Abstract
The irrigation water requirements and sensitivity to water deficits of ornamental plants is of great interest to horticultural producers for planning irrigation strategies. The effect of different deficit irrigation strategies on physiological and morphological parameters in geranium plants was studied in different growth phases to evaluate how such strategies can be safely used and to ascertain whether the flowering phase is sensitive to deficit irrigation. Pelargonium × hortorum L.H. Bailey plants, grown in a controlled growth chamber, were subjected to four irrigation treatments: control (100 % water field capacity throughout the experiment), sustainable deficit irrigation (75 % water field capacity throughout the experiment), and two regulated deficit irrigation treatments that included water stress during the vegetative growth phase or during the flowering development phase. Although the total amount of irrigation water was similar in the three deficit irrigation treatments (around 80 % of the control value), the lowest values for both height and flowering were found when deficit irrigation was applied during flowering. This indicates that plant quality does not only depend on the amount of water applied but also on the time when the reduction is applied, and that flowering is the most sensitive phase to water stress. Evapotranspiration was related to the formation of inflorescences and to increased plant height. When the irrigation strategy was changed, plants increased or decreased their water consumption and stomatal conductance to adjust to the new conditions by regulating stomatal opening, although, in general, the values of both parameters remained below those observed in the control plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regulated deficit irrigation (RDI) is an irrigation tool based on our physical knowledge of plant responses to water stress (Chaves et al. 2007). In RDI, water input is withheld or reduced for specific periods during the crop cycle, sufficiently to reduce vegetative growth but not so much as to reduce the economic value of the crop (Dry et al. 2001; Cameron et al. 2006). The main principle behind RDI is that plant sensitivity to water stress is not constant during the growth season, and that intermittent water stress in specific periods may be beneficial in terms of saving water and improving water use efficiency (Girona et al. 2005; Intringiolo and Castel 2005; Goldhamer et al. 2006).
In the last two decades, interest in irrigation techniques based on RDI procedures has been centred on fruit and nut crops, where it has been applied successfully (Goldhamer and Beede 2004; Ruiz-Sánchez et al. 2000). However, its application to ornamental crops has so far received relatively limited attention (Cameron et al. 2006; Álvarez et al. 2009). Cameron et al. (1999, 2006) demonstrated the feasibility of applying RDI to container-grown ornamental plants and reported that RDI has the potential to improve commercial crop quality in ornamental species by reducing excessive growth and promoting a more compact habit. However, according to Silber et al. (2007), RDI reduces the decorative value of leucadendron, although the response depends on the growth phase when deficit irrigation (DI) is applied (stress timing) and the duration of the same during each growth phase. However, periods of water stress during vegetative phases increase flowering intensity in carnation plants (Álvarez et al. 2009).
In this sense, differences in sensitivity to deficit irrigation (DI) between different species and cultivars (Savé et al. 2000; Clary et al. 2004; Zollinger et al. 2006) and even between growth stages have been observed in many plants (Sionit et al. 1987; Mingeau et al. 2001). Numerous works in ornamental plants, Hansen and Petersen (2004), Henson et al. (2006), Katsoulas et al. (2006), Chylińsky et al. (2007), Silber et al. (2007), De Lucia (2009), Álvarez et al. (2009), Sánchez-Blanco et al. (2009), Bolla et al. (2010), Andersson (2011) and Bernal et al. (2011), have demonstrated that the extent of growth and flowering inhibition increases as the severity of DI increases. It has been suggested that the appropriate scheduling of DI with regard to the stage of development may also determine different growth responses. Therefore, the importance of factors, such as the degree of water stress imposed and the timing and duration of reduced irrigation, have been discussed (Hassanein and Dorion 2006). All these factors are usually related to physiological parameters such as stomatal conductance, photosynthesis, leaf temperature or plant weight, which are indicative of the stress applied (Sharp et al. 2009). Nevertheless, the relationships between them depend on the growth conditions. Hence, many studies using plants grown in pots revealed that changes in stomatal conductance are the main cause of decreased photosynthesis, while in field conditions using longer-term drying cycles, perturbations in metabolism appeared to be one of major factors for the reduction of photosynthesis (Liang et al. 1997).
Geranium is one of the most widely grown ornamental plants in the world, frequently in potted plant form. Pelargonium hortorum has remained very popular with consumers for many years, mainly because of its flowers and drought tolerance (Lang and Trellinger 2001). Its principal characteristic as a potted plant is the presence of a large number of red and scented flowers surrounded by thick green foliage. The leaves are “zoned” with a dark scalloped band halfway down the leaf blade and parallel to the leaf margin, which adds to the plants ornamental value. However, the response of geranium to deficit irrigation has been relatively little studied. In a previous works on P. hortorum (Hassanein and Dorion 2006; Chyliński et al. 2007), deficit irrigation was applied throughout the whole experiment, although it is plausible to consider that geranium sensitivity to water stress may be related to individual growth phases.
The physiological and morphological response of potted geranium plants to different irrigation levels and during drought recovery was studied by Sánchez-Blanco et al. (2009). The information provided was important for elaborating deficit irrigation strategies that allow irrigation amounts to be changed in accordance with the requirements of successive phenological phases. But it is also necessary to optimize the duration and the timing of water reductions in each species to avoid any negative effects on ornamental quality.
The aims of this research were to study the effects of different deficit irrigation strategies, such as sustainable deficit irrigation applied throughout the growth season, and RDI applied in different growth phases (during the flowering phase or outside the flowering phase) on physiological and morphological parameters, to determine the extent to which these strategies can modify water consumption, water relations, growth and quality in potted geranium plants and whether the flowering phase was sensitive to deficit irrigation.
Materials and methods
Plant material and experimental conditions
Single rooted cuttings (4- to 5-cm tall and with 6–7 leaves) of Pelargonium × hortorum L.H. Bailey (zonal geranium) were transplanted into 14 × 12 cm pots (1.2 L) filled with a mixture of sphagnum peat, perlite and coconut fibre (6:3:1) and amended with osmocote plus (2 g L−1 substrate) (14:13:13 N, P, K+ microelements).
The experiment was conducted in a controlled growth chamber, where the environmental conditions were selected to simulate natural conditions, bearing in mind the conditions necessary for flowering (Armitage et al. 1981; Blanchard and Runkle 2011). The temperature in the canopy was 24 °C during the light phase and 18 °C during darkness. Relative humidity (RH) ranged between 65 and 80 %. A mean photosynthetic active radiation (PAR) of 250 μmol m−2 s−1 at canopy height was supplied during the light phase (08–00 hrs). Although the radiation levels in the growth chamber were lower than those applicable in the field, it was assumed that the specific PAR levels used were of secondary importance compared with the contrast in irrigation treatments. All the plants were watered daily to container capacity prior to starting the treatments, which lasted 5 weeks.
Treatments
To determine the maximum water-holding capacity of the substrate, all the pots were uniformly mixed and packed to a bulk density of 0.165 g cm−3. The substrate surfaces were covered with aluminium foil to prevent water evaporation and the lower parts were submerged, to half of the pot’s height, in a water bath and then were left to equilibrate overnight. The next day, the pots were removed and left to drain freely until drainage became negligible. The fresh weight was then recorded and calculated for each individual pot and considered as the weight at field capacity (WFC). At the end of the experiment, the substrate was dried in an oven at 105 °C until constant weight to obtain the dry weight and calculate the volumetric water content. Later, the difference between the fresh weight and oven-dry weight was measured and the volumetric water content was calculated (61 %), which was considered as the substrate’s field capacity.
Plants were submitted to four irrigation treatments: container capacity (control) and three DI treatments. Summarised data of the different treatments are presented in Table 1. All plants were irrigated daily and the electrical conductivity of the water applied was 0.8 dS m−1.
Four treatments were considered: control (C), irrigated at 100 % WFC throughout the experiment; sustainable deficit irrigation (SDI), irrigated at 75 % WFC throughout the experiment; regulated deficit irrigation I (RDI I), irrigated at 75 % throughout the experiment, except during the flowering phase when plants were irrigated at 100 %; regulated deficit irrigation II (RDI II), irrigated at 100 % throughout the experiment, except during the flowering phase when plants were irrigated at 75 %.
The experimental period lasted 24 weeks. During this period, each individual plant was weighed daily and the volume of irrigation water required to refill the pot to its pre-determined level of WFC (100 or 75 %) was calculated and added to each plant.
Irrigation was controlled by the decrease in weight of the pot and without compensation for any increase in plant growth, which was disregarded, since it was negligible compared with WFC (15–30 vs. 800 g). The plants in the experiment were considered to be in the flowering phase when more than 80 % of the plants of the control treatment had buds.
Growth and plant water measurements
At the end of the experimental period, the substrate was gently washed from the roots of five plants per treatment and the plants were divided into shoots (i.e. leaves and stems) and roots. Leaf number and leaf area (cm2) were determined in the same plants by measuring all mature and recently expanded leaves. Leaf number was directly counted and leaf area was determined using a leaf area meter (AM 200; ADC BioScientific Ltd., Herts, England). Plants were oven-dried at 80 °C immediately after the leaf area measurements until they reached a constant weight to measure the respective dry weights.
In addition, the root to shoot ratio was determined in these plants and calculated by dividing root dry weight by the sum of leaf and stem dry weight. Throughout the experiment, plant height, plant width and the number of leaves lost were measured in 24 plants per treatment every week. Plant height was taken as the vertical distance from substrate to the highest inflorescence, plant width was the horizontal distance between the two most distant leaves and the number of leaves lost was calculated by the accumulated sum of fallen or completely dry leaves in each plant.
The number of inflorescences per plant was recorded weekly in 24 plants per treatment and included any buds developed to the point of showing flower colour, and the percentage of plant flowering (with one or more floral buds) was determined to schedule the irrigation. The cumulative number of inflorescences was equal to the sum of the total inflorescences in each plant until that moment. Leaf and flower colour was measured with a Minolta CR-10 colorimeter, which provided the colour coordinates lightness (L*), chroma (C*) and hue angle (h°) (McGuire 1992), using three leaves and three flowers for each plant and five plants per treatment. Leaf colour was measured in the external (darker) and internal zones (lighter).
Evapotranspiration (ET) was measured gravimetrically throughout the experimental period in 24 plants per treatment, using the difference in weights (weight after irrigation and weight before irrigating again), using a balance (Analytical Sartorius, Model 5201; capacity 5.2 kg and accuracy of 0.01 g).
Seasonal changes in leaf water potential (Ψl), leaf turgor potential (Ψt), leaf osmotic potential at full turgor (Ψ100s), stomatal conductance (gs) and net photosynthesis (P n) were determined in five plants per treatment during the central hours of illumination. Leaf water potential was estimated according to Scholander et al. (1965), using a pressure chamber (Model 3000; Soil Moisture Equipment Co., Santa Barbara, CA, USA) in which, leaves were placed in the chamber within 20 s of collection and pressurised at a rate of 0.02 MPa s−1 (Turner 1988). Leaves from the Ψl measurements were frozen in liquid nitrogen (−196 °C) and stored at −30 °C. After thawing, the osmotic potential was measured in the extracted sap using a WESCOR 5520 vapour pressure osmometer (Wescor Inc., Logan, UT, USA), according to Gucci et al. (1991). Leaf turgor potential was estimated as the difference between leaf water potential (Ψl) and leaf osmotic potential. Leaf osmotic potential at full turgor (Ψ100s) was estimated as indicated above for leaf osmotic potential, using excised leaves with their petioles placed in distilled water overnight to reach full saturation. Leaf stomatal conductance (g s) and net photosynthetic rate (P n) were determined in attached leaves using a gas exchange system (LI-6400; LI-COR Inc., Lincoln, NE, USA). P n/g s ratio was used as an estimation of the intrinsic water use efficiency.
Statistical analyses of data
In the experiment, 24 plants were randomly attributed to each treatment. The data were analysed by one-way ANOVA using Statgraphics Plus for Windows 5.1 software. Ratio data were subjected to an arcsine square-root transformation before statistical analysis to ensure homogeneity of variance. Treatment means were separated with Duncan’s Multiple Range Test (P ≤ 0.05).
Results
Plant growth and ornamental parameters
Water deficit had a significant effect on biomass accumulation (Table 2). Aerial dry weight, the number of leaves and total leaf area decreased with deficit irrigation, regardless of the time when the reduction was applied. However, root dry weight was not modified and the root/shoot ratio increased in the plants grown under deficit irrigation conditions.
Water deficit was seen to significantly alter plant height and width, although the changes differed depending on the time when deficit irrigation was applied (Fig. 1). No pronounced differences in plant height were observed during the experiment between control and RDI I treatment (when deficit irrigation was applied outside the flowering phase) (Fig. 1a). The smallest values of plant height were found in the RDI II treatment (when deficit irrigation was applied during flowering), which shows that this phase is the most sensitive to water stress. Plant height was inhibited a few weeks after application of the deficit irrigation onwards in SDI and, especially, in RDI II (Fig. 1a). The behaviour of plant width was similar to that of plant height, although the differences between treatments were less marked (Fig. 1b). Control plants lost the highest number of leaves per plant during most of the experiment, although the same plants also had the highest number of green leaves (Fig. 1c). Weeks 4–5, 14–15 and, especially, 18–20, when the highest leaf loss was observed, coincided with maximum flowering and the highest number of open inflorescences per plant. Deficit irrigation affected the flowering of geranium plants, with a significant decrease in RDI II compared with the control (Fig. 2). Flowering, as assessed by the evolution of the number of inflorescences, was also affected by the timing of stress (Fig. 2a). During the first flowering phase (weeks 1.5–5.8), plants of the RDI II treatment produced fewer inflorescences than the controls, while there were no significant differences between C, SDI and RDI I, although flowering in the last two treatments lasted less time than in the controls (Fig. 2a). In the second flowering phase (11.8–15.4), plants of the RDI II treatment continued to show a lower flowering intensity and the plants of the SDI and RDI I treatments flowered earlier than the controls. The lowest accumulated number of inflorescences per plant was seen in the plants of RDI II treatment, while in SDI and RDI I no significant differences with respect to the control were observed (Fig. 2b).
Evolution of plant height (a), plant width (b) and leaf loss (c) in P. hortorum plants subjected to different irrigation treatments during the experimental period. Values are means (n = 24) and the vertical bars indicate standard errors. Symbols represent the different treatments: control (filled circles), SDI (open circles), RDI I (filled triangles) and RDI II (open triangles). Vertical lines indicate irrigation changes and numbers at the top of the figure indicate threshold levels of WFC after irrigation in the plants of both RDI treatments. Asterisks indicate significant differences between treatments
Evolution of number of inflorescences (a) and cumulative number of inflorescences per plant (b) in P. hortorum plants subjected to different irrigation treatments during the experimental period. Values are means (n = 24) and the vertical bars indicate standard errors. Symbols represent the different treatments: control (filled circles), SDI (open circles), RDI I (filled triangles) and RDI II (open triangles). Vertical lines indicate irrigation changes and numbers at the top of the figure indicate threshold levels of WFC after irrigation in the plants of both RDI treatments. Asterisks indicate significant differences between treatments
In general, no great differences in the leaf and flower colour parameters were observed in the deficit irrigation treatments compared with the control (Online Resource 1). The leaf external zone remained darker (lower L* value) and less vivid green (lower C*) compared with leaf internal zone throughout the experimental period (Online Resource 1a, 1b). The hue angle values recorded in both zones confirmed the green colour of the foliage and suggested the absence of chlorosis and necrosis (Online Resource 1c). Plants maintained their differences in h° values between leaves and flowers during the experimental period. Deficit irrigation did not affect the colour contrast between green leaves and red flowers.
Water consumption
The average amount of water added to each pot during the whole experimental period was 7.89 L for the control and 6.26, 6.25 and 6.32 L for SDI, RDI I and RDI II plants (Fig. 3a). The total irrigation amount was similar in the three deficit irrigation treatments, approximately 80 % of the amount of water supplied in the control treatment. However, the timing of deficit irrigation varied and depended on the phase of the plants.
Evolution of daily evapotranspiracion (ET) in P. hortorum plants subjected to different irrigation treatments during the experimental period. Values are means (n = 24) and the vertical bars indicate standard errors. Symbols represent the different treatments: control (filled circles), SDI (open circles), RDI I (filled triangles) and RDI II (open triangles). Vertical lines indicate irrigation changes in the plants and numbers at the top of the figure indicate threshold levels of WFC after irrigation in the plants of both RDI treatments
The daily evapotranspiration is shown in several figures (Fig. 3b: all treatments; 3c: C and SDI; 3d: C, SDI and RDI I; 3e: C, SDI and RDI II). Water consumption varied during the experiment (Fig. 3b). During the 4 weeks following the beginning of the treatments, the daily ET in all treatments reached its maximum value, even though environmental conditions (temperature, RH, light and VPD) were constant throughout the experiment. Evapotranspiration was higher in control plants than in plants of the SDI treatment (Fig. 3c). However, these differences were not constant during the whole experiment. At the beginning of the experiment, differences between treatments were greater and during some specific periods (weeks 7–8 and from week 15 onwards) the consumption of SDI plants was similar to that of control plants, despite the lower levels of water in the substrate.
In the RDI treatments, when the irrigation pattern was changed, the plants increased or decreased their water consumption (ET) and adjusted to the new conditions, but with some particular characteristics (Fig. 3d, e). When plants were exposed to deficit irrigation after normal irrigation conditions (striped area), humidity readjustment took several days, although plants of both RDI treatments restricted their ET, the day after the change in irrigation and their ET matched that of plants that had been exposed to deficit irrigation since the beginning of the experiment. During this phase, the ET of RDI plants (I and II) was equal to that of SDI plants. This was particularly marked after the first change of irrigation. Once well-watered conditions were restored (shaded area), the humidity in the substrate immediately recovered. In contrast, ET values in the RDI plants increased more slowly and were still significantly lower than that in control plants.
Water relations
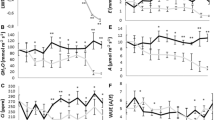
Leaf water potential values were always higher in the control than in the SDI treatment (Fig. 4a), while the Ψl values changed in the RDI treatments according to the irrigation applied in each phase. Leaf turgor potential (Ψt) was similar to Ψl and decreased slightly when plants received less water (Fig. 4c). However, no differences in Ψ100s between treatments during the experimental period were observed (Fig. 4d).
Evolution of leaf water potential (Ψl, a), leaf turgor potential (Ψt, b) and leaf osmotic potential at full turgor (Ψ100s, c) in P. hortorum plants subjected to different irrigation treatments during the experimental period. Values are means of five plants per treatments and the vertical bars indicate standard errors. Symbols represent the different treatments: control (filled circles), SDI (open circles), RDI I (filled triangles) and RDI II (open triangles). Vertical lines indicate irrigation changes and numbers at the top of the figure indicate threshold levels of WFC after irrigation in RDI treatments. Asterisks indicate significant differences between treatments
The values of the stomatal conductance and photosynthesis net rate during the period can be seen in Fig. 5. In general, g s values were higher in control plants and lower in plants submitted to deficit irrigation (Fig. 5a). In the plants of both RDI treatments, when the change in irrigation involved a reduction in the amount of water, g s decreased as a result of stomatal opening regulation. When irrigation was increased, g s increased, although the plants did not reach the values of the control plants. Such reductions with respect to the control plants were also observed in the photosynthesis levels, although the differences were less pronounced (Fig. 5b). In general, the plants of SDI and RDI I treatments showed higher P n/g s ratios (intrinsic water use efficiency) than control plants throughout the experimental period (Fig. 5c).
Evolution of stomatal conductance (g s, a), net photosynthetic rate (P n, b) and intrinsic water use efficiency (P n/g s, c) in P. hortorum plants subjected to different irrigation treatments during the experimental period. Values are means of five plants per treatments and the vertical bars indicate standard errors. Symbols represent the different treatments: control (filled circles), SDI (open circles), RDI I (filled triangles) and RDI II (open triangles). Vertical lines indicate irrigation changes and numbers at the top of the figure indicates threshold levels of WFC after irrigation in the plants of both RDI treatments. Asterisks indicate significant differences between treatments
Discussion
Plant growth is usually decreased when soil water availability is limited. In our experiment, deficit irrigation, regardless of the time of application, decreased aerial dry weight, the number of leaves per plant and total leaf area, which may be an adaptive role, restricting the evaporative surface area (Sharp 1996). In contrast, the root/shoot ratio increased as a result of DI treatments largely because the reductions in shoot growth were not matched by an equivalent loss of root development (Sánchez-Blanco et al. 2004). This response could speed up the establishment of ornamental plants in gardening or landscaping projects (Franco et al. 2006; 2011). The same responses were found by Jaleel et al. (2008) in C. roseus, by Henson et al. (2006) and Hassanein and Dorion (2006) in P. hortorum, by Andersson (2001) in P. zonale, by Álvarez et al. (2011) in C. citrinus and by Andersson (2011) in I. walleriana and Petunia × hybrid. The reduction in growth was not accompanied by colour modifications or a greater loss of leaves. The last parameter was not related with deficit irrigation, since the leaf loss was greatest during the last weeks in all treatments, even in the controls which had received enough irrigation water to prevent wilting, and was probably due to the increased number of inflorescences in conjunction with other factors such as ontogeny (Brawner 2003). According to Hassanein and Dorion (2006), leaf area is affected before wilting and leaf loss, which only begin to be affected when water stress is severe (Bargali and Tewari 2004).
The colorimetric values measured suggest that deficit irrigation levels had little effect on leaf and flower colour, and so did not reduce the quality of geranium as an ornamental plant. In a previous study, Sánchez-Blanco et al. (2009) suggested that geranium plants can cope with water shortages without losing their ornamental value.
In geranium, as other ornamental plants, there is a tendency for the first shoots to grow so long that the flowers extend a long way from the foliage, which lowers the commercial value of plants. In general, deficit irrigation decreases plant height more than width, representing a greater reducing effect on vertical than on horizontal growth, so flowers are closer to the foliage. Aesthetically and commercially, an increase in foliage size in relation to plant height gives the plant a compactness and architectural equilibrium that are much appreciated by customers. In addition, this is one the positive aspects of deficit irrigation, since height reduction makes plant management and later transplantation easier (Lang and Trellinger 2001; Van Iersel and Nemali 2004).
The application of water deficit saves water and reduces excessive growth in ornamental plants (Álvarez et al. 2009). However, plants subjected to water deficit may reduce flowering intensity, bring forward, or delay flowering and shorten the same (Cuevas et al. 2009; Bernal et al. 2011; Álvarez et al. 2012). However, in our experiment, plant quality or flowering did not only depend on the amount of applied water but also on the time when the reduction was applied. Similar responses have been cited by Sharp et al. (2009) in Rhododendron, when responses depended on the phases during which the deficit irrigation was applied.
The floriculture market appreciates plants with leaves and flowers with intense colour, a high root to shoot ratio and a certain relationship between plant height and width. However, the attractiveness and commercial value of P. hortorum is primarily associated with flowering, so that the fewer flowers per plant and the shorter flowering of the RDC II plants must be considered to be negative aspects.
Plants are able to adapt to a reduced moisture level within the growing medium and, as a result, transpiration is reduced. In our conditions, daily evapotranspiration varied during the experiment and depended mainly on the available water content. Several works have studied the evolution of water consumption in ornamental plants under different environmental conditions, levels of water stress or substrates. For example, Montero et al. (2001) found that transpiration in zonal geranium was closely related to radiation. Nevertheless, a relation between ET and both temperature and vapour pressure deficit (VPD) has been described in other studies (Bakker 1991; Álvarez et al. 2009). In our case, other parameters, including the formation of inflorescences and increase in plant height, had an effect on the behaviour of ET, since environmental conditions (temperature, RH, light and VPD) were constant throughout the experiment.
Reductions in water consumption under deficit irrigation have been attributed to the reduction in leaf area (Atkinson and Crisp 1983) and to lower stomatal conductance (Pinhero et al. 1997; Bolla et al. 2010). In our experiment, the control showed higher daily ET values than SDI, which may be explained by greater plant growth (larger transpiration area), the higher levels of stomata conductance and greater amount of water available in the control compared with SDI. The differences between the ET values obtained in the control and SDI varied throughout the experiment and might also be explained by differences in the date of flowering. Maximum ET values were found during the time when inflorescences were forming, because the plants were physiologically more active (Lorenzo et al. 1996; Bañón et al. 2009).
All the ornamental species studied by García-Navarro et al. (2004) responded to water stress by reducing daily water consumption, although the time before this response was observed and the intensity of the same varied between species. When these authors compared average daily ET in relation to leaf area rather than ET per plant, they found that while all species reduced ET per plant, some species did so in relation to leaf area, but others did not. At the beginning of our experiment, geranium plants of the DI treatments reduced the ET values in relation to leaf area compared with control plants, but such differences were less marked as the experiment progressed. The same observation was made for the evolution of the stomatal conductance values. Maximum g s values were found at the beginning of the experiment in all treatments, when ET was also highest. In this case, evapotranspiration was practically equivalent to transpiration, as evaporation from the soil was very low. Moreover, at the beginning of the experiment, leaf area was much lower, so transpiration values in relation to leaf area were much higher than during the rest of the experiment. This behaviour explains the lower water potential values measured at that time. The close relationship found between g s, ET and Ψh at the beginning of the experiment continued during the rest of the experimental period, as was observed by Colom and Vazzana (2003), Jaleel et al. (2008), Lenzi et al. (2009) and Bolla et al. (2010).
Plants from the RDI treatments modified stomatal conductance to adjust to irrigation changes, although, in general, g s remained below that of the control plants. This could be due to acclimation to the previous water-deficit situations (Leskovar 1998; Liptay et al. 1998; Franco et al. 2001; Vilagrosa et al. 2003; Bruce et al. 2007; Cameron et al. 2008; Walter et al. 2011).
Plants under irrigation deficit exhibited moderate water stress levels as indicated by the leaf potential and turgor values. In addition, the degree to which deficit irrigation was imposed did not point to any osmotic adjustment. In a previous experiment, limited osmotic adjustment was observed only in geranium plants that received 60 % less water than the control (Sánchez-Blanco et al. 2009). However, deficit irrigation caused a decrease in stomatal conductance, which suggests that geranium has very sensitive stomata (Arora et al. 1998). A reduction in stomatal opening could lead to a lower photosynthetic rate at some moments during the experimental period. However, differences in stomatal conductance between treatments did not seem to be followed by similar changes in photosynthetic rate. In this sense, plants submitted to SDI and RDI I treatments are able to increase their intrinsic water use efficiency, i.e. plants maintain acceptable photosynthetic rates despite reduced stomatal opening respect to the control. CO2 assimilation remains proportionally higher than water vapour loss from the stomata as an additional drought acclimatation mechanism. Previous studies in a variety of ornamental species indicated that P n/g s can be modified under deficit irrigation (increasing or decreasing) (Rasoul Sharifi and Rundel 1993; Mugnai et al. 2005; Jaleel et al. 2008; Álvarez et al. 2009; Bolla et al. 2010).
Conclusions
Despite the fact that the amounts of water provided to P. hortorum plants in the deficit treatments were similar, their behaviour differed, depending on the phase when deficit irrigation was applied, even though deficit irrigation was moderate. Deficit irrigation applied outside the flowering phase brought this phase forward, but did not decrease flowering intensity; it also increased the root/shoot ratio and the width to height ratio, providing a better plant, besides saving 20 % of water. Deficit irrigation during the flowering phase is not to be recommended, because the intensity and duration of flowering are reduced. This finding should be borne in mind when deciding irrigation strategies for use in this kind of plant.
Author contribution
S Álvarez performed the experiment, carried out statistical analysis and wrote the article. MJ Sánchez-Blanco and S Bañón designed and instructed the research work. MJ Sánchez-Blanco also coordinated the study and provided study material and facilities for the experiments. The three authors were involved in data interpretation and paper preparing. All authors have read and approved the final manuscript.
Abbreviations
- C:
-
Control
- C*:
-
Chroma
- DI:
-
Deficit irrigation
- EC:
-
Electrical conductivity
- ET:
-
Evapotranspiration
- g s :
-
Stomatal conductance
- h°:
-
Hue angle
- L*:
-
Lightness
- P :
-
Significance
- PAR:
-
Photosynthetic active radiation
- PRD:
-
Partial root drying
- P n :
-
Net photosynthesis
- RDI:
-
Regulated deficit irrigation
- RH:
-
Relative humidity
- SDI:
-
Sustainable deficit irrigation
- VPD:
-
Vapour pressure deficit
- WFC:
-
Water at field capacity
- Ψl :
-
Leaf water potential
- Ψt :
-
Leaf turgor potential
- Ψ100s :
-
Leaf osmotic potential at full turgor
References
Álvarez S, Navarro A, Bañón S, Sánchez-Blanco MJ (2009) Regulated deficit irrigation in potted Dianthus plants: effects of severe and moderate water stress on growth and physiological responses. Sci Hortic 122:579–585
Álvarez S, Navarro A, Nicolás E, Sánchez-Blanco MJ (2011) Transpiration, photosynthetic responses, tissue water relations and dry mass partitioning in Callistemon plants during drought conditions. Sci Hortic 129:306–312
Álvarez S, Gómez-Bellot MJ, Castillo M, Bañón S, Sánchez-Blanco MJ (2012) Osmotic and saline effect on growth, water relations, and ion uptake and translocation in Phlomis purpurea plants. Environ Exp Bot 78:138–145
Andersson NE (2001) Weight controlled irrigation of potted plants. Acta Hortic 559:371–375
Andersson NE (2011) The influence of water stress and air velocity on growth of Impatiens walleriana and Petunia x hybrid. Sci Hortic 128:146–151
Armitage AM, Carlson WH, Flores JA (1981) The effect of temperature and quantum flux density on the morphology, physiology and flowering of hybrid geranium. J Am Soc Hortic Sci 106:643–647
Arora R, Dharmalingam SP, Bradford CB (1998) Water-stress-induced heat tolerance in geranium leaf tissues: a possible linkage through stress proteins? Physiol Plant 103:24–34
Atkinson D, Crisp CM (1983) The effect of some plant growth regulators and herbicides on root system morphology and activity. Acta Hortic 136:21–28
Bakker J (1991) Leaf conductance of four glasshouse vegetable crop as affected by air humidity. Agric For Meteorol 55:33–36
Bañón S, Miralles J, Navarro A, Sánchez-Blanco MJ (2009) Influence of paclobutrazol and substrate on daily evapotranspiration of potted geranium. Sci Hortic 122:572–578
Bargali K, Tewari A (2004) Growth and water relation parameters in drought-stressed Coriaria nepalensis seedlings. J Arid Environ 58:505–512
Bernal M, Estiarte M, Peñuelas J (2011) Drought advances spring growth phenology of the Mediterranean shrub Erica multiflora. Plant Biol 13:252–257
Blanchard MG, Runkle ES (2011) Quantifying the thermal flowering rates of eighteen species of annual bedding plants. Sci Hortic 128:30–37
Bolla A, Voyiatzis D, Koukourikou-Petridou M, Chimonidou D (2010) Photosynthetic parameters and cut-flower yield of rose ‘Eurored’ (H.T.) are adversely affected by mild water stress irrespective of substrate composition. Sci Hortic 126:390–394
Brawner F (2003) Geraniums: the complete encyclopedia. Schiffer Publishing Ltd., PA, pp 832–836
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Cameron RWF, Harrison-Murray RS, Scott MA (1999) The use of controlled water stress to manipulate growth of container-grown Rhododendron cv. Hoppy. J Hortic Sci Biotech 74:161–169
Cameron RWF, Harrison-Murray RS, Atkinson CJ, Judd HL (2006) Regulated deficit irrigation: a means to control growth in woody ornamentals. J Hortic Sci Biotech 81:435–443
Cameron R, Harrison-Murray R, Fordham M, Wilkinson S, Davies W, Atkinson C, Else M (2008) Regulated irrigation of woody ornamentals to improve plant quality and precondition against drought stress. Ann Appl Biol 153:49–61
Chaves MM, Santos TP, Souza CR, Ortuño MF, Rodrigues ML, Lopes CM (2007) Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann Appl Biol 150:237–252
Chyliński WK, Łukaszewska AJ, Kutnik K (2007) Drought response of two bedding plants. Acta Physiol Plant 29:399–406
Clary J, Savé R, Biel C, De Herralde F (2004) Water relations in competitive interactions of Mediterranean grasses and shrubs. Ann Appl Biol 144:149–155
Colom MR, Vazzana C (2003) Photosynthesis and PSII functionality of drought-resistant and drought-sensitive weeping lovegrass plants. Environ Exp Bot 49:135–144
Cuevas J, Pinillos V, Cañete ML, González M, Alonso F, Fernández MD, Hueso JJ (2009) Optimal levels of postharvest deficit irrigation for promoting early flowering and harvest dates in loquat (Eriobotrya japonica Lindl.). Agric Water Manage 96:831–838
De Lucia B (2009) Response of potted Australian ornamental plants to different soil water conditions. Acta Hortic 807:277–282
Dry PR, Loveys BR, McCarthy MG, Stoll M (2001) Strategic irrigation management in Australian vineyards. J Int Sci Vigne vin 35:129–139
Franco JA, Bañón S, Fernández JA, Leskovar DI (2001) Effect of nursery regimes and establishment irrigation on root development of Lotus creticus seedlings following transplanting. J Hortic Sci Biotech 76:174–179
Franco JA, Martínez-Sánchez JJ, Fernández JA, Bañón S (2006) Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J Hortic Sci Biotech 81:3–17
Franco JA, Bañón S, Vicente MJ, Miralles J, Martínez-Sánchez JJ (2011) Root development in horticultural plants grown under abiotic stress conditions: a review. J Hortic Sci Biotech 86:543–556
García-Navarro MC, Evans RY, Savé R (2004) Estimation of relative water use among ornamental landscape species. Sci Hortic 99:163–174
Girona J, Mata M, Marsal J (2005) Regulated deficit irrigation during the kernel-filling period and optimal irrigation rates in almond. Agric Water Manag 75:152–167
Goldhamer DA, Beede RH (2004) Regulated deficit irrigation effects on yield, nut quality and water-use efficiency of mature pistachio trees. J Hortic Sci Biotech 79:538–545
Goldhamer DA, Viveros M, Salinas M (2006) Regulated deficit irrigation in almonds: effects of variations in applied water and stress timing on yield and yield components. Irrig Sci 24:101–114
Gucci R, Xiloyannis C, Flore JA (1991) Gas exchange parameters, water relations and carbohydrate partitioning in leaves of field-grown Prunus domestica following fruit removal. Physiol Plant 83:497–505
Hansen CW, Petersen KK (2004) Reduced nutrient and water availability to Hibiscus rosa-sinensis ‘Cairo Red’ as a method to regulate growth and improve post-production quality. Eur J Hortic Sci 69:159–166
Hassanein A, Dorion N (2006) Determining morphological and physiological parameters for the selection of drought-tolerant geraniums (Pelargonium × hortorum L. H. Bailey). J Hortic Sci Biotech 81:707–713
Henson DY, Newman SE, Hartley DE (2006) Performance of selected herbaceous annual ornamentals grown at decreasing levels of irrigation. HortScience 41:1481–1486
Intringiolo DS, Castel JR (2005) Effects of regulated deficit irrigation on growth and yield of young Japanese plum tress. J Hort Sci Biotech 80:117–182
Jaleel CA, Gopi R, Sankar B, Gomathinayagam M (2008) Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress. C R Biol 331:42–47
Katsoulas N, Kittas C, Dimokas G, Lykas Ch (2006) Effect of irrigation frequency on rose flower production and quality. Biosys Eng 96:237–244
Lang H, Trellinger K (2001) Geraniums. In: Gaston M, Konjoian P, Kunkle L, Wilt M (eds) Tips on regulating growth of floriculture crops. Ohio Florists’ Associations Services Inc, Columbus, pp 88–95
Lenzi A, Pittas L, Martinelli T, Lombardi P, Tesi R (2009) Response to water stress of some oleander cultivars suitable for pot plant production. Sci Hortic 122:426–431
Leskovar DI (1998) Root and shoot modification by irrigation. HortTechnology 13:562–568
Liang J, Zhang J, Wong MH (1997) Can stomatal closure caused by xylem ABA explain the inhibition of leaf photosynthesis under soil drying? Photosynthesis Res 51:149–151
Liptay A, Sikkema P, Fonteno W (1998) Transplant growth control through water deficit stress: a review. HortTechnology 8:540–543
Lorenzo P, Medran E, Sánchez-Guerreto MC, Muñoz R (1996) Greenhouse crop transpiration, an implement to soilless irrigation management. Acta Hortic 458:113–119
McGuire RG (1992) Reporting of objective colour measurements. HortScience 27:1254–1255
Mingeau M, Perrier C, Ameglio T (2001) Evidence of drought-sensitive periods from flowering to maturity on highbush blueberry. Sci Hortic 89:23–40
Montero JL, Antón A, Muñoz P, Lorenzo P (2001) Transpiration from geranium grown under high temperatures and low humidities in greenhouses. Agric For Meteorol 107:323–332
Mugnai S, Vernieri P, Malorgio F, Serra G (2005) Response of some ornamental shrubs to different soil water conditions. Ad Hortic Sci 19:94–100
Pinhero RG, Rao M, Paliyath G, Mur D, Fletcher R (1997) Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol induced chilling tolerance of maize seedling. Plant Physiol 114:659–704
Rasoul Sharifi M, Rundel PW (1993) The effect of vapour pressure deficit on carbon isotope discrimination in the desert shrub Larrea tridentata (Creosote Bush). J Exp Bot 44:481–487
Ruiz-Sánchez MC, Torrecillas A, Pérez-Pastor A, Domingo R (2000) Regulated deficit irrigation in apricot trees. Acta Hortic 537:759–766
Sánchez-Blanco MJ, Ferrández T, Navarro A, Bañón S, Alarcón JJ (2004) Effects of irrigation and air humidity preconditioning on water relations, growth and survival of Rosmarinus officinalis plants during and after transplanting. J Plant Physiol 161:1133–1142
Sánchez-Blanco MJ, Álvarez S, Navarro A, Bañón S (2009) Changes in leaf water relations, gas exchange, growth and flowering quality in potted geranium plants irrigated with different water regimes. J Plant Physiol 166:467–476
Savé R, Biel C, De Herralde F (2000) Leaf pubescence, water relations and chlorophyll fluorescence in two subspecies of Lotus creticus L. Biol Plant 43:239–244
Scholander PF, Hammel HT, Bradstreet ED, Hemingsen EA (1965) Sap pressure in vascular plants. Science 148:339–346
Sharp RE (1996) Regulation of plant growth responses to low soil water potential. Hortic Sci 31:36–38
Sharp RG, Else MA, Cameron RW, Davies WJ (2009) Water deficits promote flowering in Rhododendron via regulation of pre and post initiation development. Sci Hortic 120:511–517
Silber A, Levi M, Cohen M, David N, Shtaynmetz Y, Assouline S (2007) Response of Leucadendron ‘Safari Sunset’ to regulated deficit irrigation: effects of stress timing on growth and yield quality. Agricu Water Manage 87:162–170
Sionit N, Patterson DT, Coffin RD, Mortenson DA (1987) Water relations and growth of the weed, goosegrass (Eleusine indica), under drought stress. Field Crops Res 17:163–173
Turner NC (1988) Measurement of plant water status by the pressure chamber technique. Irrig Sci 9:289–308
Van Iersel MW, Nemali KS (2004) Drought stress can produce small but not compact marigolds. HortScience 39:1298–1301
Vilagrosa A, Bellot J, Vallejo VR, Gil-Pelegrín E (2003) Cavitation, stomatal conductance, and leaf dieback in seedlings of two co-ocurring Mediterranean shrubs during an intense drought. J Exp Bot 54:2015–2024
Walter J, Nagy L, Hein R, Rascher U, Beierkuhnlein C, Willner E, Jentsch A (2011) Do plants remember drought? Hints towards a drought-memory in grasses. Environ Exp Bot 71:34–40
Zollinger N, Kjelgren R, Cerny-Koenig T, Kopp K, Koenig R (2006) Drought responses of six ornamental herbaceous perennials. Sci Hortic 109:267–274
Acknowledgments
This work was supported by the Spanish Ministry of Science and Innovation (AGL 2008-05258-C02-1-2, AGL 2011-30022-C02-01) and Fundación Séneca (15356/PI/10).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Aroca.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Álvarez, S., Bañón, S. & Sánchez-Blanco, M.J. Regulated deficit irrigation in different phenological stages of potted geranium plants: water consumption, water relations and ornamental quality. Acta Physiol Plant 35, 1257–1267 (2013). https://doi.org/10.1007/s11738-012-1165-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1165-x