Abstract
The present study aimed to uncover the interconnected mechanisms underlying salt tolerance of Atriplex nummularia, a potential fodder plant for saline agriculture. The plants were grown in gravel/hydroponic quick check system in the greenhouse and irrigated with various seawater salinities (sws) (0, 25, 50, 100 and 150 %). Raising NaCl salinity stimulated the plant growth, which was highest at 50 % sws. Growth stimulation was mainly due to positive water balance, bestowed by plant ability to adjust osmotically and to minimize water loss via transpiration. Osmotic adjustment was mainly achieved by substantial accumulation of Na+ and Cl−. This was associated concurrently with sharp decrease in K+ and NO3 − concentrations, resulting into ion imbalance. However, the plants were able to maintain adequate ion ratios in their roots and juvenile leaves, where the metabolic activities are expected to be highest. Salt-induced reduction in transpiration rates was coincided with progressive decrease in net photosynthesis (P N). This reduction was proportionally higher than those observed for photosynthesis, leading to improve photosynthetic water use efficiency (PWUE). Reduction in NO3 − concentration may contribute to the overall reduction in total soluble protein (TSP), total N content and hence net photosynthetic rates. However, photosynthetic nitrogen use efficiency (PNUE) and nitrogen use efficiency (NUE) were transiently increased, peaked at moderate salinities, indicating that the plants could effectively regulate nitrogen utilization for C-assimilation machinery. Thus, plant’s ability to maintain carbon and nitrogen assimilation in equilibrium through well-coordinated regulatory mechanisms could be considered as a key determinant for salt tolerance in A. nummularia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant growth and productivity are severely threatened by increasing soil salinity and water scarcity worldwide. At least 6 % of the world’s total area is saline and more than 30 % of irrigated land is salt-affected to various degrees (FAO 2011). The intensive use of the precious natural recourses (i.e., land and water) along with high evapotranspiration and inefficient irrigation systems inevitably accelerates the secondary salinization, particularly, in arid climates (Lieth and Mochtschenko 2002; Munns 2005). The rapidly growing demands of the progressively increasing population seem to enforce the improvement of salt resistance of our conventional crops by genetic manipulation (Flowers 2004), but the outcomes remain not significant so far (Läuchli and Grattan 2007). An alternative approach is to utilize the naturally salt-tolerant halophytes for sustainable crop production (Lieth et al. 1999). This would also provide the access to use saline water, reducing thereby the pressure on the limited fresh water resources (Rozema and Flowers 2008).
One promising candidate with a high potential to become a cash crop fodder is Atriplex nummularia Lind. (Chenopodiaceae). Owing to its extraordinary high salt resistance (Osman and Ghassaeli 1997), and adequate fodder quality within the range of conventional forage sources (El Mourid et al. 2001), it has been introduced specifically to increase forage productivity of salt-affected soils in many Mediterranean countries (Le Houérou 1995). Although investigations of A. nummularia irrigated with highly saline water have been reported, knowledge about the level of salt resistance (threshold) and responses to salt stress (interrelating salt-resistance mechanisms) has been lacking so far. Understanding the physiological, biochemical and molecular mechanisms underlying salt resistance of this species could provide basic information required for its sustainable utilization on a large scale.
Adaptation to NaCl salinity is complex and comprises a wide range of morphological, physiological and biochemical mechanisms (Flowers and Colmer 2008). These mechanisms are closely related to the four major constraints of plant growth on saline substrates: (1) water deficit, (2) restriction of CO2 uptake, (3) ion toxicity, and (4) nutrient imbalance (Eisa et al. 2012). Osmotic stress caused by low water potential in saline soils interferes with the plant ability to take up water, creating water deficit (Munns 2005; Koyro et al. 2011). This would induce a rapid stomatal closure to avoid water loss via transpiration, which in turn leads to a low photosynthetic rate due to restricted CO2 availability (stomatal limitation) (Huchzermeyer and Koyro 2005; Flexas et al. 2007). High NaCl salinity may cause specific ion toxicity as disproportionate presence of Na+ and/or Cl− in cellular compartments can inhibit several enzymatic systems (Tester and Davenport 2003). In addition, it may negatively impact the acquisition of essential nutrients as Na+ and Cl− competitively inhibits the uptake of other cations and anions, respectively (Liu et al. 2006). This leads, consequently, to extreme ion ratios (e.g., Na+/K+, Cl−/NO3 −) within the plant, altering a wide range of important metabolic processes that plant growth is crucially depending on (Munns and Tester 2008).
One possibility to overcome the deleterious effects of salinity is the maintenance of water potential gradient between plant and soil together with a selective ion uptake and compartmentation (Flowers and Colmer 2008). A well-characterized response of several halophytes including Atriplex is their ability to utilize the massive accumulation of inorganic ions (mainly Na+ and Cl−) in their tissues to adjust osmotically (Ramos et al. 2004; Aghaleh et al. 2009). Although this mechanism is energy-efficient to adjust osmotically, it may lead to ion toxicity and/or nutritional imbalance (Koyro et al. 2008). Therefore, adaptation by salt inclusion requires a precise coordination between mechanisms which operate at cellular, intracellular, tissue or organ level to sustain ion homeostasis within the plant (Apse and Blumwald 2007; Koyro et al. 2011). One of the means to control ion influx is selective ion absorption and transport in favor of K+ versus Na+ (or NO3 − over Cl−) at the root level (Wang et al. 2004; Huang et al. 2006).
In general, salt-secreting Chenopods like A. nummularia exhibit a weak selective uptake capacity, and accumulate high amounts of Na+ in their shoots, particularly the adult leaves (Yeo and Flowers 1986). This is presumed to restrict toxic ion deposition into the photosynthesizing juvenile leaves (Munns 2002). Exclusion of accumulated toxic ions via bladder hairs is a common strategy in many Atriplex species. The activity of these bladder hairs is highly selective, secreting mostly Na+ and Cl−, assuring thereby adequate K+/Na+ ratio, particularly, within the photosynthetic leaf tissues (Kelley et al. 1982; Glenn et al. 1999).
Another metabolic response to hyperosmotic salinity is the synthesis and accumulation of organic (compatible) solutes in the cytoplasm to maintain an osmotic equilibrium across the tonoplast (Wyn Jones and Gorham 2002; Ashraf and Foolad 2007). Typically accumulated in this response are sugars, sugar alcohols, nitrogen-containing solutes such as amino acids and quaternary amino-acid derivatives (like proline) (Mansour 2000; Murakeozy et al. 2003). These solutes have been reported to function in osmoregulation, preservation of photosynthesis, protection of membrane integrity, stabilization of enzymes/proteins, detoxification of reactive oxygen species and as a reducing equivalent (Ashraf and Foolad 2007; Koyro et al. 2012). Nevertheless, the biosynthesis of organic metabolites is an energy-consuming process, supported mainly by carbon skeletons derived from photosynthesis. Salt resistance requires, therefore, a fine regulatory crosstalk between carbon and nitrogen metabolism within the plant to meet the requirements of growth and survival under stress conditions.
To get precise information about salinity resistance threshold and gain a better understanding of salt-resistance mechanisms of A. nummularia, this work aims to investigate biomass accumulation, water and ion relations, CO2 gas exchange, accumulation of compatible solutes and metabolites (carbohydrates, amino acids, soluble proteins) under long-term NaCl treatment to determine the main physiological features leading to salt resistance of A. nummularia. Special emphasis was taken to the role that the bladder hairs play in the regulation of internal salt concentrations of the leaves. Considering the relation between carbon and nitrogen metabolism, we also assessed how A. nummularia plants alter their carbon partitioning and allocation to sustain both growth and osmotic adjustment under high saline condition.
Materials and methods
Plant material, culture, growth conditions and harvest
Seeds of A. nummularia were washed with running tap water for 24–48 h and sown in sand soil mixture in an environment-controlled greenhouse. Five weeks later, individual plants of uniform size were transferred into a soilless (gravel/hydroponic) culture (quick check system) (Fig. 1) according to Koyro (2003) in the greenhouse. The plants were irrigated with a basic nutrient solution (Epstein 1972) using a drip irrigation system. Growth conditions were as follows: photoperiodic conditions of 16 h light/8 h dark, temperature of 25 ± 2 °C during the day and 15 ± 2 °C during the night, relative humidity of 60–70 % and light intensity in the range of 250 μE m−2s−1 at the plant level. Salt treatments started 2 weeks later by raising NaCl concentration in the nutrient solution in increments of 50 mol m−3 NaCl each day until the final concentration was attained. There were altogether five salinity levels [control, 125, 250, 500 and 750 mol m−3 NaCl; equivalent to 0, 25, 50, 100 and 150 % seawater salinity (sws)]. The quick check system was programmed to irrigate the plants daily every 4 h for 30 min starting at midnight. The experiment was performed for a total period of 11–12 weeks.
At the harvest time, the plants (three replicates each treatment) were separated into roots (R), adult leaves (La), and juvenile leaves (Lj). The root segments were washed for 1–2 min with ice-cold 0.2 mM CaSO4 solution, then for 1–2 min with distilled water to remove the excess of salts in the free spaces. Subsequently they were blotted carefully with tissue paper to remove the surface water. The fresh weight of all plant organs were directly determined. The washed plant materials were oven-dried at 105 °C for 48 h and reweighed. Representative specimens (200–300 mg) from each plant organ were pooled and stored at −80 °C for quantitative chemical analyses.
Osmotic potential and CO2 gas exchange
The osmotic potential of the press sap of all plant organs was measured with the freeze-point depression method using a cryo-osmometer (Osmomat 030, Genotec GMBH, Berlin).
Gas exchange measurements were taken 4 and 8 weeks after the initiation of salt treatment porometrically using a LI-COR 6200 portable photosynthesis system (LI-COR, Lincoln, NE, USA). The net photosynthetic rate (P N), transpiration (E) and stomatal resistance (R s) of the third or fourth fully expanded leaves were measured between 0900 and 1500 hours under saturating irradiation (2,000 μmol photon m−2s−1). Water use efficiency (PWUE) of the photosynthesis was estimated as the ratio of assimilation to transpiration rates.
Mineral ion contents
To provide precise physiological interpretations of salt-regulation mechanisms in A. nummlaria, ion contents of the root, leaf tissues and bladder hairs were determined separately. The adaxial and abaxial surfaces of the leaves (adult and juvenile) were rinsed in 25 ml distilled water (bladder hair fractions). These fractions were sealed in air-tight vials and stored at 4 °C for ion analyses. The fresh weight of these washed leaves was determined and the ion contents of the bladder hairs were calculated on fresh weight base. Approximately 0.1 g of milled dried material from the roots as well as washed leaves (adult and juvenile) was ashed in a muffle furnace at 550 °C for 12 h. The ashes were then extracted with HNO3 (32 %) according to Steubing and Fangmeier (1992). Plant extractions as well as bladder hair fractions were then diluted and filtered. Na+, K+, Ca2+ and Mg2+ concentrations were measured using an atomic absorption spectrophotometer (Perkin Elmer model PE 2100). The selective absorption (SAK:Na) and selective transport (STK:Na) capacity for K+ over Na+ was calculated according to the following equations (Pitman 1965; Wang and Zhu 1994):
Anion (Cl−, NO3 −) concentrations in the press sap of the root and leaves as well as in the bladder hair fractions were determined using an ion chromatographic system (Meteohm AG, Herisau, Switzerland). The separation process was carried out using an IC anion column (Hamilito PRP-X100, 250 mm) based on a polystyrene–divinylbenzene co-polymer.
Total soluble carbohydrates (TSC), protein (TSP) and amino acids (TAA)
Total soluble carbohydrate (TSC) in the press sap were assayed spectrophotometrically according to Masuko et al. (2005). The press sap was also used to determine the total soluble protein (TSP) spectrophotometrically according to Bradford (1976).
For amino-acid determination, freeze-dried pulverized samples of about 0.1 g from R, La, Lj were homogenized and extracted according to Högy (2002). The extractions were then derivatized with 9-fluorenylmethyl chloroformate (FMOC-Cl) according to (Einarsson et al. 1983). Amino-acid concentrations were determined using a reversed-phase high-performance liquid chromatograph (RP-HPLC, Varian, USA) equipped with a Varian autosampler model 410 and a Varian 210 pump. The separation was performed using an analytical ODS column (Aminotag 80 TM, 150 × 4.6 mm) protected by a PR-8 guard column (10 × 3.2 mm). The derivatives were analyzed using a HPLC fluorescence detector (Shimadzu RF-535) with a wavelength of 260 nm as excitation and 310 nm as emission wavelength.
Carbon and nitrogen contents
Carbon (C) and nitrogen (N) contents of the R, La and Lj were determined using a Vario MAX-CNS analyzer (Elementar Analyse system GmbH, Germany). Nitrogen-use efficiency (NUE) was calculated as:
The photosynthetic nitrogen-use efficiency (PNUE, μmol CO2 mol−1 leaf N S−1) was estimated using the following equation (Debez et al. 2006):
Statistical analysis
All data sets were subjected to a one-way-ANOVA analysis using the SPSS for Windows statistical data analysis package (SPSS Inc., 2002, release 16, Chicago, IL, USA). Tukey’s post-hoc test was employed to determine if significant (P ≤ 0.05) differences occurred between individual salinity treatments.
Results
Plant growth and biomass production
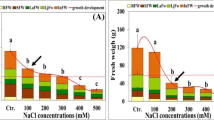
The growth of all plant organs (expressed as fresh weight) was strongly stimulated with increasing water salinity relative to controls, with maximum growth occurred at moderate salinity level (50 % sws) (Fig. 2a, b). A. nummularia plants displayed conspicuous growth and continued to develop new leaves even at the highest salinity treatment (150 % sws), where their fresh weights were still higher compared with the controls. Significant (P ≤ 0.05) increases of about 550 and 111 % in plant fresh weight were observed at moderate (50 % sws) and high (100 % sws) salinity level, respectively. These increases were mainly a result of increased shoot (stem and leaves) rather than root fresh weights (Fig. 2a, b). Consequently, the shoot/root ratio was significantly enhanced with raising NaCl salinity, reached maximum at 50 % sws. Salinity resistance threshold (water salinity causes the initial significant reduction in maximum expected yield according to Shannon and Grieve 1999) and C50 (water salinity which reduces the maximum yield by 50 %) were at salinity levels higher than 100 % sws (Fig. 2b).
Development and growth responses of A. nummularia plants to varying seawater salinities (a) and plant fresh weight at different water salinity (b). R root, St stem, La adult leaves, Lj juvenile leaves. The dotted line marks the C50 value. Each column represents the mean of nine replicates and the bars represent standard errors. Columns with the same letter are not significantly different at P ≤ 0.05, Tukey’s post-hoc test
Mineral element composition and contents
Raising water salinity progressively and significantly (P ≤ 0.05) increased Na+ concentrations of all organs (Fig. 3a). Whatever the salinity treatment, foliar Na+ concentrations (particularly those of the adult leaves) were generally higher than those of the root. High salinity level resulted in five- to tenfold increases in Na+ concentrations relative to controls depending on the plant organs.
Effect of increasing NaCl salinity on Na+ (a), K+ (b), Cl− (c), and NO3 − (d) concentrations of different organs. R roots, La adult leaves, Lj juvenile leaves. Each value represents the mean of nine replicates and the bars represent standard errors. Bars with the same letter are not significantly different at P ≤ 0.05, Tukey’s post-hoc test
Elevating water salinity led to a steep rise in Na+ concentrations inside the bladder hairs of both adult and juvenile leaves (fresh weight base) (Fig. 4). This effect was more pronounced for the bladders of juvenile leaves, where Na+ accumulated up to sevenfold relative to the controls.
Contrarily, tissue K+ concentrations decreased transiently in all plant organs with increasing NaCl salinity, although, these reductions were more pronounced in the leaves, especially juvenile ones. Maximum reductions in tissue K+ concentrations (50, 75 and 80 % for R, La and Lj, respectively) were noted at moderate salinities (Fig. 3c). Along with salt-induced Na+ increments, this led to a significant (P ≤ 0.05) increase in the Na+/K+ ratio within all plant organs. Even at the highest salinity level, the roots exhibited distinctly the lowest Na+/K+ ratios (being 7.4), while the adult leaves had the highest ratio (being 27.8).
Selective absorption in favor of K+ versus Na+ (SAK:Na) was very low (0.10 ± 0.02) under control conditions (Table 1). Raising water salinity resulted into significant (P ≤ 0.05) increases in SAK: Na up to 113 folds at seawater salinity level (Table 1). Selective transport capacity of K+ over Na+ (STK:Na) was generally <1 at the whole range of salinities with one exception; STK:Na for the Lj was higher than one under control conditions (Table 1). However, STK:Na of the juvenile leaves was remarkably higher as compared to that of the adult leaves at the whole range of salinity treatments (Table 1).
Negligible amounts of K+ were detected inside the bladder hairs of both adult and juvenile leaves under control conditions (Fig. 4). Elevating water salinity led to a transient decrease in K+ excretion. As a result, Na+/K+ ratio in the bladder hairs increased markedly with increasing water salinity reached maximum (24.0 and 74.2 for the bladders of the adult and juvenile leaves, respectively) at the highest salinity level. STK:Na from the juvenile leaves to the corresponding bladder hairs was lowest (Table 1).
Tissue Ca2+ and Mg2+ concentrations of all plant organs were transiently declined in response to external NaCl salinity (data not shown).
Cl− concentrations showed a similar tendency to that of Na+, gradually incremented in all plant organs as water salinity rose (Fig. 3c). However, the leaves tended to accumulate comparatively lower Cl− than Na+ at the whole range of salinities. Cl− excretion from the leaves via bladder hairs increased consistently as NaCl concentration in the nutrient solution rose, the effect was much pronounced for the juvenile ones (Fig. 4). NaCl salinity lowered transiently and significantly (P ≤ 0.05) NO3 − concentrations of all plant organs, with maximum reduction observed at low and moderate salinities (Fig. 3d). Intense salinity treatment (150 % sws) led to substantial NO3 − accumulation in the roots and adult leaves, where its concentration exceeded the control levels (Fig. 3d).
CO2-gas exchange
The net photosynthesis rate (P N) was steadily and significantly (P ≤ 0.05) declined as water salinity increased, reached only about 27 % of the control values at the highest salinity treatment (Table 2). Elevating water salinity gradually and significantly (P ≤ 0.05) increased the stomatal resistance (R s), with maximum increase (eightfold relative to the controls) observed at the highest water salinity level (Table 2). As a consequence, the ratio of internal to external CO2 concentration (C i/C a) was distinctly reduced from 0.5 ± 0.04 (control conditions) to 0.22 ± 0.04 at the highest salinity treatment.
Salt-induced increase in R s was correlated with a substantial reduction in the transpiration rates (E), which reached a minimum at the highest salinity level (Table 2). Reduction in the transpiration rates was, however, proportionately much greater than that of photosynthetic rates, leading to improve the photosynthetic water-use efficiency (PWUE) with increasing water salinity. Compared to the control, WUE was enhanced by about 107 % in plants grown at 150 % sws (Table 2).
Nitrogen and carbon contents (relations)
On average, total nitrogen contents of control plants ranged between 2.7 and 5.3 % (dry weight basis), with maximum N content observed in Lj (Fig. 5a). Increasing water salinity transiently decreased N content of all organs, with significant (P ≤ 0.05) reductions occurred at 50 % sws (for R and La) and at 100 % sws (for Lj). N contents were reduced by 28, 35 and 13 % for R, La and Lj, respectively, in response to high salinity treatment (Fig. 5a).
Effect of various NaCl-salinity on nitrogen contents (a), nitrogen-use efficiency (NUE) (b), C/N ratio (c) and total soluble carbohydrate contents (d) of the roots (R), adult leaves (La) and juvenile leaves (Lj). Each value represents the mean of nine replicates and the bars represent standard errors. Bars with the same letter are not significantly different at P ≤ 0.05, Tukey’s post-hoc test
The same trend was also observed for carbon contents, although with less adverse effect as compared to nitrogen, resulted into a transient increase in C/N ratio in all plant organs with increasing NaCl salinity (Fig. 5c). Both growth NUE and PNUE were significantly (P ≤ 0.05) highest at moderate salinities (25 and 50 % sws), but lowest at full strength salinity (Fig. 5b; Table 1, respectively).
TSC, TSP, total amino acids (TAA), and proline (Pro) contents
On average, TSC contents ranged between 88.9 (root) and 95.5 (adult leaves) mmol l−1 press sap under control conditions (Fig. 5d). TSC content of the roots was notably increased as water salinity rose, exceeded clearly the initial values at highest salinity level. As for the leaves, TSC contents of La significantly (P ≤ 0.05) declined, while those of Lj were slightly (statistically not significant) enhanced with elevated water salinity (Fig. 5d).
TSP content ranged from 0.2 (root) to 0.9 (juvenile leaves) mg l−1 press sap in control plants (Fig. 6a). Elevating substrate salinity transiently decreased TSP content of both La and Lj (Fig. 6). Salinities above 25 % sws enhanced the TSP contents of Lj, but did not significantly affect those of La. Unlikely, TSP content of the roots increased gradually and significantly (P ≤ 0.05) as the external salinity rose (Fig. 6a).
Effect of increasing NaCl salinity on total soluble protein (a) and proline (b) contents of different plant organs. R roots, La adult leaves, Lj juvenile leaves. Each value represents the mean of nine replicates and the bars represent standard errors. Bars with the same letter are not significantly different at P ≤ 0.05, Tukey’s post-hoc test
Increasing NaCl salinity transiently decreased the TAA content of both adult and juvenile leaves, with lowest TAA content observed at moderate salinity (Table 3). Higher salinities significantly augmented TAA content of the leaves, especially, the juvenile ones. As for the roots, TAA content increased gradually with the rise of salinization level, with maximum increase (ca. 96 %) at the highest salinity treatment (Table 3).
Proline concentration was very low (constituting only 3–5 % of the TAA content) in all plant organs under control condition (Fig. 6b). Its concentration was slightly (statistically not significant) enhanced with increasing salinity up to 50 % sws. Higher salinities, however, induced a progressive accumulation, particularly in the juvenile leaves. The highest salinity treatment caused significant increases of seven, six and eightfold in proline concentrations of R, La and Lj, respectively (Fig. 6b).
Osmotic adjustment
As can be seen in Fig. 7, osmotic value (mOsmol) of all plant organs increased clearly as water salinity increased. Sums of osmotically active solute concentrations (Na+, K+, Cl−, NO3 −, TSC, TSP, TAA and others) were sufficient, from quantitative point of view, to explain more than 95 % of the osmotic potentials in all plant organs (Fig. 7). Under control conditions, not only TSC, K+ but also Na+ (to lesser extent) contributed to a considerable part of the osmotic potential, particularly in the root, where they accounted for about 26.2, 20.8, and 22.3 %, respectively. The involvement of organic solutes in the osmotic potential decreased, whilst that of Na+ and Cl− increased in response to raising water salinity (Fig. 7).
Concentrations of osmotically active substances in different plant organs at control (a) and 150 % sws (b). R roots, La adult leaves, Lj juvenile leaves. Na sodium, Cl chloride, K potassium, NO 3 nitrate, TSC total soluble carbohydrates, TAA total amino acids and other (Ca, Mg, SO4, PO4, oxalate). X symbols mark the corresponding osmotic value of the press saps
Discussion
Although salinity is a growth-limiting factor for most plant species, it strongly stimulated the growth of A. nummularia, with optimal growth (amounted to ~550 % of the control) occurring at 50 % sws. Similar results have been reported for this species (Ramos et al. 2004; Silveira et al. 2009) and many other halophytes (Khan et al. 2000; Debez et al. 2006).
The mechanisms leading to growth improvement at moderate NaCl levels are not fully understood. However, in agreement with Nerd and Pasternak (1992), salt-induced growth stimulation in A. nummularia might be largely the consequence of increased tissue water content. This is strongly supported by a marked decrease in dry-matter accumulation and a significant increase in leaf succulence (unpublished data). Increasing leaf succulence in response to salinity is an important adaptive strategy that might contribute to the regulation of internal ion concentrations in halophytes such as A. nummularia (Koyro et al. 2008).
Supraoptimal salinities inhibited slightly the growth of A. nummularia, a response that is quite common in many halophytes (Geissler et al. 2009; Eisa et al. 2012). However, the plants were able to express high growth potentials even at 150 % sws, where their fresh weights were still greater than the respective controls (111 % increases). Together, these results indicate that A. nummularia is a highly tolerant and productive species, particularly, under high saline conditions.
The initial adverse effect of salinity on plant growth is usually due to osmotic effect resulting from low substrate water potential. Results of this study showed that osmotic potential of all plant organs fell gradually and became more negative as the external salinity rose (Fig. 7). This indicates that A. nummularia was able to maintain, even under high saline conditions, a sufficient water influx. Similar results have been reported for A. nummularia (Silveira et al. 2009), A. triagularis (Karimi and Ungar 1984), A. semibaccata (De Villiers et al. 1996), A. prostrate (Wang et al. 1997) and A. griffithii (Khan et al. 2000). As expected, lowering osmotic potential was associated concurrently with a substantial accumulation of Na+ and Cl−, particularly in the aerial parts. Their participations at the total osmotic potential increased significantly from 23 to 45 and 9 to 13 % (for Na+ and Cl−, respectively) under control conditions to 40–60 and 30–50 % at full-strength salinity. This indicates clearly that A. nummularia behaves as a salt includer, utilizes controlled accumulation of Na+ (balanced by Cl−) to adjust osmotically (Munns 2005). Osmotic adjustment using inorganic ions is cost-effective (with respect of energy and resources spent) compared to biosynthesis of organic solutes (Koyro and Huchzermeyer 1999; Marcum 2006).
Another mechanism to cope with salt-induced water imbalance is the progressive decline in transpiration rates (Table 2). Similar characteristics of water conservation have also been reported for other halophytic species (Liu and Stützel 2002). Together with the ability to adjust osmotically, these indicate that A. nummularia can efficiently control its water relations, maintaining thereby a positive water balance at a wide range of salinities. Therefore, one can presume that hyperosmotic conditions are not a limiting factor for the growth of A. nummularia under saline conditions.
Reduction in transpiration rates was coincided with a progressive decrease in net photosynthetic rates (P N). Many earlier reports showed that plant photosynthetic capacity was inhibited by salinity (Bayuelo-Jimenez et al. 2003; Koyro et al. 2006). Interestingly, A. nummularia plants continued to grow and photosynthesize, although at a reduced rate, even at high water salinity (Table 2). The mechanisms by which halophytes maintain growth and photosynthesis as well as cause–effect relationship between growth and photosynthesis under hyperosmotic salinity could be difficult to explain. Salt-induced reduction in photosynthetic rates is likely to be a consequence of an enhanced stomatal closure, leading to a substantial reduction in CO2 diffusion to the carboxylation sites (Khan et al. 2000; Geissler et al. 2009). This interpretation is supported by the linear correlation between net photosynthesis (P N), transpiration rate (E), stomatal resistance (R s) and the internal CO2 concentration (Table 2). Similarly, a positive correlation between photosynthetic rates and stomatal conductance has been found in Atriplex prostrata (Wang et al. 1997), A. nummularia and A. hastata (Dunn and Neales 1993) and A. centralasiatica (Qiu et al. 2003). The reduction in transpiration rates was proportionally larger than that in photosynthetic rates, resulting into enhanced PWUE. Such an improvement in PWUE has been also reported for several halophytic species in response to salinity (Naidoo et al. 1995) and considered as an important feature for the long-term survival in saline environments (Naidoo and Mundree 1993; Koyro 2000).
However, suppression of photosynthesis at high salinities may attribute also to impaired biochemical and photochemical capacities of the leaves (Sobrado 2005). This may be due to an inhibition (synthesis or activity) of several enzymes related to photosynthesis such as Rubisco (Rivelli et al. 2002). Alterations in photosynthesis as described above could also be explained either by nutrient deficiency or ion toxicity usually observed under saline conditions (Navarro et al. 2008; Abogadallah 2010). Of all macro nutrients, photosynthesis is most critically restricted by nitrogen availability. In this study, NO3 − concentration decreased sharply in all plant organs in response to moderate salinities. Apparently, this might be due to the antagonism of NO3 − by Cl− in the external substrate (Feigin et al. 1987; Zhu 2002) or a dilution effect due to salt-induced enhanced succulence. Low NO3 − concentration seems to be responsible for the reduction in TSP and total nitrogen contents (Figs. 5a, 6a), indicative of suppressed NO3 − assimilation rates under saline conditions (Feigin et al. 1991; Al-Rawahy et al. 1992).
Expectedly, the competition between chloride and nitrogen under saline conditions greatly alters vital processes such as photosynthesis, since a large fraction of leaf nitrogen (~60–80 %) is invested in the photosynthetic machinery (Evans and Seemann 1989; Makino and Osmond 1991). Surprisingly, PNUE and NUE were highest at moderate salinities (25–50 % sws). This clearly indicates that A. nummularia plants could effectively regulate nitrogen partitioning for carbon assimilation machinery to enable an optimal photosynthetic capacity and, hence, growth and development at moderate salinities. On the other hand, incorporation of NO3 − into downstream products is usually linked with a high metabolic cost and intimately depends on the leaf capacity to supply carbon skeleton (Pace et al. 1990). As a consequence, this would alter the allocation of photoassimilates between sugars and amino-acid biosynthesis. Salt-induced increasing in C/N ratio with elevating substrate salinity (Fig. 5b) reflects an enhanced carbon flux toward the synthesis of sugars at the cost of N assimilation. Together, these reveal that A. nummularia plants could maintain C- and N-assimilation processes in equilibrium through well-coordinated regulatory mechanisms to enable optimal growth and development under saline conditions.
Raising water salinity also resulted into a substantial decrease in K+ concentration. Its participation in the osmotic adjustment fell from 21, 4, and 12 % under control condition to 5, 1, and 3 % (for R, La, and Lj, respectively) at the highest water salinity. Similar results have been observed previously, and interpreted as a consequence of competition between K+ and Na+ uptake or due to changes in membrane integrity caused by displacement of Ca2+ by Na+ (Cramer et al. 1985; Silveira et al. 2009). Thus, Na+/K+ ratio enhanced significantly in all plant organs in response to salinity. Nevertheless, the plants were able to maintain a relatively low Na+/K+ ratio in their roots and juvenile leaves (where the metabolic demands are expected to be greatest and the sensitivity to Na+ is highest) at the whole range of salinities (compared to respective external solution). This reveals that A. nummularia may exert some control over ion absorption and transport in these organs. Of which, selective absorption of K+ over Na+ (SAK:Na) that substantially increased from 0.10 under control conditions reached about 100 at high salinity treatment (Table 1). Further, the selective transport capacity in favor of K+ (STK:Na) from root to shoot (Table 1) was very weak (below one at the whole range of salinities). This reflects a preferential transport of Na+ to the shoot where more than 80 % of the total sodium content was accumulated. Such ion distribution between root and shoot confirms that A. nummularia is typically a salt includer (Flowers et al. 1977). It is worth noting that STK:Na from root to adult leaves was lower than to the juvenile ones at the whole range of salinities. This indicates a preferential Na+ sequestration into the adult leaves, which might function as ion sinks to restrict or at least delay excessive accumulation of Na+ and Cl− in the actively photosynthesizing tissues (Jeschke 1984; Koyro 2002).
Low salt concentrations in the active metabolic sites of the leaves is directly linked to their capacity to remove or compartmentalize the surplus of salts (mainly Na+ and Cl−) into the bladder hairs (Freitas and Breckle 1992). Here, we observed that Na+ and Cl− concentrations in the bladder hairs (fresh weight base) were correlated with those of the leaf tissue and external solution (Fig. 4). Quite remarkably, the juvenile leaves were able to compartmentalize more Na+ and Cl− into their bladder hairs compared to the adult ones at the whole range of salinities (Fig. 4). This may be due to the fact that the formation of these bladder hairs occurs only in the early stages of the leaf development, so that the ratio between hair density and leaf mass is highest in this stage (Kelley et al. 1982). This confirms the significant role that the bladder hairs play to withdraw Na+ and Cl− from the leaves, particularly, the juvenile photosynthetically active ones (Schirmer and Breckle 1982). As for the adult leaves, this mechanism seems to be less effective. Therefore, ion dilution as a result of increasing leaf succulence may be the main strategy operating in the adult leaves to avoid the toxic effects of harmful ions (Debez et al. 2006).
At the cellular level, low K+/Na+ ratio may not occur into the cytoplasm, in case that Na+ and Cl− are effectively compartmentalized into the vacuoles. This would help maintaining adequate ion ratios in the cytosol and provide an energetically cheap osmotic driving force for water uptake (Tester and Davenport 2003). However, this process by itself is energy-consuming (accumulation of Na+ occurs against a concentration gradient) and usually accompanied by the synthesis of compatible osmolytes (extra energy requirements) in the cytoplasm to balance the low osmotic potential in the vacuole (Slama et al. 2007). This might explain growth reduction at the highest water salinity (Huchzermeyer and Koyro 2005; Geissler et al. 2009).
Osmotically active organic solutes such as amino acids have been reported to accumulate in response to salinity (Misra and Gupta 2005). In this study, high water salinity level was connected with a noticeable increase in the TAA concentrations (Table 3). Data of TAA contents were consistent with those of total soluble protein, suggesting that increased-amino acid pool in the leaves of A. nummularia plants grown at hyperosmotic salinity was due to a decreased rate of protein synthesis or an enhanced proteolysis (Forment et al. 2002; Pessarakli et al. 2005). In agreement with other studies (Storey and Wyn Jones 1979; Bajji et al. 1998), increasing TAA content under hyperosmotic salinity was entirely the consequence of a marked increase in proline levels (Fig. 6b). Proline accumulation, a common response to hyperosmotic salinity, is considered to be involved in salt-resistance mechanism as was reported for A. spongiosa (Storey and Wyn Jones 1979) and A. halimus (Martínez et al. 2005). Although proline accumulated to high concentrations (five to sevenfold) at the highest salinity level, its concentration (ca 80–150 μmol g−1 DW) was insufficient to account for a significant contribution to the total osmotic adjustment (Fig. 7). This may not, however, preclude its importance as osmolyte, because it is thought to be synthesized and restricted mainly in the cytoplasm (merely about 10 % of cell volume). Thus, its significance as a cytoplasmic osmolyte seems to be higher than is assumed from its concentration on dry-weight basis.
Likewise, TSC contents were generally raised in A. nummularia (except for adult leaves) as water salinity rose. Several hypotheses have been mentioned to explain carbohydrates accumulation, even with suppressed photosynthesis, under hyperosmotic salinity. TSC accumulation is believed to result primarily from decreased export due to shortage of energy source (e.g., ATP) (Munns and Termaat 1986) or disturbance of carbohydrate metabolism, regulated by various synthesizing and degrading enzymes which may be ion-specifically controlled (Rathert 1982; Marschner 1995; Singh et al. 1996). Carbohydrate accumulation has been commonly documented and is considered to play an important role in osmotic adjustment in salt-tolerant plants (Popp and Smirnoff 1995; Bajji et al. 2001; Murakeozy et al. 2003). As can be seen in Fig. 7, TSC concentration does not seem to play an important direct role as osmolyte in A. nummularia in the sense of osmotic adjustment as their involvement at osmotic potential decreased from 26, 6, and 5 % under control condition to 5, 3, and 4 % at high water salinity (for R, La, Lj, respectively). In addition to their role as osmolytes, proline and carbohydrates are presumed to be osmoprotectant involved in stabilizing cellular membranes, protecting proteins and enzymes, acting as a stress signal or as free-radical scavengers (Vinocur and Altman 2005; Ashraf and Foolad 2007). They may also function as nitrogen and carbon sources during limited photosynthesis under stress conditions (Wang and Showalter 2004).
Results of the present study indicate that A. nummularia is highly salt-tolerant species in terms of biomass production under saline conditions, especially in arid climates. The delicate balance among osmotic adjustment, salt accumulation, salt compartmentation, and salt excretion via bladder hairs, along with a mutual coordination and complementation of carbon and nitrogen metabolism and allocation of cellular components within the plant appear to be important components of its high salt resistance. Growth reduction at supraoptimal salinities is presumably due to low supply of photoassimilates as a consequence of impaired photosynthetic capacity. This worse effect is aggravated by the diversion of assimilates away from the synthetic processes involved in cell growth and development to maintenance processes. Further investigations are needed to decipher the relation between photosynthesis, respiration, plant growth and biomass accumulation under salt stress. Finally, it is worth to mention that A. nummularia does not only offer the possibility of being an alternative promising fodder crop under moderate salinities but also, through understanding of its physiology, may provide possible routes to enhance salt resistance in other crops.
Author contribution
Designing and conducting the entire experimental work, photosynthesis measurements, and writing the manuscript were taken care by Sayed Hussin and Hans-Werner Koyro. The chemical and statistical analysis was carried out by Nicole Geissler.
Abbreviations
- C i/C a :
-
Ratio of internal to external CO2 concentration
- E :
-
Transpiration rate
- La:
-
Adult leaves
- Lj:
-
Juvenile leaves
- NUE:
-
Nitrogen-use efficiency
- P N :
-
Net photosynthetic rate
- PNUE:
-
Photosynthetic nitrogen use efficiency
- Pro:
-
Proline
- PWUE:
-
Photosynthetic water use efficiency
- R s :
-
Stomatal resistance
- SAKNa :
-
Selective absorption of K+ over Na+
- STKNa :
-
Selective transport of K+ over Na+
- sws:
-
Seawater salinity
- TAA:
-
Total amino acids
- TSC:
-
Total soluble carbohydrates
- TSP:
-
Total soluble protein
References
Abogadallah GM (2010) Antioxidative defense under salt stress. Plant Signal Behav 5:369–374
Aghaleh M, Niknam V, Ebrahimzadeh H, Razavi K (2009) Salt stress effects on growth, pigments, proteins and lipid peroxidation in Salicornia persica and S. europaea. Biol Plant 53:243–248
Al-Rawahy SA, Stroehlein JL, Pessarakli M (1992) Dry matter yield and nitrogen-15, Na+, Cl− and K+ content of tomatoes under sodium chloride stress. J Plant Nutr 15:341–358
Apse MP, Blumwald E (2007) Na+ transport in plants. FEBS Lett 581:2247–2254
Ashraf M, Foolad MA (2007) Improving plant abiotic-stress resistance by exogenous application of osmoprotectants glycine betaine and proline. Environ Exp Bot 59:206–216
Bajji M, Kinet J, Lutts S (1998) Salt stress effects on roots and leaves of Atriplex halimus L. and their corresponding callus cultures. Plant Sci 137:131–142
Bajji M, Lutts S, Kient JM (2001) Water deficit effects on solute contribution to osmotic adjustment as a function of leaf aging in three durum wheat (Triticum durum Defs.) cultivars performing differently in arid conditions. Plant Sci 160:669–681
Bayuelo-Jimenez JS, Debouck DG, Lynch JP (2003) Growth, gas exchange, water relations and ion composition of Phaseolus species grown under saline conditions. Field Crop Res 80:207–498
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cramer GR, Läuchli A, Polito VS (1985) Displacement of Ca2+ by Na+ from the plasmalemma of root cells: a primary response to stress. Plant Physiol 79:207–211
De Villiers AJ, Von Teichman I, Van Rooyen MW, Theron GK (1996) Salinity induced changes in anatomy, stomatal counts and photosynthetic rate of Atriplex semibaccata. S Afr J Bot 62:270–276
Debez A, Saadaoui D, Ramani B, Ouerghi Z, Koyro H-W, Huchzermeyer B, Abdelly C (2006) Leaf H+-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ Exp Bot 57:285–295
Dunn GM, Neales TF (1993) Are the effect of salinity on growth and leaf gas-exchange related? Photosynthetica 29:33–42
Einarsson S, Josefsson B, Lagerkvist S (1983) Determination of amino acids with 9-fluorenylmethylchloroformate and reversed-phase high-performance liquid chromatography. J Chromatogr 282:609–618
Eisa S, Hussin S, Geissler N, Koyro HW (2012) Effect of NaCl salinity on water relations, photosynthesis and chemical composition of Quinoa (Chenopodium quinoa Willd.) as a potential cash crop halophyte. Aust J Crop Sci 6:357–368
El Mourid M, Malki M, Sbeita A, Chiriyaa A, Nefzaoui A, Shideed K, Awawedha F, Hassan SH, Sweidan Y (2001) Crop livestock integration: alternatives to stop desertification in arid regions of WANA. In: Expert meeting: scientific research and its role in combating desertification and stabilizing Sand Dunes. Taghit, Algeria, November 4–6
Epstein E (1972) Mineral nutrition of plants: principles and perspectives. Wiley, New York
Evans JR, Seemann JR (1989) The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In: Briggs WR (ed) Photosynthesis. Alan R Liss Inc., New York, pp 183–205
FAO (2011) Land and plant nutrition management service. http://www.fao.org/ag/agl/agll/spush
Feigin A, Rylski I, Meiri A, Shalhevet J (1987) Response of melon and tomato plants to chloride-nitrate ratios in saline nutrient solutions. J Plant Nutr 10:1787–1794
Feigin A, Pressman E, Imas P, Miltau O (1991) Combined effects of KNO3 and salinity on yield and chemical composition of lettuce and Chinese cabbage. Irrig Sci 12:223–230
Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30:1284–1298
Flowers TJ (2004) Improving crop salt resistance. J Exp Bot 55:307–319
Flowers TJ, Colmer TD (2008) Salinity resistance in halophytes. New Phytol 179:945–963
Flowers TJ, Troke PF, Yeo AR (1977) Mechanism of salt resistance in halophytes. Ann Rev Plant Physio 28:89–121
Forment J, Naranjo MA, Roldán M, Serrano R, Vicente O (2002) Expression of Arabidopsis SR-like splicing proteins confers salt resistance to yeast and transgenic plants. Plant J 30:511–519
Freitas H, Breckle SW (1992) Importance of bladder hairs for salt resistance of field-grown Atriplex species from a Portuguese salt marsh. Flora 187:283–297
Geissler N, Hussin S, Koyro H-W (2009) Interactive effects of NaCl salinity, elevated atmospheric CO2 concentration on growth, photosynthesis, water relations and chemical composition of the potential cash crop halophyte Aster tripolium L. Environ Exp Bot 65:220–231
Glenn E, Brown JJ, Blumwald E (1999) Salt-tolerant mechanisms and crop potential of halophytes. Crit Rev Plant Sci 18:227–255
Högy P (2002) Wirkungen erhöhter CO2- und/oder Ozonkonzentrationen auf den Ertrag und die Qualität landwirtschaftlicher Nutzpflanzen: dissertation. University of Giessen, Germany
Huang Y, Zhang G, Wu F, Chen J, Zhou M (2006) Differences in physiological traits among salt-stressed barley genotypes. Commun Soil Sci Plan 37:557–570
Huchzermeyer B, Koyro H-W (2005) Salt and drought stress effects on photosynthesis. In: Pessarakli M (ed) Handbook of plant and crop stress, 2nd edn. Marcel Dekker Inc., New York, pp 751–778
Jeschke WD (1984) K+-Na+ exchange at cellular membranes, intercellular compartmentation of cations, and salt resistance. In: Staples RC, Toeniessen GH (eds) Salinity tolerance in plants strategies for crop improvement. Wiley, New York, pp 37–66
Karimi SH, Ungar IA (1984) The effect of salinity on the ion content and water relations of Atriplex triangularis. In: Tiedemann AR, McArthur ED, Stutz HC, Stevens R, Johnson KL (eds) Proceeding of the symposium on the biology of Atriplex and related Chenopods, pp 124–130
Kelley DB, Goodin JR, Miller DR (1982) Biology of Atriplex. In: Sen DN, Rajpurohit KST (eds) Contribution to the ecology of halophytes. Task Veg Sci 2. Dr. W. Junk Publishers, Hague, pp 79–107
Khan MA, Ungar IA, Showalter AM (2000) Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophytes Atriplex griffithii var. stocksii. Ann Bot Lond 85:225–232
Koyro H-W (2000) Effect of high NaCl-salinity on plant growth, leaf morphology, and ion composition in leaf tissues of Beta vulgaris ssp. maritima. J Appl Bot Angew Bot 74:67–73
Koyro H-W (2002) Mechanisms of adaptation to high NaCl-salinity in halophytes: a review. In: Lieth H (ed) Sustainable use of halophytes. Kluwer, Dordrecht
Koyro H-W (2003) Study of potential cash crop halophytes in a quick check system. Task Veg Sci 38:5–17
Koyro H-W, Huchzermeyer B (1999) Influence of high NaCl-salinity on growth, water and osmotic relations of the halophyte Beta vulgaris ssp. maritima: development of a quick check. In: Hamdy A, Lieth H, Todorovic M, Moschenko M (eds) Halophytes uses in different climates I. Backhuys Publishers, Leiden, pp 89–103
Koyro H-W, Geissler N, Hussin S, Huchzermeyer B (2006) Mechanisms of cash crop halophytes to maintain yield and reclaim soils in arid areas. In: Khan MA, Weber DJ (eds) Task Veg Sci 40: ecophysiology of high salinity tolerant plants. Springer, Berlin, pp 345–366
Koyro H-W, Geissler N, Hussin S, Debez A, Huchzermeyer B (2008) Strategies of halophytes to survive in a salty environment. In: Khan NA, Singh S (eds) Abiotic stress and plant responses. I.K. International Publishing House, New Delhi, pp 83–104
Koyro H-W, Geissler N, Seenivasan R, Huchzermeyer B (2011) Plant stress physiology; physiological and biochemical strategies allowing to thrive under ionic stress. In: Pessarakli M (ed) Handbook of plant and crop stress, 3rd edn. CRC press, Taylor & Francis Group, West Palm Beach, pp 1051–1094
Koyro H-W, Ahmed P, Geissler N (2012) Abiotic stress response in plants: an overview. In: Ahmed P, Prasad MNV (eds) Environmental adaptation and stress tolerance of plants in the era of climate change. Springer, Dordrecht
Läuchli A, Grattan SR (2007) Plant growth and development under salinity stress. In: Jenks MA, Hasegawa PM, Jain SM (eds) Advances in molecular breeding toward drought and salt tolerant crops. Springer, Dordrecht
Le Houérou HN (1995) Forage halophytes in the Mediterranean basin. In: Chouker-Allah R, Malcolm CV, Hamdy A (eds) Halophytes and biosaline agriculture. Marcel Dekker Inc, New York, pp 115–136
Lieth H, Mochtschenko M (2002) Halophyte uses in different climates IV: cashcrop halophytes for future halophytes growers. Backhuys Publishers, Leiden
Lieth H, Moschenkom M, Lohmann M, Koyro H-W, Hamdy A (1999) Halophytes uses in different climates I: ecological and physiological studies. In: Lieth H (ed) Progress in biometeorology, vol 3. Backhuys Publishers, Leiden
Liu F, Stützel H (2002) Leaf expansion, stomatal conductance and transpiration of vegetable amaranth (Amaranthus spp.) in response to soil drying. J Am Soc Hortic Sci 127:878–883
Liu X, Duan D, Li W, Tadano T, Khan A (2006) A comparative study on responses of growth and solute composition in halophytes Suaeda salsa and Limonium bicolor to salinity. In: Khan MA, Weber DJ (eds) Ecophysiology of high salinity tolerant plants. Springer, Netherlands, pp 135–143
Makino A, Osmond B (1991) Effect of nitrogen nutrition on nitrogen partitioning between chloroplast and mitochondria in pea and wheat. Plant Physiol 96:335–362
Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plantarum 43:491–500
Marcum KB (2006) Saline tolerance physiology in grasses. In: Khan MA, Weber DJ (eds) Ecophysiology of high salinity tolerant plants. Task Veg Sci 40. Springer, Dordrecht, pp 157–172
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, New York
Martínez JP, Kinet JM, Bajji M, Lutts S (2005) NaCl alleviates polyethylene glycol-induced water stress in the halophyte species Atriplex halimus L. J Exp Bot 56:2421–2431
Masuko T, Minami A, Iwasaki N, Majima, Nishimura S-I, Lee YC (2005) Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal Biochem 339:69–72
Misra N, Gupta AK (2005) Effect of salt stress on proline metabolism in two high yielding genotypes of green gram. Plant Sci 169:331–339
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Munns R, Termaat A (1986) Whole plant responses to salinity. Aust J Plant Physiol 13:143–160
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Murakeozy EP, Nagy Z, Duhaze C, Bouchereau A, Tuba Z (2003) Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J Plant Physiol 160:395–401
Naidoo G, Mundree SG (1993) Relationship between morphological and physiological responses to water logging and salinity in Sporobolus virginicus (L.) Kunth. Oecologia 93:360–366
Naidoo G, Jhanke J, Von Willert DJ (1995) Gas exchange responses of the C4 grass, Sporobolus virginicus (Poaceae) to salinity stress. In: Khan MA, Ungar IA (eds) Biology of salt tolerant plants. Department of Botany, University of Karachi, Karachi, pp 121–130
Navarro A, Banón S, Conejero W, Sánchez-Blanco MJ (2008) Ornamental characters, ion accumulation and water status in Arbutus unedo seedlings irrigated with saline water and subsequent relief and transplanting. Environ Exp Bot 62:364–370
Nerd A, Pasternak D (1992) Growth, ion accumulation, and nitrogen fractioning in Atriplex barclayana grown at various salinities. J Range Manage 45:164–166
Osman AE, Ghassaeli F (1997) Effects of storage conditions and presence of fruiting bracts on the germination of Atriplex halimus and Salsola Vermiculata. Exp Agric 33:149–155
Pace GH, Volk RJ, Jackson WA (1990) Nitrate reduction in response to CO2-limited photosynthesis: relationship to carbohydrate supply and nitrate reductase activity in maize seedlings. Plant Physiol 92:286–292
Pessarakli M, Marcum KB, Kopec DM (2005) Growth responses and nitrogen-15 absorption of desert saltgrass under salt stress. J Plant Nutr 28:1441–1452
Pitman MG (1965) Transpiration and the selective uptake of potassium by barley seedlings (Hordeum vulgare cv. Bolivia). Aust J Biol Sci 18:987–999
Popp M, Smirnoff N (1995) Polyol accumulation and metabolism during water deficit. In: Smirnoff N (ed) Environment and plant metabolism: flexibility and acclimation. BIOS Scientific Publishers, Oxford, pp 199–215
Qiu N, Lu Q, Lu C (2003) Photosynthesis, photosystem II efficiency and the xanthophylls cycle in the salt-adapted halophyte Atriplex centralasiatica. New Phytol 159:479–486
Ramos J, Lopez MJ, Benlloch M (2004) Effect of NaCl and KCl salts on the growth and solute accumulation of the halophyte Atriplex nummularia. Plant Soil 259:163–168
Rathert G (1982) Influence of extreme K:Na ratios and high substrate salinity on plant metabolism of crops differing in salt tolerance. V: ion-specific salinity effects on invertase in leaves of bush bean and sugar beet plants. J Plant Nutr 5:97–110
Rivelli AR, Lovelli S, Perniola M (2002) Effect of salinity on gas exchange, water relations growth of sunflower (Helianthus annuus). Funct Plant Biol 29:1405–1415
Rozema J, Flowers TJ (2008) Crops for a salinized world. Science 322:1478–1480
Schirmer U, Breckle SW (1982) The role of bladders for salt removal in some Chenopodiaceae (mainly Atriplex species). In: Sen DN, Rajpurohit KS (eds) Contribution to the ecology of halophytes. Dr. W. Junk publisher, Hauge, pp 215–231
Shannon MC, Grieve CM (1999) Tolerance of vegetable crops to salinity. Sci Hortic 78:5–38
Silveira JAG, Araújo SAM, Lima JPMS, Viegas RA (2009) Roots and leaves display contrasting osmotic adjustment mechanisms in response to NaCl-salinity in Atriplex nummularia. Environ Exp Bot 66:1–8
Singh AK, Chakravarthy D, Singh TPK, Singh HN (1996) Evidence for a role of l-proline as a salinity protectant in the cyanobacterium Nostoc muscorum. Plant Cell Environ 19:490–494
Slama I, Ghnaya T, Messedi D, Hessini K, Labidi N, Savoure A, Abdelly C (2007) Effect of sodium chloride on the response of the halophyte species Sesuvium portulacastrum grown in mannitol-induced water stress. J Plant Res 120:291–299
Sobrado MA (2005) Leaf characteristics and gas exchange of the mangrove Laguncularia racemosa as affected by salinity. Photosynthetica 43:217–221
Steubing L, Fangmeier A (1992) Pflanzenökologisches Praktikum. Eugen Ulmer-Verlag, Stuttgart
Storey R, Wyn Jones RG (1979) Responses of Atriplex spongiosa and Suaeda monoica to salinity. Plant Physiol 63:156–162
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot Lond 91:503–527
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotech 16:1–10
Wang L-W, Showalter AM (2004) Cloning and salt-induced, ABA-independent expression of choline mono-oxygenase in Atriplex prostrata. Physiol Plantarum 120:405–412
Wang SM, Zhu XY (1994) Studies on the characteristics of ion absorption and distribution in Puccinellia tenuiflora. Acta Pratacul Sin 3:39–43
Wang L, Showalter A, Ungar A (1997) Effect of salinity on growth, ion content, and cell wall chemistry in Atriplex prostrata (Chenopodiaceae). Am J Bot 84:1247–1255
Wang S, Wan C, Wang Y, Chen H, Zhou Z, Fu H, Sosebee RE (2004) The characteristics of Na, K and free proline distribution in several drought-resistant plants of the Alxa Desert, China. J Arid Environ 56:525–539
Wyn Jones RG, Gorham J (2002) Intra- and intercellular compartmentation of ions: a study in specificity and plasticity. In: Läuchli A, Lüttge U (eds) Salinity: environment–plants–molecules. Kluwer, Dordrecht, pp 159–180
Yeo AR, Flowers TJ (1986) Salinity resistance in rice (Oryza sativa L.) and a pyramiding approach to breeding varieties for saline soils. In: Turner NC, Passioura JB (eds) Effect of drought on plant growth. Salts in soils. CSIRO, Melbourne, pp 161–173
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgments
The financial support of the German Academic Exchange Service (DAAD) is gratefully acknowledged. The authors are indebted to Mr. Gerhard Mayer, Mrs. Angelika Bölke, Mr. Jürgen Franz and Mr. Wolfgang Stein for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Bavaresco.
Rights and permissions
About this article
Cite this article
Hussin, S., Geissler, N. & Koyro, HW. Effect of NaCl salinity on Atriplex nummularia (L.) with special emphasis on carbon and nitrogen metabolism. Acta Physiol Plant 35, 1025–1038 (2013). https://doi.org/10.1007/s11738-012-1141-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1141-5