Abstract
The paper mainly studied the short-term influences of experimental warming, nitrogen addition, and their combination on physiological performance of P. tabulaeformis seedlings. Free air temperature increase system of infrared heaters was used to raise monthly average soil and air temperature by 2.6 and 2.1 °C above the ambient. NH4NO3 solution was added for a total equivalent to 25 g N m−2 a−1. Experimental warming and nitrogen addition induced a significant increase in leaf nitrogen concentration, A max, Φ, antioxidant enzymes activities, ASA and free proline contents, but both of them sharply decreased AOS and MDA level. Interestingly, the interaction of warming and nitrogen fertilization further improved leaf nitrogen concentration, A max, Φ, and antioxidant compounds accumulation, and also resulted in lower rate of O2 − production than either single warming or fertilization. Obviously, the beneficial effects of warming and N fertilization alone on leaf physiology of P. tabulaeformis seedlings were magnified by the combination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing concentrations of CO2 and several other radiatively active gases have the potential to induce global warming. It is estimated by IPCC (2007) that the average global surface temperature will increase by 2–4.5 °C above the pre-industrial levels by 2100. The expected rapid change of climate is likely to affect growth, morphology, and physiology of plants. Previous studies showed that growth, photosynthesis, leaf N content, and biochemical reaction of plants were directly and indirectly influenced by warming (Hansen et al. 2006; Tegelberg et al. 2008). On the other hand, nitrogen deposition is another crucial environmental change, and it is seriously affecting terrestrial and aquatic ecosystems. Nitrogen also acts as one of the key resources likely to regulate plant responses to climate warming (Lewis et al. 2004).
Substantial efforts are made to study ecological and biological effect of climate warming and nitrogen deposition on plant growth and physiology (Nakaji et al. 2001; Pérez et al. 2005; Larssen et al. 2006; Han et al. 2009). It is generally accepted that warming always promotes plant photosynthetic capacity in cold regions (Han et al. 2009). Similarly, nitrogen load usually be helpful for photosynthesis of trees unless the amount of available N in forest soil exceeds the requirement for tree growth (Yao and Liu 2006). Leaf photosynthesis is closely related to leaf N content, and high leaf N content always improves plant photosynthesis (Brown et al. 1996). On the other hand, active oxygen species (AOS) metabolism was also closely related to photosynthesis (Foyer et al. 2002). When absorbed light energy could not be exploited by photosynthetic machinery, AOS were always synthesized and accumulated by plant. Accumulation of AOS can attack membrane lipids, cause oxidized fatty acid reaction products like malondialdehyde (MDA), and disrupt normal metabolism (Benson and Bremner 2004). In order to control AOS, antioxidant defense systems composed of both enzymatic and non-enzymatic antioxidants are developed. AOS and antioxidant defense systems are deeply affected by environment, such as temperature and nutrient status (Ahmad and Hellebust 1988; Tegelberg et al. 2008). Antioxidant systems, reactive species and MDA are important aspects of plant physiology, which likely influence photosynthesis and plant growth, but in previous studies, little attention had been paid to these aspects. Therefore, our study would be helpful to understanding the combined effects on conifer tree species.
Warming and nitrogen deposition are expected to increase simultaneously in the future. In recent years, a few studies concerning the combinative effects of warming and N addition on soil microorganism, nitrogen mineralization, carbon sequestration, and plant have been done (Mäkipää et al. 1999; Majdi and Öhrvik 2004; Flury and Gessner 2011). However, the interactive effects of warming and nitrogen deposition on plant physiology, such as photosynthesis, leaf nitrogen, especially AOS metabolism are poorly understood.
Tibetan Plateau and its surrounding subalpine ecosystems are susceptible to climate change, so the future climate warming and nitrogen deposition must bring great influences on the subalpine coniferous forest, especially on seedlings (Wang 2004). Pinus tabulaeformis is an important species in the southeast of the Qinghai-Tibetan Plateau of China, and widely used in reforestation programs at present. Our previous study shows that warming and nitrogen addition increase plant growth, and their combination further promote growth of P. tabulaeformis seedlings (Zhao and Liu 2009). We hypothesize that warming combined with nitrogen will be more helpful to leaf physiology than warming and nitrogen alone. Changes in leaf morphology, N concentration, photosynthesis and antioxidant metabolism were studied in P. tabulaeformis seedlings in order to better understand the mechanisms of plants to adapt to nitrogen deposition and global warming in the subalpine forest ecosystems in the Eastern Tibetan Plateau.
Materials and methods
Experiment design and plant material
The experiment was conducted outdoors during the growing season from April to October 2007 in Maoxian Ecological Station of Chinese Academy of Sciences, Sichuan Province, China (31°41′N, 103°53′E, 1,820 m a.s.l.). Mean annual temperature, precipitation and evaporation are 8.9 °C, 900 mm, and 795.8 mm, respectively. Experiment design followed Zhang et al. (2005) using 165 cm × 15 cm overhead infrared heaters (Kalglo Electronics Inc., Bethlehem, PA, USA) to generate a warmed environment. There were four blocks, and each block contained a pair of 2 m × 2 m plots (a warmed plot and a control plot). The warmed plot was continuously heated by an infrared heater suspended 1.5 m above middle of the plots. The control plot of each pair had a ‘dummy’ heater of the same shape and size as the infrared heater suspended 1.5 m high in order to simulate the shading effects of the heater. The distance between the control plot and the warmed plot was 6 m in order to avoid heating the control plot.
Each 2 m × 2 m plot was divided into four 1 m × 1 m subplots, and indigenous soil of the plots until the depth of 50 cm was replaced by the sieved topsoil from a forest. PVC pipes (20-cm diameter and 50-cm depth) were buried vertically in each subplot ground for planting experimental seedlings. In March 2007, 320 healthy uniform 2-year-old P. tabulaeformis seedlings were selected based on the plant height, basal diameter, and fresh weight (16.89 ± 0.25 cm, 3.62 ± 0.45 mm, 7.95 ± 0.42 g, respectively) in a local tree nursery, and randomly transplanted into PVC pipes (ten seedlings in each subplot). The seedlings grown in two diagonal subplots in each plot were weekly watered with 200 ml 2.7 mM ammonium nitrate solution (for a total equivalent to 25 g N m−2 a−1) to the soil surface of the PVC pipes, and seedlings in the other two subplots were watered with the equivalent water. Nitrogen amount was based on previous studies (Nakaji et al. 2001; Yao and Liu 2006).
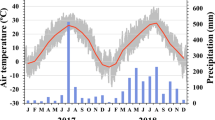
Artificial warming and nitrogen addition was conducted from 15 April to 15 October 2007. During the experimental period, infrared heaters were continually powered on for 24 h 1 day, and seedlings were watered frequently as needed. The infrared heaters significantly increased both mean air (at 20 cm aboveground) and soil (at 10 cm depths) temperature of warmed plots by 2.1 and 2.6 °C above those of control plots (Fig. 1), respectively, and the warming effects of infrared heaters over the soil surface were equal (Zhao and Liu 2009; Loik and Harte 1997). Infrared heaters did not significantly change the average soil moisture between warmed plots and control plots (Fig. 1). The four treatments in this study were: (1) unwarmed unfertilized (UU); (2) unwarmed fertilized (UF); (3) warmed unfertilized (WU); (4) warmed fertilized (WF).
Specific leaf area (SLA) and total leaf nitrogen content
Current-year needles of two randomly selected seedlings within each subplot were collected in June 2007, and the needles were then mixed for a composite sample for the following determination. The mean value of two composite samples of each treatment from the same block was used as a replicate for statistical analysis.
Needles were digitally scanned into a personal computer and then analyzed with a UTHSCSA ImageTool analysis system to determine the leaf areas. These needles were dried at 70 °C for 48 h to determine the dry weight. Then dried needles were ground and analyzed for total needle nitrogen concentration by the Kjeldahl method using an automatic total N analyzer (Kjeltec 2200, Nils Foss, Denmark). SLA and area-based nitrogen concentration were derived based on the measured data.
Photosynthetic parameters
Light-response curve was measured on fully expanded, exposed current-year leaves under controlled optimal conditions using an open-mode portable photosynthesis system (Model LI-6400, Li-Cor, Inc., Lincoln, NE, USA) to characterize warming and nitrogen fertilization induced shifts in carbon acquisition in June 2007. Response to PAR were measured at 0, 20, 50, 80, 100, 200, 300, 400, 600, 800, 1,000, 1,200, 1,600, 1,800, and 2,000 μmol m−2 s−1 using the 6,400 artificial light source and temperature was held at 25 °C and the humidity at 40 % during the measurements. The light-response curve of photosynthesis was fitted with a non-rectangular hyperbola (Hirose and Werger 1987):
where P n is the net photosynthetic rate, Φ is the initial slope of the curve, I is the photosynthetic photon flux density (PPFD), P max is the light-saturated rate of photosynthesis, θ is the convexity and R d is the dark respiration rate. First, from linear regression of the photosynthetic rate on PPFD at 0–200 μmol m−2 s−1, Φ, R d, and LCP were obtained as the slope, y-intercept and the x-intercept of these regressions, respectively. Then, a non-rectangular hyperbola was fitted to the whole curve using the Φ and R d values to obtain P max and θ (Hikosaka et al. 2004). Each individual per subplot were randomly selected for determination and the mean value of two individuals for each treatment within the same blocks was used as the replicate for statistical analysis. After the measurements, the measured leaves were collected and the projected leaf area was measured as described above. The photosynthetic parameters are based on the projected leaf area.
Leaf sampling
On 28 June 2007, fully expanded current-year needles were randomly selected from four seedlings within each subplot, and then mixed for a composite sample for the following physiological determination. The mean value of two composite samples of each treatment from the same block was used as a replicate for the statistical analysis.
The rate of superoxide anion radical (O2 −) production, hydrogen peroxide (H2O2) and malondialdehyde (MDA) content
The rate of superoxide radical production (O2 −) was measured as described by Ke et al. (2002), by monitoring the nitrite formation from hydroxylamine in the presence of O2 −. With 1.5 ml of 65 mM potassium phosphate (pH 7.8), 0.5 g samples were ground and centrifuged at 5,000×g for 10 min. Then, 0.5 ml of the supernatant was incubated with 0.45 ml of 65 mM phosphate buffer (pH 7.8) and 0.5 ml of 10 mM hydroxylamine hydrochloride at 25 °C for 20 min. After incubation, 8.5 mM sulfanilamide and 3.5 mM α-naphthylamine were added to the incubation mixture. After reaction at 25 °C for 20 min, the absorbance in the aqueous solution was read at 530 nm. A standard curve with NO2 − was used to calculate the production rate of O2 − from the chemical reaction of O2 − and hydroxylamine.
Hydrogen peroxide (H2O2) was determined as described by Prochazkova et al. (2001). 0.5 g needles were homogenized with 5 ml cooled acetone in a cold room (10 °C), filtered and then mixed with 2 ml titanium reagent and 5 ml ammonium solution to precipitate the titanium–hydrogen peroxide complex. Reaction mixture was centrifuged at 10,000×g for 10 min and precipitate was dissolved with 5 ml 2 M H2SO4. The above mixture was re-centrifuged and supernatant was read at 415 nm.
The thiobarbituric acid (TBA) test was used to determine the lipid peroxidation. 0.5 g needles were ground with 5 ml of 20 % (w/v) trichloroacetic acid (TCA) and the homogenate was centrifuged at 3,500×g for 20 min. Then 2 ml of the aliquot of the supernatant was mixed with 2 ml of 20 % TCA containing 0.5 % (w/v) TBA and 100 μl 4 % (w/v) butylated hydroxytoluene in ethanol. The mixture was heated at 95 °C for 30 min and then quickly cooled on ice. The contents were centrifuged at 10,000×g for 15 min and the absorbance was measured at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The concentration of MDA was calculated using an extinction coefficient of 155 mM−1 cm−1. Results were expressed as n mol g−1 FW.
Antioxidant enzymes activities
Needles (1.0 g) were homogenized under ice-cold conditions in 3 ml of extraction buffer [50 mM phosphate buffer (pH 7.4), 1 mM EDTA, 1 g (polyvinylpyrrolidone) PVP and 0.5 % (v/v) Triton X-100]. The homogenates were centrifuged at 10,000×g for 30 min at 4 °C, and the supernatant was used for the following assays.
Superoxide dismutase (SOD) activity was determined according to the method of Becana et al. (1986). One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of nitro blue tetrazolium (NBT) reduction, measured with a scanning spectrophotometer (Unicam UV-330, Thermo Spectronic, England, UK) at 560 nm.
Peroxidase (POD) activity was based on the determination of guaiacol oxidation (extinction coefficient 26.6 mM−1 cm−1) at 470 nm by H2O2 (Ekmekci and Terzioglu 2005). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 20. 1 mM guaiacol, 12.3 mM H2O2, and enzyme extract in a 3 ml volume.
Catalase (CAT) activity was determined by measuring the decrease in absorption at 240 nm in a reaction solution of 50 mM potassium phosphate buffer (pH 7.2), 10 mM H2O2 and 50 μl enzyme extract (Kato and Shimizu 1987). CAT activity was calculated using the extinction coefficient (40 mM−1 cm−1) for H2O2.
Ascorbate peroxidase (APX) activity was measured using fresh extracts by measuring the reduction of ascorbic acid (ASA) after oxidization by APX in the presence of H2O2 (Nakano and Asada 1981). The reduction of ASA was obtained by reading the absorbance decrease at 290 nm (extinction coefficient 2.8 mM−1 cm−1).
Soluble protein content was determined followed by methods of Bradford (1976), using bovine serum albumin as a calibration standard.
The content of proline and ascorbic acid (ASA)
The free proline content was determined according to the method described by Bates et al. (1973). 1 g needles were homogenized using a pestle and mortar with 5 cm3 of sulfosalicylic acid (3 % w/v). After centrifugation (5 min at 20,000×g) 0.5 cm3 of the supernatant was incubated at 100 °C for 60 min with 0.5 cm3 of glacial acetic acid and 0.5 cm3 of ninhydrin reagent. After cooling, 1 cm3 of toluene was added to the mixture and the absorbance of the chromophore containing toluene was recorded at 520 nm.
ASA was determined as described by Hodges et al. (1996). Current-year needles (0.5 g) were homogenized in 5 ml of cold 5% (w/v) m-phosphoric acid and centrifuged at 10,000g for 15 min. About 300 μl of supernatant was incubated for 5 min in a 700 μl total volume of 100 mM KH2PO4 and 3.6 mM EDTA. Color was developed with 400 μl of 44 % o-phosphoric acid, 400 μl of 65 mM α, α′-dipyridyl in 70 % ethanol, and 200 μl of 110 mM FeCl3. The reaction mixtures were then incubated at 40 °C for 1 h and quantified at 525 nm.
Statistical analysis
Two-way analysis of variance (ANOVA) was used to detect the effects of warming, N fertilization and their interactions. Individual treatment means were compared with Duncan’s test to identify whether they were significantly different at the 0.05 probability level. Because sunlight, wind, temperature, rain and soil type of the four blocks were uniform, the block effects on determined parameters were not significant (data not shown). Therefore, we did not emphasize the block effect in the present study. All statistical analyses were carried out using the software for Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA), version 10.0.
Result
SLA
All treatments significantly changed SLA of the seedlings, but no significant interaction between warming and N fertilization was observed. SLA was clearly decreased by warming, whereas nitrogen addition significantly increased SLA (Fig. 2). SLA of seedlings treated with the combination of warming and N fertilization was lower than that of seedlings with UU and UF treatment, but it was higher than that of seedlings with WU treatment (Fig. 2).
Effects of elevated temperature and N fertilization on specific leaf area (SLA) (a), and area-based nitrogen concentration (b) of P. tabulaeformis seedlings. The bars with different letters are significantly different from each other (P < 0.05). Values are means of four replicates ± SE. W experimental warming effect, F N fertilization effect, W × F the interactive effect of warming and N fertilization. NS not significant at the level of P = 0.05, significant at the level of *P = 0.05, significant at the level of **P = 0.01, significant at the level of ***P = 0.001
Leaf nitrogen content
Warming and N addition had significant effects on area-based leaf nitrogen content of seedlings (Fig. 2). WU and UF treatment significantly increased N concentration and WF treatment further increased leaf nitrogen concentration (Fig. 2).
Photosynthetic properties
Analyses of repeated measures indicated that artificial warming had significant effects on all of the measured photosynthetic parameters (Fig. 3). Warming induced greater A max, Φ, LCP, and R d of P. tabulaeformis seedlings compared with the control (Fig. 3). Nitrogen addition also significantly increased A max and Φ, but did not obviously affected LCP and R d of seedlings (Fig. 3). Though no significant interactive effects of warming combined with N fertilization on any one of these properties was observed, WF treatment further increased A max and Φ of the seedlings than warming or N fertilization alone (Fig. 3).
Effects of elevated temperature and N fertilization on maximum net photosynthetic rate (A max) (a), apparent quantum yield (Φ) (b), photosynthetic light compensation point (LCP) (c), and dark respiration rate (R d) (d) of P. tabulaeformis seedlings. The bars with different letters are significantly different from each other (P < 0.05). Values are means of four replicates ± SE. W experimental warming effect, F N fertilization effect, W × F the interactive effect of warming and N fertilization. NS not significant at the level of P = 0.05, significant at the level of *P = 0.05, significant at the level of **P = 0.01, significant at the level of ***P = 0.001
AOS and MDA contents
Artificial warming induced oxidative decline, the level of H2O2 and O2 − remarkably reduced by warming, N fertilization and the combination (Fig. 4). On the other hand, warming, N fertilization and the combination also resulted in clear reduction in MDA content (Fig. 4). Moreover, compared to warming and N fertilization alone, the combination further decreased the rate of O2 − production and MDA concentration.
Effects of elevated temperature and N fertilization on H2O2 content (a), the rate of O2 − (b), and MDA content (c) of P. tabulaeformis seedlings. The bars with different letters are significantly different from each other (P < 0.05). Values are means of four replicates ± SE. W experimental warming effect, F N fertilization effect, W × F the interactive effect of warming and N fertilization. NS not significant at the level of P = 0.05, significant at the level of *P = 0.05, significant at the level of **P = 0.01, significant at the level of ***P = 0.001
Antioxidant defense systems
Warming clearly increased SOD activity and the contents of ASA and free proline (Fig. 5). N fertilization significantly affected SOD, POD, APX, ASA and free proline, resulting in higher activities of these antioxidant enzymes and more content of the two antioxidants (Fig. 5). WF treatment induced greater contents of ASA and proline than WU and UF treatments.
Effects of elevated temperature and N fertilization on a SOD, b POD, c CAT and d APX activities, and e ASA content, f free proline content of P. tabulaeformis seedlings. The bars with different letters are significantly different from each other (P < 0.05). Values are means of four replicates ± SE. W experimental warming effect, F N fertilization effect, W × F the interactive effect of warming and N fertilization. NS not significant at the level of P = 0.05, significant at the level of *P = 0.05, significant at the level of **P = 0.01, significant at the level of ***P = 0.001
Discussion
Warmer temperature might increase leaf thickness, cause greater tissue density in leaf, and consequently reduce SLA of plant (Pandey et al. 2007). Similarly, SLA of P. tabulaeformis seedlings was remarkable reduced by experimental warming (Fig. 2). However, SLA of P. tabulaeformis seedlings was significantly increased by N fertilization, which was well agreed with the result of Knops and Reinhart (2000). In their study, higher SLA induced by nitrogen addition could enhance the aboveground competition of plant species for light (Knops and Reinhart 2000). No previous study focused on interaction of warming and N fertilization on SLA of plant leaves. Because the effects of warming and N fertilization on SLA of P. tabulaeformis seedlings were contrary, and might compensate each other, the combinative effect was not significant on SLA. In addition, SLA of P. tabulaeformis seedlings under WF treatment was lower than that of seedlings under UU treatment, probably due to the negative effect of warming is higher in absolute value than the positive effect of N fertilization on SLA.
Leaf N content was often enhanced by N addition due to higher soil N availability (Hobbie et al. 2001; Cao et al. 2008). Leaf N content of P. tabulaeformis seedlings was increased by N fertilization regardless of warming. It was reported that warming increased mineralization of the forest floor and N availability in soil solution leading to higher N level in leaf tissue (Van Cleve et al. 1990). Consistent with the previous result (Tingey et al. 2003), leaf N concentration of P. tabulaeformis seedlings was clearly increased by warming. The positive effects of warming and nitrogen addition on leaf N concentration were magnified by the combination, resulting in increased accumulation of nitrogen in leaves of P. tabulaeformis seedlings (Fig. 2).
Light-response curve and their resulting coefficients, obtained within physiological characterization of gas exchanges at the leaf level, may be important tools for simulation models aimed at the prediction of potential plant behavior in response to environmental conditions (Avola et al. 2008). Warming was reported to increase the activity or the amount of Rubisco, and provide more optimal temperature conditions for photosynthesis (Wang et al. 1995). It is also reported that most of the nitrogen is used for synthesizing components of the photosynthetic apparatus, and net assimilation rates increase linearly with leaf N concentration (Sugiharto et al. 1990; Brown et al. 1996). Consistent with the previous studies (Wang et al. 1995; Chen et al. 2005), warming and N fertilization significantly increased A max and Φ in P. tabulaeformis seedlings (Fig. 3). Compared to the WU and UF treatment, the WF treatment induced higher A max and Φ of P. tabulaeformis seedlings probably due to the highest leaf N concentration among all treatments. Therefore, it was possible that artificial warming combined with N fertilization further improved carbon acquisition and light utilization of P. tabulaeformis, with a consequent greater increase in the dry matter (Fig. 6; Zhao and Liu 2009).
Effects of elevated temperature and N fertilization on total biomass of P. tabulaeformis seedlings. The bars with different letters are significantly different from each other (P < 0.05). Values are means of four replicates ± SE (Zhao and Liu 2009)
Soil warming generally increased the value of light compensation point (LCP) in literature (Wang et al. 1995). Similarly, our results also implied that warming was favorable for improving LCP of P. tabulaeformis. On the other hand, both the WU and WF treatment significantly increased R d of needles, due to a direct effect of temperature on respiratory enzymes or changes in leaf morphology and structure (Farrar and Williams 1991; Zha et al. 2002).
Photosynthetic capacity has great influences on AOS and the scavenging system (Foyer et al. 2002). Nitrogen nutrition can improve light reaction and dark reaction of photosynthetic organization, and reduce deoxidization capacity (Xiao et al. 1998). In this experiment, warming and nitrogen addition significantly reduced AOS and MDA level in needles of P. tabulaeformis seedlings (Fig. 4). The combination of warming and N fertilization induced the lowest level of O2 − probably due to high photosynthetic capacity, since photosynthesis could consume light energy in order to prevent the generation of AOS (Foyer et al. 2002). Our result suggested warming combined with N addition was more favorable for alleviating the risk of natural oxidative damage than the single treatment.
Tegelberg et al. (2008) reported that antioxidant enzymes activities could be increased by warming in order to balance and control the oxygen toxicity. In the present study, except SOD activity, the activities of determined antioxidant enzymes were not significantly changed by warming, suggesting that elevated soil and air temperature by about 2 °C could not induce oxygen toxicity and alternation in most of the antioxidant enzymes. On the other hand, the activities of SOD, POD and APX of P. tabulaeformis seedlings were enhanced by nitrogen supply regardless of environmental temperature (Fig. 5). Similar result was also observed by Yao and Liu (2006), and the activities of SOD, POD, CAT and APX in needles of P. asperata seedlings were higher at the presence of N addition.
ASA and proline could function as a hydroxyl radical scavenger to prevent membrane damage and protein denaturation (Ain-Lhout et al. 2001; Burkey et al. 2006). Present study showed that warming significantly increased the contents of ASA and free proline (Fig. 5). Schonhof et al. (2007) also reported that warming stimulated the accumulation of ASA in broccoli. However, in another study, free proline content in wheat seedlings was decreased by warming (Öncel et al. 2000). The different results were likely attributed that deferent species responded differently on proline accumulation to environmental temperature (Öncel et al. 2000). Similar to Sánchez et al. (2002), seedlings with nitrogen supply had higher proline content, because it was a nitrogen-storage compound and the synthesis and accumulation of proline could be stimulated by nitrogen supply (Ahmad and Hellebust 1988). The combination of warming and nitrogen fertilization further increased the contents of proline and ASA than warming or N fertilization treatment alone (Fig. 5). These results further confirmed that climate warming combined with N fertilization might be more favorable for plant antioxidant defensive system, and providing plants with an advantage in response to potential environmental stress.
In conclusion, the leaf morphology of P. tabulaeformis seedlings was differently changed by warming and nitrogen addition. The warmer condition significantly reduced SLA of the seedlings regardless of nitrogen treatments. In contrast, nitrogen addition increased SLA under both the control and warmed plots. Our results also suggested that the combination of warming and N fertilization (WF) further increased leaf nitrogen content and photosynthetic ability (A max and Φ), compared to these parameters of P. tabulaeformis seedlings under UF and WU treatments. In addition, the WF treatment also caused greater content of two important antioxidants (proline and ASA) and the lower rate of O2 − production. Thus, we conclude that future warming combined with N deposition will further improve the photosynthetic capacity and reduce the risk of oxidative damage of plant, at least in a short term.
Author contribution
Chunzhang Zhao has major contribution in lab and field experiment, data collection and analysis, and manuscript preparation. Qing Liu has designed and supervised the research project.
Abbreviations
- A max :
-
Maximum rate of photosynthesis
- AOS:
-
Active oxygen species
- APX:
-
Ascorbate peroxidase
- ASA:
-
Ascorbic acid
- CAT:
-
Catalase
- H2O2 :
-
Hydrogen peroxide
- LCP:
-
Photosynthetic light compensation point
- Φ:
-
Apparent quantum yield
- LMR:
-
Leaf mass ratio
- MDA:
-
Malondialdehyde
- O2 − :
-
Superoxide radical
- POD:
-
Peroxidase
- R d :
-
Dark respiration rate
- SLA:
-
Specific leaf area
- SOD:
-
Superoxide dismutase
- UF:
-
Unwarmed fertilized
- UU:
-
Unwarmed unfertilized
- WF:
-
Warmed fertilized
- WU:
-
Warmed unfertilized
References
Ahmad I, Hellebust JA (1988) The relationship between inorganic nitrogen metabolism and proline accumulation in osmoregulatory response of two euryhaline microalgae. Plant Physiol 88:348–354
Ain-Lhout F, Zunzunegui M, Barradas MCD, Tirado R, Clavijo A, Garcia Novo F (2001) Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 230:175–183
Avola G, Cavallaro V, Patanè C, Riggi E (2008) Gas exchange and photosynthetic water use efficiency in response to light, CO2 concentration and temperature in Vicia faba. J Plant Physiol 165:796–804
Bates LS, Waldren RP, Teare IK (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208
Becana M, Aparicio-Tejo P, Irigoyen JJ, Snchez-Daz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol 82:1169–1171
Benson EE, Bremner D (2004) Oxidative stress in frozen plant: a free radical point of view. In: Lane FB, Benson EE (eds) Life in frozen state. CRC Press Inc., FL, USA, pp 256–269
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown KR, Thompson WA, Weetman GF (1996) Effects of N addition rates on the productivity of Picea sitchensis, Thuja plicata, and Tsuga heterophylla seedlings. Trees 10:189–197
Burkey KO, Neufeld HS, Souza L, Chappelka AH, Davison AH (2006) Seasonal profiles of leaf ascorbic acid content and redox state in ozone-sensitive wildflowers. Environ Pollut 143:427–434
Cao B, Dang TL, Yü X, Zhang S (2008) Effects of [CO2] and nitrogen on morphological and biomass traits of white birch (Betula papyrifera) seedlings. For Ecol Manag 254:217–224
Chen SP, Bai YF, Zhang LX, Han XG (2005) Comparing physiological responses of two dominant grass species to nitrogen addition in Xilin River Basin of China. Environ Exp Bot 53:65–75
Ekmekci Y, Terzioglu S (2005) Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pestic Biochem Physiol 83:69–81
Farrar JF, Williams ML (1991) The effects of increased atmospheric carbon dioxide and temperature on carbon partitioning, source-sink relations and respiration. Plant Cell Enviorn 14:819–830
Flury S, Gessner MO (2011) Experimentally simulated global warming and nitrogen enrichment effects on microbial litter decomposers in a marsh. Appl Environ Microb 77(3):803–809
Foyer CH, Vanacker H, Gornez LD, Harbinson J (2002) Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: review. Plant Physiol Biochem 40:659–668
Han C, Liu Q, Yang Y (2009) Short-term effects of experimental warming and enhanced ultraviolet-B radiation on photosynthesis and antioxidant defense of Picea asperata seedlings. Plant Growth Regul 58:153–162
Hansen AH, Jonasson S, Michelsen A, Julkunen-Tiitto R (2006) Long-term experimental warming, shading and nutrient addition affect the concentration of phenolic compounds in arctic-alpine deciduous and evergreen dwarf shrubs. Oecologia 147:1–11
Hikosaka K, Kato MC, Hirose T (2004) Photosynthetic rate and partitioning of absorbed light energy in photoinhibited leaves. Physiol Plant 121:699–708
Hirose T, Werger MJA (1987) Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solodage alissima stand. Physiol Plant 70:215–222
Hobbie EA, Olszyk DM, Rygiewicz PT, Tingey DT, Johnson MG (2001) Foliar nitrogen concentrations and natural abundance of 15 N suggest nitrogen allocation patterns of Douglas-fir and mycorrhizal fungi during development in elevated carbon dioxide concentration and temperature. Tree Physiol 21:1113–1122
Hodges DM, Andrew CJ, Johnson DA, Hamilton RI (1996) Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol Plant 98:685–692
IPCC (2007) Climate change 2007: the physical science basis. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Contribution of working group I to the fourth assessment report of the intergovernmental panel of climate change. Cambridge University Press, Cambridge
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can J Bot 65:729–735
Ke D, Wang A, Sun G, Dong L (2002) The effect of active oxygen on the activity of ACC synthase induced by exogenous IAA. Act Bot Sin 44:551–556 (in Chinese)
Knops JMH, Reinhart K (2000) Specific leaf area along a nitrogen fertilization gradient. Am Midl Nat 144(2):265–272
Larssen T, Lydersen E, Tang D, He Y, Gao J, Liu H, Duan L, Seip HM, Vogt RD, Mulder J, Shao M, Wang Y, Shang H, Zhang X, Solberg S, Aas W, Økland T, Eilertsen O, Angell V, Liu Q, Zhao D, Xiang R, Xiao J, Luo J (2006) Acid rain in China. Environ Sci Technol 40(2):418–425
Lewis JD, Lucash M, Olszyk DM, Tingey DT (2004) Relationships between needle nitrogen concentration and photosynthetic responses of Douglas-fir seedlings to elevated CO2 and temperature. New Phytol 162:355–364
Loik ME, Harte J (1997) Changes in water relations for leaves exposed to a climate-warming manipulation in the Rocky Mountains of Colorado. Environ Exp Bot 37:115–123
Majdi H, Öhrvik J (2004) Interactive effects of soil warming and fertilization on root production, mortality, and longevity in a Norway spruce stand in Northern Sweden. Global Change Biol 10:182–188
Mäkipää R, Karjalainen T, Pussinen A, Kellomäki S (1999) Effects of climate change and nitrogen deposition on the carbon sequestration of a forest ecosystem in the boreal zone. Can J For Res 29:1490–1501
Nakaji T, Fukami M, Dokiya Y, Izuta T (2001) Effects of high nitrogen load on growth, photosynthesis and nutrient status of Cryptomeria japonica and Pinus densiflora seedlings. Trees 15:453–461
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate- specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Öncel I, Keles Y, Üstün AS (2000) Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ Pollut 107:315–320
Pandey R, Chacko PM, Choudhary ML, Prasad KV, Pal M (2007) Higher than optimum temperature under CO2 enrichment influences stomata anatomical characters in rose (Rose hybrida). Sci Hortic 113:74–81
Pérez P, Morcuende R, Martin del Molino I, Martinez-Carrasco R (2005) Diurnal changes of Rubisco in response to elevated CO2, temperature and nitrogen in wheat grown under temperature gradient tunnels. Exp Bot 53:13–27
Prochazkova D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Sánchez E, Ruiz JM, Romero L (2002) Proline metabolism in response to nitrogen toxicity in fruit of French Bean plants. Sci Hortic 93:225–233
Schonhof I, Kläring HP, Krumbein A, Claußen W (2007) Schreiner M, Effect of temperature increase under low radiation conditions on phytochemicals and ascorbic acid in greenhouse grown broccoli. Agric Ecosyst Environ 119:103–111
Sugiharto B, Miyata K, Nakamoto H, Sasakawa H, Sugiyama T (1990) Regulation of expression of carbon-assimilating enzymes by nitrogen in maize leaf. Plant Physiol 92:963–969
Tegelberg R, Julkunen-Tiitto R, Vartiainen M, Paunonen R, Rousi M, Kellomäki S (2008) Exposures to elevated CO2, elevated temperature and enhanced UV-B radiation modify activities of polyphenol oxidase and guaiacol peroxidase and concentrations of chlorophylls, polyamines and soluble proteins in the leaves of Betula pendula seedlings. Environ Exp Bot 62:308–315
Tingey DT, Mckane RB, Olszyk DM, Johnson MG, Rygiewicz PT, Henrylee E (2003) Elevated CO2 and temperature alter nitrogen allocation in Douglas-fir. Global Change Biol 9:1038–1050
Van Cleve K, Oechel WC, Hom JL (1990) Response of black spruce (Picea mariana) ecosystems to soil temperature modification in interior Alaska. Can J For Res 20:1530–1535
Wang KY (2004) Processes of Subalpine Forest Ecosystems in the West of Sichuan. Sichuan Publishing House of Science and Technology, Chengdu (in Chinese)
Wang KY, Kellmäki S, Laitinen K (1995) Effects of needle age, long-term temperature and CO2 treatments on the photosynthesis of Scots pine. Tree Physiol 15:211–218
Xiao K, Zhang RX, Qian WP (1998) The physiological mechanism of senescence and photosynthetic function decline of flag leaf in wheat regulated by nitrogen nutrition. Plant Nutr Fertil Sci 4:371–378 (in Chinese)
Yao XQ, Liu Q (2006) Changes in photosynthesis and antioxidant defenses of P. asperata seedlings to enhanced ultraviolet-B and to nitrogen supply. Physiol Plant 129:364–374
Zha T, Wang KY, Ryyppö A, Kellmäki S (2002) Impact of needle age on the response of respiration in Scots pine to long-term elevation of carbon dioxide concentration and temperature. Tree Physiol 22:1241–1248
Zhang W, Parker KM, Luo Y, Wan S, Wallace LL, Hu S (2005) Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Global Change Biol 11:266–277
Zhao CZ, Liu Q (2009) Growth and photosynthetic responses of two coniferous species to experimental warming and nitrogen fertilization. Can J For Res 38:1–12
Acknowledgments
This study is funded jointly by the Key Program of the National Natural Science Foundation of China (31100446 and 31070533), the forefront project of Chengdu Institute of Biology, and the Chinese Academy of Sciences (Y0B2021100). We are also indebted to Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization and Ecological Restoration, Biodiversity Conservation Key Laboratory of Sichuan Province and Maoxian Ecological Station, Chengdu Institute of Biology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Franklin.
Rights and permissions
About this article
Cite this article
Zhao, C., Liu, Q. Effects of soil warming and nitrogen fertilization on leaf physiology of Pinus tabulaeformis seedlings. Acta Physiol Plant 34, 1837–1846 (2012). https://doi.org/10.1007/s11738-012-0982-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-0982-2