Abstract
Zinc deficiency and salinity are well-documented soil problems and often occur simultaneously in cultivated soils. Usually, plants respond to environmental stress factors by activating their antioxidative defense mechanisms. The antioxidative response of wheat genotypes to salinity in relation to Zn nutrition is not well understood. So, we investigated the effect of Zn nutrition on the growth, membrane permeability and sulfhydryl group (–SH groups) content of root cells and antioxidative defense mechanisms of wheat plants exposed to salt stress. In a hydroponic experiment, three bread wheat genotypes (Triticum aestivum L. cvs. Rushan, Kavir, and Cross) with different Zn-deficiency tolerance were exposed to adequate (1 μM Zn) and deficient (no Zn) Zn supply and three salinity levels (0, 60, and 120 mM NaCl). The results obtained showed that adequate Zn nutrition counteracted the detrimental effect of 60 mM NaCl level on the growth of all three wheat genotypes while it had no effect on the root and shoot growth of ‘Rushan’ and ‘Kavir’ at the 120 mM NaCl treatment. At the 0 and 60 mM NaCl treatments, Zn application decreased root membrane permeability while increased –SH group content and root activity of catalase (CAT) and superoxide dismutase (SOD) in ‘Rushan’ and ‘Kavir’. In contrast, Zn had no effect on the root membrane permeability and –SH group content of ‘Rushan’ and ‘Kavir’ exposed to the 120 mM NaCl treatment. At all salinity levels, ‘Cross’ plants supplied with Zn had lower root membrane permeability and higher –SH group content compared to those grown under Zn-deficient conditions. At the 0 and 60 salinity levels, Zn-deficient roots of Kavir and Rushan genotype leaked significantly higher amounts of Fe and K than the Zn-sufficient roots. In contrast, at the 120 mM treatment, Zn application had no effect or slightly increased Fe and K concentration in the root ion leakage of these wheat genotypes. For ‘Cross’, at all salinity levels, Zn-deficient roots leaked significantly higher amounts of Fe and K compared with the Zn-sufficient roots. The differential tolerance to salt stress among wheat genotypes examined in this study was related to their tolerance to Zn-deficiency, –SH group content, and root activity of CAT and SOD. Greater tolerance to salinity of Zn-deficiency tolerant genotype ‘Cross’ is probably associated with its greater antioxidative defense capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crops grown in arid and semi-arid regions are often exposed to adverse environmental conditions, particularly salt stress (Marschner 1995). Soil salinity is an important abiotic stress aggravated by modern intensive agricultural practices and the increasing irrigation needs. About 20 % of the cultivated areas and nearly half of all irrigated lands across the world are affected by salinity (Zhu 2001).
The reduction in plant growth in saline environments could be due to either reduced water potential or the toxic effects of Na+ and Cl− ions on plant metabolism (Yeo and Flowers 1983). Salinity causes an imbalance of the cellular ions resulting in ion toxicity, nutrient deficiency (e.g., Ca and K deficiencies) and osmotic stress (Khan et al. 2000; Munns 2002), thus affecting the plant growth, morphology, and survival (Locy et al. 1996). High Na and Cl levels reduce absorption of essential nutrients such as K and also can be toxic for plants (Koyro 2006; Parida and Das 2005).
Salinity induces oxidative damages in plant tissues (Bartosz 1997; Rout and Shaw 2001), although the origin of oxidative stress is still unknown (Arbona et al. 2003). It seems that salt stress limits gas exchange and CO2 supply to the leaf (Harris and Outlaw 1991; Fendina et al. 1994) and thereby causes the over-reduction of the photosynthetic electron transport chain (Osmond and Grace 1995). This induces the generation of reactive oxygen species (ROS), such as singlet oxygen, superoxide anion, hydrogen peroxide, and hydroxyl radical (Asada 1994; Gossett et al. 1994). The generation of ROS is a common feature of many environmental stresses including salinity (Dionisio-Sese and Tobita 1998; Chen et al. 2000; Neill et al. 2002) that damages macromolecules in cells such as proteins, nucleic acids, and lipids (Imlay 2003).
On the other hand, zinc (Zn) is an essential micronutrient, which its deficiency is common in wheat growing areas of the world particularly in alkaline soils (Cakmak et al. 1996; Takkar and Walker 1993; Welch et al. 1991; White and Zasoski 1999). In alkaline soils, Zn is fixed or precipitated in unavailable forms. Furthermore, alkaline soils are often associated with high level of salt accumulation. Soil salinity may reduce Zn uptake by plant due to strong competition by salt cations at the root surface (Tinker and Lauchli 1984).
Similar to salt stress, Zn deficiency induces oxidative stress in plants by affecting both generation and detoxification of O2 free radicals (Cakmak 2000). An increase in the level of reactive O2 species (ROS) has been considered as a major reason for impairment of various cellular functions in Zn-deficient plants (Cakmak 2000). Several studies have reported that Zn deficiency may enhance production of \( {\text{O}}_{2}^{\cdot - } \) during photosynthetic electron transport (Pinton et al. 1994; Cakmak et al. 1995; Cakmak and Engels 1999). Zinc is required for detoxification of ROS including superoxide radical and H2O2 (Cakmak 2000).
Plant responses to salinity and Zn nutrition have been widely studied (Khoshgoftarmanesh et al. 2006a, b). In addition, the effect of Zn nutrition on growth and yield of wheat has been reported in previous studies (Khoshgoftarmanesh et al. 2006a, b); however, the effect of Zn nutrition on alleviating salt-induced oxidative damages in wheat is still poorly understood. Therefore, the aim of this study was to investigate the effect of Zn on the growth and antioxidative responses of wheat plants under salt stress conditions. Considering that wheat genotypes respond differently to Zn nutrition, three wheat genotypes varying in Zn-deficiency tolerance were used.
Materials and methods
Plant growth
Seeds of three bread wheat genotypes (Triticum aestivum L. cvs. Rushan, Kavir, and Cross) with different Zn-deficiency tolerance (Khoshgoftarmanesh et al. 2006a) were surface sterilized by 1 % (v/v) sodium hypochlorite and germinated in quartz sand, moistened with distilled water. In the previous field and pot experiments, ‘Kavir’ was categorized as a Zn-deficiency sensitive wheat genotype while ‘Cross’ was reported to be Zn-deficiency tolerant genotype (Khoshgoftarmanesh et al. 2006a, b). In these studies, Zn-deficiency tolerance has been measured as the ratio of yield at Zn-deficient conditions to the yield capacity at adequate Zn supply. ‘Rushan’ has been reported as a Zn-deficiency tolerant genotype in the field experiments (Khoshgoftarmanesh et al. 2006a, b). After 2 weeks, 32 seedlings per replicate were transferred to 14-l containers with continuously aerated nutrient solution. The containers were wrapped with black polyethylene to prevent light from reaching the roots and solution. The nutrient solution had the following composition: 0.88 mM K2SO4, 2.0 mM Ca(NO3)2, 0.25 mM KH2PO4, 1.0 mM MgSO4, 0.1 mM KCl, 1 μM H3BO3, 0.5 μM MnSO4, 0.2 μM CuSO4, 0.02 μM (NH4)6Mo7O24, 100 μM FeEDTA and 2 mM MES [2-(N-morpholino)ethanesulfonicacid] adjusted to pH 6 with KOH as a pH buffer. Two Zn rates (0 and 1 μM Zn in the form of ZnSO4·7H2O) and three salinity levels (0, 60, and 120 mM NaCl) were used. The first group of plants was cultivated on the standard nutrient solution containing 1 μM Zn (Zn-sufficient plants), and the second group (Zn-deficient plants) was cultivated on the standard nutrient solution without Zn addition. After 9 days, three salinity levels were created by adding 4 M NaCl to standard nutrient solution gradually to achieve 0, 60, and 120 mM concentrations, respectively. Nutrient solutions were replaced every two days. After 45 days, the wheat seedlings were harvested. The roots and shoots were washed with deionized water and dried at 70 °C for 48 h. Shoot and root dry matter yields were determined for each replicate.
Plant shoot samples were dried for 48 h at 70 °C, weighed, and digested by microwave (USEPA 1995). About 0.500 g of plant shoot samples were digested in APCU-40 75 ml TFM Teflon vessel of microwave (Milestone Srl, START D, Sorisole, Italy) using 5 ml of HNO3 and 3 ml of H2O2, then were filtered through Whatman no. 42 filters, transferred to 50-ml volumetric flasks, and diluted with deionized water.
For quality control, reagent blanks and a NIST Standard Reference Materials (NIST No. 1515 apple leaves) were included. Concentration of Zn in the digest solutions was measured by atomic absorption spectrometry (AAS) (PerkinElmer 800, PerkinElmer, Wellesley, MA) (Chapman and Partt, 1961). All plant tissue concentrations are expressed on a dry weight basis.
Reactive sulfhydryl group assay
The –SH groups were measured in the roots of intact wheat seedlings as described by Welch and Norvell (1993) using sulfhydryl-reactive reagent DTNB [5,5′-dithio-bis(2-nitrobenzoic acid)]. We measured the –SH groups that diffuse to the exterior surface of the root cells. The roots of wheat seedling were immersed in 75 ml sulfhydryl reaction buffer (0.2 M Tris–HCl plus 0.02 M Na–EDTA adjusted to pH 8.2 with NaOH). A 1.0-ml of 10 mM DTNB dissolved in absolute methanol was added to the reaction buffer at time zero. The roots were removed from the reaction buffer after 15 min. Reactive sulfhydryl groups in 2.0-ml aliquots of sulfhydryl buffer solution were immediately measured using spectrophotometry for increasing absorption at 412 nm. Concentrations of sulfhydryl were calculated from standard curves prepared from Cys standards made immediately before the assay.
Root leakage conductivity
Root cell-membrane permeability was assessed by modified method described by Yan et al. (1996). The 1-cm washed root segments were placed in a beaker containing 10 ml deionized water. The root samples were immersed at 30 °C for 3 h and then, the conductivity of the solution was measured. The samples were boiled for 2 min, cooled to room temperature (25 °C) and then, their EC was measured. The electrolyte leakage was calculated as follows:
where C1 and C2 are the electrolyte conductivities measured before and after boiling, respectively.
Ion leakage
Thirty-five days after imbibition, sub-samples of seedlings were transferred from nutrient solution to 2 l aerated 0.5 mM CaSO4 plus 0.01 mM H3BO3 for 15 min to remove nutrient solution on the root surfaces. The roots of wheat seedling were then rinsed in water and transferred to polyethylene pots (400 ml) containing ion leakage solution (0.5 mM CaSO4 and 0.01 mM H3BO3). The wheat roots were completely immersed in aerated solution. After 4 h, the wheat seedlings were removed and ion leakage solutions were collected. Concentrations of Fe and K in the leakage solutions were measured by the atomic absorption spectroscopy (AAS) (PerkinElmer 800, PerkinElmer, Wellesley, MA).
Catalase (CAT) assay
The fresh roots were homogenized in a pestle and mortar with 100 mM Tris–HCl buffer (pH 8) containing 2 mM EDTA, 5 mM dl-dithiothreitol, 10 % glycerol, 100 mM sodium borate, 4 % (w/v) insoluble polyvinylpyrrolidone (PVP), and 1 mM phenylmethylsulphonylfluoride (PMSF). The homogenate was filtered through muslin cloth and centrifuged at 12,000g for 40 min. The supernatant was stored in separate aliquots at −80 °C, prior to enzyme analysis. Total protein content was measured as described by Bradford (1976). Catalase (EC 1.11.1.6) activity was determined by spectrophotometry (Aebi 1984). The decomposition of H2O2 was monitored by the decrease in absorbance at 240 nm. For the assay, a 10 mM potassium phosphate buffer (pH 7.0) and 10 mM H2O2 were used.
Superoxide dismutase (SOD) assay
Activity of SOD (EC 1.15.1.1) was assayed according to the method described by Nishikimi et al. (1972) and expressed as units of SOD g−1 FW. The assay mixture contained 1.2 ml sodium pyrophosphate buffer (pH 8.3, 0.052 M), 0.1 ml 186 μM phenazine methosulphate, 0.3 ml 300 μM nitroblue tetrazolium (NBT), 0.2 ml NADH (780 μM), 100 μg protein, and water in a total volume of 3 ml. The reaction was started by the addition of NADH. After incubation at 30 °C for 90 s, 1.0 ml glacial acetic acid was added and stirred vigorously. The colour intensity of the chromogen in the reaction mixture was measured by mean values of spectrophotometry for increasing absorption at 560 nm. An enzyme-free system was used as a negative control.
Statistical analysis
The experiment was set up in a completely randomized factorial design; each treatment contained four replicates. All values reported in this work are means of three independent experiments. No significant difference was found between the experiments in the measured parameters. Therefore, the combined analysis of three experiments was performed. Analysis of variance procedures were conducted with the PROC GLM procedure of SAS (SAS Institute 2000). Means were separated using Fisher’s protected least significant difference (LSD) method at P < 0.05.
Results
Shoot and root dry matter production
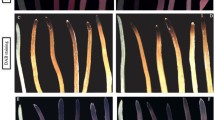
Irrespective of Zn supply and Zn-deficiency tolerance of genotypes, increasing salinity significantly (P < 0.05) reduced shoot dry weight (Fig. 1). A significant (P < 0.05) difference was found among the studied wheat genotypes in shoot dry matter yield. Zinc nutrition resulted in significant increase of shoot dry matter weight of all the studied wheat genotypes grown in nutrient solutions containing 0 and 60 mM NaCl concentrations. At the 120 mM NaCl treatment, Zn application had no effect on the shoot dry matter yield of Rushan and Kavir genotypes while it significantly (P < 0.05) increased the shoot growth of ‘Cross’ (Fig. 1).
In both with and without Zn treatments, ‘Rushan’ produced the highest root dry matter yield (Fig. 2). By increasing salinity, the root dry matter yield was decreased although the magnitude of this decrease differed among the wheat genotypes. At the 0 and 60 mM NaCl levels, wheat plants supplied with adequate Zn produced greater root biomass compared with those grown at the Zn-free nutrient solutions. At the 120 mM treatment, Zn application had no significant effect or slightly decreased the root dry matter weight of ‘Rushan’ and ‘Kavir’ while increased in ‘Cross’.
Zn concentration in shoots of wheat
Increasing NaCl concentration in the nutrient solution resulted in significant increase of Zn concentration in the shoot of all three wheat genotypes (Table 1) although the magnitude of this increase was dependent on the genotype. In ‘Rushan’, regardless of salinity level, addition of Zn significantly increased shoot Zn concentration. In ‘Kavir’, Zn nutrition had no effect on shoot Zn concentration at the 0 and 60 mM NaCl levels and increased it at the 120 mM NaCl treatment. Zinc nutrition had no significant effect on shoot Zn concentration of ‘Cross’.
Root membrane permeability
Increasing salinity significantly enhanced root cell membrane permeability at both Zn levels and in all genotypes (Fig. 3). At both Zn treatments, salinity-induced membrane permeability became more pronounced in ‘Kavir’. In Rushan and Kavir genotypes, Zn application decreased root membrane permeability at the 0 and 60 mM NaCl treatments, whereas it had no effect on the root membrane permeability of these genotypes at the 120 mM treatment. At all salinity levels, ‘Cross’ plants supplied with Zn had lower root membrane permeability compared with those grown at the Zn-free nutrient solution (Fig. 3).
Reactive sulfhydryl group concentration
The studied wheat genotypes varied significantly (P < 0.05) in the root reactive –SH group concentration (Fig. 4). Irrespective of wheat genotype, significant reduction in concentration of reactive –SH groups was found by salinity. At the 0 and 60 mM NaCl treatments, wheat plants grown at the Zn-free nutrient solution had less concentration of –SH groups in their roots compared with those plants grown at the Zn-containing nutrient solutions. At the 120 mM salinity level, Zn application had no effect on root –SH group concentration in ‘Kavir’ and ‘Rushan’ while increased in ‘Cross’ (Fig. 4).
The CAT and SOD activity of wheat roots
The root CAT and SOD activity varied significantly among wheat genotypes (Figs. 5, 6). The highest and lowest root activity of CAT and SOD was found in Cross and Kavir genotypes, respectively. Salt stress increased activity of CAT and SOD in roots of all wheat genotypes. At all salinity levels, wheat plants supplied with Zn had higher root CAT and SOD activity compared to those grown at the Zn-free nutrient solution.
Root ion leakage
Nutrient concentration in the root ion leakage varied significantly among the wheat genotypes (Figs. 7, 8). At the 120 mM NaCl treatment, ‘Kavir’ plants leaked greater quantities of K and Fe from their roots than did the roots of ‘Cross’ and ‘Rushan’ plants. Salinity increased Fe and K concentrations in the root ion leakage solution of all wheat genotypes particularly ‘Kavir’ (Figs. 7, 8). At the 0 and 60 mM NaCl levels, Zn-deficient roots of ‘Kavir’ and ‘Rushan’ leaked significantly higher amounts of Fe and K than Zn-sufficient roots. At the 120 mM treatment, Zn application had no effect on Fe and K concentrations in the root ion leakage of these two wheat genotypes. For ‘Cross’, at all salinity levels, Zn-deficient roots leaked significantly higher amounts of Fe and K than Zn-sufficient roots.
Discussion
Increasing NaCl salinity significantly reduced shoot and root dry matter weight of all wheat genotypes although the magnitude of this decrease was genotype-dependent. The reduction in the shoot and root biomass of wheat plants exposed to salt stress was in accordance to their sensitivity to Zn-deficiency stress as reported earlier (Khoshgoftarmanesh et al. 2006a). In the previous field and pot experiments, ‘Kavir’ was categorized as a Zn-deficiency sensitive wheat genotype while ‘Cross’ was reported to be Zn-deficiency tolerant genotype. In these studies, Zn-deficiency tolerance has been measured as the ratio of yield at Zn-deficient conditions to the yield capacity at adequate Zn supply. ‘Rushan’ has been reported as a Zn-deficiency tolerant genotype in the pot and field experiments (Khoshgoftarmanesh et al. 2006a, b) but as a Zn-deficiency sensitive genotype in nutrient solution culture studies (Khoshgoftarmanesh et al. 2006a). In this experiment, ‘Kavir’ had the greatest reduction in its shoot and root growth when exposed to different levels of salinity. In contrast, ‘Rushan’ and ‘Cross’ plants were more tolerant to salinity and thus, less reduction in their growth was observed under saline conditions.
At the 0 and 60 mM NaCl treatments, Zn nutrition significantly increased the shoot and root growth of Kavir and Rushan genotypes while at the 120 mM NaCl treatment, Zn application had no significant effect on the shoot dry matter weight of these genotypes. This indicates that at the 120 mM level, salinity was a greater limiting factor than Zn deficiency in growth of ‘Kavir’ and ‘Rushan’. On the other hand, Zn nutrition increased shoot dry matter production of ‘Cross’ at all salinity levels. It seems that due to Zn-deficiency tolerance of ‘Cross’ under saline conditions, even at the highest salinity level (120 mM), Zn nutrition improved the crop growth. This is in accordance with our previous findings indicating greater salt-tolerance of ‘Cross’ than ‘Rushan’ and ‘Kavir’ especially under Zn-deficient conditions (Khoshgoftarmanesh et al. 2006a). In contrast, Zn nutrition had no effect on root growth of ‘Cross’ plants at the 120 mM NaCl concentration. This result suggests that in Cross genotype root growth is more sensitive to salinity than shoot growth.
The detrimental effect of salinity on growth of wheat plants could be associated with the oxidative stress induced by ROS (Khan and Panda 2002). The activated oxygen species are produced when plants are subjected to environmental stresses such as salinity (Dionsio-Sese and Tobita 1998). These reactive oxygen species can seriously induce oxidative damage and impair membrane integrity (Cakmak 2000). Impaired membrane integrity induces more ion leakage from the roots and causes plant growth reduction.
The effect of salinity on the ion leakage of root cells was similar to its effect on the plant shoot growth. Ion leakage was measured as an indicator of root membrane permeability. Another evidence for increased root membrane permeability of plants exposed to salinity is increase of Fe and K concentrations in the root leakage solution of wheat genotypes. Increase in membrane permeability of root cells under Zn deficiency is a well-known phenomenon (Welch et al. 1982). Impaired root membrane integrity and elevated membrane permeability to inorganic ions in Zn-deficient wheat plant were first demonstrated by Welch et al. (1982). They reported that Zn deficiency caused greater leakage of P and Cl from root cells than it did in Zn-sufficient plants and concluded that Zn has a direct effect on the structural integrity of biomembranes. The present study shows that this effect of Zn deficiency on membrane permeability of root cells is dependent on the genotype used and the level of salt stress. The wheat genotypes tested in this study showed large variation in membrane permeability under Zn deficiency (Fig. 3). At the 0 and 60 mM NaCl levels, Zn-supplied ‘Kavir’ and ‘Rushan’ plants showed lower membrane permeability than those grown at the Zn-free treatment. Application of Zn partly counteracted adverse effects of 60 mM level of salinity on membrane permeability in Kavir and Rushan genotypes. Among the wheat genotypes examined, the lowest root leakage of K and Fe was found for Cross genotype, the most Zn-deficiency tolerant wheat genotype, while ‘Kavir’ showed the highest membrane permeability.
On the other hand, Zn nutrition could partly reduce the oxidative damages induced by salinity on the root cell membranes (Fig. 3). These results emphasize the role of Zn in protection and maintenance of cellular integrity in plants and imply that an adequate Zn supply is critically important for protection of cells against salinity-induced oxidative damage (Cakmak 2000). This indicates that Zn application could protect these genotypes against oxidative damages caused by 60 mM level of salinity.
One of the major mechanisms affecting crop salt tolerance is elevated antioxidative defense capacity of plants (Cakmak 2000). Possibly, the tolerant genotype ‘Cross’ has higher antioxidative defense capacity. The evidence for this suggestion is greater content of –SH groups as well as greater activity of CAT and SOD in the roots of Cross genotype as compared with Kavir and Rushan genotypes. Higher levels of –SH groups in Zn-deficiency tolerant wheat genotypes were also shown by Rengel (1995). The results presented here are in agreement with findings of Rengel (1995) that indicated the level of root –SH groups might be a screening parameter for high Zn-deficiency tolerance in wheat. The sulfhydryl groups represent an important component of antioxidant potential of plant cells. Most of the non-protein –SH groups in plants represent glutathione which is an important antioxidant in plant cells involved in the detoxification of ROS (Hall 2002).
An increase in the root activity of CAT and SOD in wheat plants exposed to salt stress indicates the antioxidative response of plant to salinity. Increasing salinity resulted in decrease of –SH group content and increased root activity of CAT and SOD in all studied wheat genotypes. These results suggest that under saline conditions, antioxidative defense mechanisms were activated to protect root membrane structure against oxidation. At the 0 and 60 mM NaCl levels, Zn nutrition increased concentration of –SH groups in all three wheat genotypes. Cakmak (2000) found that Zn ion can partially prevent the loss in sulfhydryl groups under stress conditions. The protective effect may be due to preventing sulfhydryl oxidation by physically capping the sulfhydryl groups. Welch and Norvell (1993) suggested that Zn can act as an antioxidant protecting sulfhydryl groups in certain membrane proteins against oxidation and formation of disulfides at the outer membranes surfaces. In fact, Zn may protect membrane protein sulfhydryl groups from oxidative damage. Welch and Norvell (1993) reported that concentration of –SH groups in the plasma membrane proteins of root cells was decreased by Zn deficiency in barley (Welch and Norvell 1993; Rengel 1995) and in wheat, especially in Zn-deficiency sensitive wheat cultivars. Our results also indicated that in Zn-deficiency sensitive Kavir genotype the oxidative damage of 60 mM NaCl salinity level on the root membrane integrity was greater than in Cross genotype. At the 120 mM salinity treatment, Zn nutrition had no effect on the –SH group concentration in ‘Rushan’ and ‘Kavir’. These results suggest that Zn2+ ions could protect reactive sulfhydryl groups in wheat plants from oxidative reactions induced by low to medium salinity levels while at high saline conditions, Zn nutrition is ineffective.
Conclusion
Our results showed that Zn supply, dependent on the crop genotype and salinity level could increase salt-tolerance of wheat. These results suggest that the increased resistance to salinity of wheat plants is probably associated with the reduced oxidative damages of salt stress on roots. Zinc nutrition increased plant resistance to salt stress by stabilizing root cell membranes, as indicated by significant reduction in the root membrane permeability and reduction of ion leakage from the roots.
The differential tolerance to salt stress in these wheat genotypes was related to their tolerance to Zn-deficiency stress. Non protein –SH groups (glutathione) and activity of antioxidant enzymes CAT and SOD appeared to be related to differential salt-tolerance among the studied genotypes. Greater salt-tolerance of ‘Cross’ is probably associated with its higher antioxidative defense capacity. It is concluded that the oxidative damage of salt stress on wheat genotypes is dependent on their tolerance to Zn-deficiency stress and the level of Zn supply.
Author contribution
Mrs. B. Daneshbakhsh contributed to all the experimental processes and was responsible for statistical analysis of data. Dr. A.H. Khoshgoftarmanesh supervised the research, contributed to interpretation of results and discussion and preparation of manuscript. Dr. H. Shariatmadari and Dr I. Cakmak supervised the research, contributed to interpretation of results and discussion.
Abbreviations
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- H2O2 :
-
Hydrogen peroxide
- SH:
-
Sulfhydryl
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Arbona V, Flors V, Garcia-Agustin P, Gomez-Cadenas A (2003) Enzymatic and non-enzymatic antioxidant responses of Carrizo citrange, salt-sensitive citrus rootstock, to different levels of salinity. Plant Cell Physiol 44:388–394
Asada K (1994) Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineauxs PM (eds) Causes of photooxidative stress and amelioration of defense system in plants. CRC Press, Boca Raton, pp 77–103
Bartosz G (1997) Oxidative stress in plants. Acta Physiol Plant 19:47–64
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Cakmak I, Engels C (1999) Role of mineral nutrients in photosynthesis and yield formation. In: Rengel Z (ed) Mineral nutrition of crops. Haworth Press, New York, pp 141–168
Cakmak I, Atli M, Kaya R, Evliya H, Marschner H (1995) Association of high light and zinc deficiency in cold induced leaf chlorosis in grapefruit and mandarin trees. J Plant Physiol 146:355–360
Cakmak I, Yilmaz A, Kalayci M, Ekiz H, Torun B, Erenoglu B, Braun HJ (1996) Zinc deficiency as a critical problem in wheat production in central Anatolia. Plant Soil 180:165–172
Chen LM, Lin CC, Kao CH (2000) Copper toxicity in the rice seedlings: changes in antioxidative enzyme activities, H2O2 level, and cell wall peroxidase activity in roots. Bot Bull Acad Sin 41:99–103
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Fendina IS, Tsonev TD, Guleva EI (1994) ABA as a modulator of the response of Pisum sativum to salt stress. J Plant Physiol 143:245–249
Gossett DR, Millhollon EP, Lucas MC (1994) Antioxidant responses to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Harris MJ, Outlaw WH (1991) Rapid adjustment of guard-cell abscisic acid levels to current leaf-water status. Plant Physiol 95:171–173
Imlay JA (2003) Pathway of oxidative damage. Annu Rev Microbiol 57:395–418
Khan MH, Panda SK (2002) Induction of oxidative stress in roots of Oryza sativa L. in response to salt stress. Biol Plant 45:625–627
Khan MA, Ungar IA, Showalter AM (2000) Effects of salinity on growth, water relations and ion accumulation in the subtropical perennial halophyte, Atriplex griffithii var. stocksii. Ann Bot 85:225–232
Khoshgoftarmanesh AH, Shariatmadari H, Karimian N (2006a) Responses of wheat genotypes to zinc fertilization under saline soil conditions. J Plant Nutr 29:1543–1556
Khoshgoftarmanesh AH, Shariatmadari H, Karimian N, van der Zee SEATM (2006b) Cadmium and zinc in saline soil solutions and their concentrations in wheat. Soil Sci Soc Am J 70:582–589
Koyro HW (2006) Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ Exp Bot 56:136–146
Locy RD, Chang CC, Neilson BL, Singh NK (1996) Photosynthesis in salt-adapted heterotrophic tobacco cells and regenerated plants. Plant Physiol 110:321–328
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signaling. Curr Opin Plant Biol 5:388–395
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun 48:849–854
Osmond CB, Grace SC (1995) Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J Exp Bot 46:1351–1362
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: review. Ecotox Environ Safe 60:324–349
Pinton R, Cakmak I, Marschner H (1994) Zinc deficiency enhanced NAD(P)H-dependent superoxide radical production in plasma membrane vesicles isolated from roots of bean plants. J Exp Bot 45:45–50
Rengel Z (1995) Sulfhydryl groups in root-cell plasma membranes of wheat genotypes differing in zinc efficiency. Physiol Plant 95:604–612
Rout NP, Shaw BP (2001) Salt tolerance in aquatic macrophytes: possible involvement of the antioxidative enzymes. Plant Sci 160:415–423
Takkar PN, Walker CD (1993) The distribution and correction of zinc deficiency. In: Robson AD (ed) Zinc in soils and plants. Kluwer Academic Publishers, Dordrecht, pp 151–166
Tinker PB, Lauchli A (1984) Advances in plant nutrition. Academic Publishers, San Diego
USEPA (1995) Method 3051: Microwave assisted acid digestion of sediments, sludges, soils, and oils. Available online at http://www.epa.gov/SW-846/pdfs/3051.pdf (verified 22 July 2004). USEPA, Washington, DC
Welch RM, Norvell WA (1993) Growth and nutrient uptake by barley (Hordeum vulgare L. cv. Herta): studies using an N-(2- hydroxyethyl) ethylene dinitrilotriaacetic acid-buffered nutrient solution technique. II. Role of zinc in the uptake and root leakage of mineral nutrients. Plant Physiol 101:627–631
Welch RM, Webb MJ, Loneragan JF (1982) Zinc in membrane function and its role in phosphorus toxicity. In: Scaife A (ed), Plant nutrition. Proceedings of the ninth international plant nutrition colloquium, Warwick University, England, August 22–27, Commonwealth Agricultural Bureaux, Farnham House, Farnham Royal, pp 710–715
Welch RM, Allaway WH, House WA, Kubota J (1991) Geographic distribution of trace element problems. In: Mortdvedt JJ, Cox FR, Shuman LM, Welch RM (eds) Micronutrients in agriculture. SSSA No. 4. Madison, pp 31–57
White JG, Zasoski RH (1999) Mapping soil micronutrients. Field Crops Res 60:11–26
Yan B, Dai Q, Liu X, Huang S, Wang Z (1996) Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179:261–268
Yeo AR, Flowers TJ (1983) Varietal differences in the toxicity of sodium ions in rice leaves. Physiol Plant 59:189–195
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Bartosz.
Rights and permissions
About this article
Cite this article
Daneshbakhsh, B., Khoshgoftarmanesh, A.H., Shariatmadari, H. et al. Effect of zinc nutrition on salinity-induced oxidative damages in wheat genotypes differing in zinc deficiency tolerance. Acta Physiol Plant 35, 881–889 (2013). https://doi.org/10.1007/s11738-012-1131-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1131-7