth of cotton. Argon J 72:407–412
Toyoshima C (2009) How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim Biophys Acta 1793:941–946
Vulkan-Levy R, Ravina I, Mantell A, Frenkel H (1998) Effect of water supply and salinity on pima cotton. Agr Water Manage 37:121–132
Widell S, Larsson C (1990) A critical evaluation of markers used in plasma membrane purification. In: Larsson C, Moller IM (eds) The plant plasma membrane. Structure, function and molecular biology. Springer, Berlin, pp 16–43
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14 Suppl:S165–S183
Yang Y, Han C, Liu Q, Lin B, Wang JW (2008) Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiol Plant 30:433–440
You SZ, Yang HQ, Zhang L, Shao XJ (2009) Effects of cadmium stress on fatty acid composition and lipid peroxidation of Malus hupehensis. Chin J Appl Ecol 28:2032–2037 (in Chinese)
Yu HG, Su WA (1996) Studies on relationship between fatty acids desaturation of PSII membrane and low temperature photoinhibation of cucumber. Acta Bioch Bioph Sin 12:227–233
Zhang XZ (1992) Crop physiological research methods. China Agricultural Press, Beijing, pp 131–207 (in Chinese)
Zhang GW, Lu HL, Zhang L, Chen BL, Zhou ZG (2011) Salt tolerance evaluation of cotton (Gossypium hirsutum L.) at its germinating and seedling stages and selection of related indices. Chin J Appl Ecol 22:2045–2053 (in Chinese)
Zhang L, Zhang GW, Wang YH, Zhou ZG, Meng YL, Chen BL (2013) Effect of soil salinity on physiological characteristics of functional leaves of cotton plants. J Plant Res 126:293–304
Zhao X, Tisdell C (2009) The sustainability of cotton production in China and in Australia: comparative economic and environmental (Working paper NO. 157). Nova Science Publishers, New York.pp,1–37
Zhong H, Lāuchli A (1993) Spatial and temporal aspects of growth in the primary root of cotton seedlings: effect of NaCl and CaCl2. J Exp Bot 44:763–771
Zhou ZS, Huang SQ, Guo K, Mehta SK, Zhang PC, Yang ZM (2007) Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. J Inorg Biochem 101:1–9
Zhou ZS, Wang SJ, Yang ZM (2008) Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 70:1500–1509
Acknowledgments
This work was supported by a grant from the National High Technology Research and Development Program of China (863 Program) (No. 2007AA10Z206).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Zwiazek.
Rights and permissions
About this article
Cite this article
Zhang, L., Chen, B., Zhang, G. et al. Effect of soil salinity, soil drought, and their combined action on the biochemical characteristics of cotton roots. Acta Physiol Plant 35, 3167–3179 (2013). https://doi.org/10.1007/s11738-013-1350-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1350-6