Abstract
The relation between orientation of cortical microtubules and the transcript levels of a 65 kDa microtubule-associated protein (VaMAP65-1) was investigated along epicotyls of azuki bean (Vigna angularis) seedlings. In addition, the effects of 1-aminocyclopropane-1-carboxylic acid (ACC), the immediate precursor of ethylene, and hypergravity, a gravitational force exceeding 1g, on the relation were examined. The percentage of cells with longitudinal microtubule increased, whereas that with transverse microtubules decreased from the apical to the basal regions of epicotyls. The transcript levels of VaMAP65-1 decreased toward the basal region. ACC induced reorientation of cortical microtubules from transverse to longitudinal directions and down-regulation of VaMAP65-1 expression. Hypergravity also induced reorientation of cortical microtubules and down-regulation of the expression. Strong correlations were observed between the percentage of cells with longitudinal or transverse microtubules and the transcript levels of VaMAP65-1. These results suggest that down-regulation of VaMAP65-1 expression is at least partly involved in the regulation of the orientation of cortical microtubules in azuki bean epicotyls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant cells have organized microtubule arrays, which are essential for cell polarization and cell division. Cortical microtubules, a characteristic structure of interphase cells, are assumed to be responsible for anisotropic expansion of plant cells by directing the orientation of cellulose microfibrils (Giddings and Staehelin 1991; Shibaoka 1994; Baskin 2001; Wasteneys 2004). For example, elongating cells in the apical region of stems have predominantly transversely oriented cortical microtubules, whereas lateral expanding cells in basal region of stems have predominantly longitudinally oriented cortical microtubules. In addition, the orientation of cortical microtubules is controlled by various factors, such as plant hormones and environmental stimuli. Longitudinal cortical microtubules are predominant in the presence of ethylene, which promotes lateral growth in shoots (Steen and Chadwick 1981). On the contrary, transverse cortical microtubules are predominant in the presence of gibberellins, which promote elongation growth of shoots (Shibaoka 1974). Hypergravity, which suppresses elongation growth and promotes lateral growth in stems, induced reorientation of cortical microtubules from transverse to longitudinal directions (Soga et al. 2006).
Microtubule-associated proteins (MAPs) play a variety of roles in organization of microtubules. γ-Tubulin complex proteins (GCPs) containing γ-tubulin, GCP2 and GCP3 are required for microtubule nucleation and proper organization of cortical microtubules (Murata et al. 2005; Pastuglia et al. 2006; Erhardt et al. 2002). Katanin has microtubule-severing activity and is involved in the reorientation of cortical microtubules (Burk et al. 2001; Bouquin et al. 2003). Recently, we revealed that the transcript levels of γ-tubulin complex (VaTUG and VaGCP3) and katanin (VaKTN1) genes were transiently increased during reorientation of cortical microtubules by ACC or hypergravity in azuki bean epicotyls (Soga et al. 2008, 2009, 2010). Thus, both nucleation and severing of cortical microtubules are assumed to be regulated at the transcriptional levels of γ-tubulin complex and katanin genes during reorientation of cortical microtubules.
Jiang and Sonobe (1993) isolated 65 kDa family of proteins referred to as 65 kDa MAP (MAP65) from tobacco cells. MAP65 proteins are required for bundling of microtubules by forming cross-bridges between adjacent microtubules. It has also been shown that the contents of MAP65 protein in upper region of azuki bean epicotyls, which have predominantly transverse cortical microtubules, are higher than those in basal region of epicotyls, which have predominantly longitudinal cortical microtubules (Sawano et al. 2000). However, the mechanism regulating the levels of MAP65 protein has not yet been elucidated. One important regulating step of the levels of MAP65 protein may be the transcriptional levels of MAP65 gene during reorientation of microtubules. To confirm this point, we investigated relation between the orientation of cortical microtubules and the transcript levels of MAP65 (VaMAP65-1) gene along epicotyls of azuki bean seedlings. We also examined effects of ethylene (ACC) and hypergravity on the relation in azuki bean epicotyls.
Materials and methods
Plant material
Seeds of azuki bean (Vigna angularis Ohwi et Ohashi cv. Erimowase) were soaked in running tap water for 1 day at 30°C and allowed to germinate on gauze spread on a plastic dish filled with water at 25°C in the dark. Five days later, seedlings with epicotyls 30–35 mm in length were selected, and used for measurement of the orientation of cortical microtubules and gene expression. For ACC treatment, a 10 mm upper region (3–13 mm below the hook) was marked with India ink. The marked seedlings were transplanted into a plastic vessel (80 mm in diameter, 100 mm in height) containing 15 ml of 5 mM MES-KOH buffer (pH 6.0) with or without ACC, and then incubated at 25°C in the dark. For hypergravity treatment, the marked seedlings were transplanted into test tubes (16 mm in diameter, 100 mm in length) containing 1.5 ml of 1 mM MES-KOH buffer (pH 6.0), and then exposed to basipetal hypergravity with a centrifuge (H-28-F, Kokusan Co., Tokyo, Japan) at 25°C in the dark. The magnitude of acceleration was regulated by changing the speed of rotation. The seedlings for 1g controls were placed on top of spinning centrifuge during incubation. All manipulations were done under dim green light (ca. 0.09 μmol m−2 s−1 at handling level).
Orientation of cortical microtubules
The orientation of cortical microtubules was analyzed by immunofluorescence microscopy. The excised segments of azuki bean epicotyls were immediately fixed with 4% (w/v) paraformaldehyde in a buffer [50 mM PIPES, 5 mM EGTA, 2 mM MgSO4, 0.5% (v/v) TritonX-100, 1% (v/v) glycerol, pH 7.3] at 25°C for 1 h after infiltration with a vacuum pump for 10 min. Then the air pressure was restored, and the samples were washed four times (15 min each) with phosphate-buffered saline (PBS) at 25°C. The segments were cut at the middle and the epidermal strips were peeled from either apical or basal end to middle. The specimens were treated with a detergent solution [1% (v/v) Nonidet P-40 in PBS] for 0.5 h, and then washed four times (15 min each) with PBS. They were incubated in a blocking solution [1% (w/v) bovine serum albumin in PBS] at 37°C and washed with PBS. These specimens were treated with FITC-conjugated monoclonal antibodies against α- and β-tubulin (F2168 and F2043, Sigma) diluted 1:100 (v/v) in PBS at 37°C for 20 min, and washed with PBS. They were then mounted on a slide glass and covered with a PBS solution containing 90% (v/v) glycerol. The specimens were observed under a fluorescence microscope (Axio Imager. A1, Carl Zeiss, Göttingen, Germany), and the images were processed with image processing software programs attached to VB-7000 (Keyence, Osaka, Japan).
The orientation of cortical microtubules adjacent to the outer tangential wall of each epidermal cell was determined. In most cells, the orientation of cortical microtubules was uniform, although it varied with the cell. Thus, we recorded the orientation of cortical microtubules in each cell. We determined the frequency of cells with cortical microtubules within a range of 90–70° (transverse), 70–20° (oblique), 20–0° (longitudinal) to the longitudinal cell axis, and in a variety of directions (random).
Transcript levels of VaMAP65-1
The epidermal tissues were collected from the excised segments, and then immediately frozen with liquid nitrogen and kept at −80°C until use. The frozen epidermal strips (fresh weight: ca. 100 mg) were homogenized in a mortar with a pestle. Total RNA was prepared using RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA), including a DNA elimination step (RNase-Free DNase Set, Qiagen). Single strand cDNA was obtained from 2 μg of total RNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) with a random hexamer primer. One μL of the reaction mixture (total volume: 20 μL) was used subsequently for PCR. Real time RT-PCR was performed with the Applied Biosystems 7500 Real Time PCR System with Power SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer’s instructions. All data were normalized with respect to 18S rRNA, which was measured as an internal standard. Primers were designed by the Primer Express program (Applied Biosystems) and had the following sequences: for VaMAP65-1 (AB624333) (forward, 5′-GAAGCAAACGGAGCTTGAAGA-3′; reverse, 5′-TTCTCCCGTGCAGCATCTG-3′) and for 18S rRNA for normalization (forward, 5′-AGTCATCAGCTCGCGTTGAC-3′; reverse, 5′-TCAATCGGTAGGAGCGACG-3′). Concentrations of primers were 50 nM. Double-strand cDNA was generated by initial treatment with 50°C for 2 min and 95°C for 10 min followed by PCR (95°C for 15 s, 60°C for 1 min) for 40 cycles. We determined the nucleotide sequence of the RT-PCR products, and confirmed the specificity of the primers.
Results
Spatial changes along epicotyls
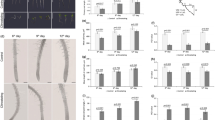
The orientation of cortical microtubules adjacent to the outer tangential wall in epidermal cells of epicotyls was analyzed by immunofluorescence microscopy. In the upper region of epicotyls, 4 types of cells, transverse, oblique, longitudinal and random, were observed, and the percentage of each type was 11.0, 16.7, 69.3 and 3.0%, respectively. Namely, cells having transverse microtubules were predominant in the upper region of epicotyls. The ratio of four types of cells varied along epicotyls. The percentage of cells with transverse microtubules decreased from the apical to the basal regions, while that of cells with longitudinal microtubules increased (Fig. 1). Cells having transverse microtubules were not observed in the basal region of epicotyls.
Changes in the orientation of cortical microtubules along epicotyls of azuki bean seedlings. Seedlings with epicotyls 30–35 mm in length were selected, and epicotyls were divided at 10-mm interval from the region 3 mm below the hook. The orientation of cortical microtubules of adjacent to the outer tangential wall of epidermal cells was analyzed by immunofluorescence microscopy. The percentage of cells with microtubules within a range of 90–70° (transverse), 70–20° (oblique), 20–0° (longitudinal) to the longitudinal cell axis, and in various directions (random) was calculated for 300 cells from 20 segments. Experiments were repeated three times. Values are mean ± SE (n = 3)
For the analysis of the transcript levels of MAP65, we cloned partial cDNA of MAP65 from azuki bean epicotyls based on the conserved regions found in common with Arabidopsis (At5g55230) and tobacco (AJ289862, AJ289863 and AJ289864). The cloned cDNA was designated VaMAP65-1 (AB624333). Figure 2 shows the transcript levels of VaMAP65-1 along epicotyls. The VaMAP65-1 gene was expressed in all regions. However, the level of VaMAP65-1 significantly decreased from the apical to the basal regions.
Changes in the expression of VaMAP65-1 gene along epicotyls of azuki bean seedlings. Epicotyls were divided into three regions as shown in Fig. 1. The expression of VaMAP65-1 gene in epidermal tissues of the region was determined by a real time RT-PCR. The values were compensated with levels of 18S rRNA as an internal standard, and expressed as percentage of levels of upper region. Values are mean ± SE (n = 3)
ACC-induced changes
Figure 3 shows orientation of cortical microtubules in epidermal cells of upper region of epicotyls after a 5-h treatment with ACC at different concentrations. In the absence of ACC, no clear changes in microtubule orientation were observed during incubation (Fig. 3). With increasing concentration of ACC, the percentage of cells with transverse microtubules was decreased, while that of cells with longitudinal microtubules was increased in a 5-h treatment (Fig. 3). In the presence of 10−6 M ACC, the percentages of cells with longitudinal, oblique and transverse microtubules were almost the same. Cells having longitudinal microtubules were predominant in the epicotyls treated with ACC more than 10−5 M. The percentage of cells with longitudinal or transverse microtubules showed significant correlations with the logarithm of the concentration of ACC (R = 0.98 and −0.99). The level of VaMAP65-1 significantly decreased with increasing concentration of ACC (Fig. 6). The expression of VaMAP65-1 showed a significant correlation with the logarithm of the concentration of ACC (R = −0.97).
Effects of ACC on the orientation of cortical microtubules in azuki bean epicotyls. A 10 mm upper region was marked with India ink, and the seedlings were treated with 5 mM MES-KOH buffer (pH 6.0) containing different concentrations of ACC for 5 h at 25°C in the dark. The percentage of microtubule orientation was calculated as indicated in Fig. 1. Values are mean ± SE (n = 3)
Hypergravity-induced changes
The orientation of cortical microtubules in epidermal cells of upper region of epicotyls grown under different gravitational conditions was analyzed. Cells having transverse microtubules were predominant in the epicotyls under 1g conditions (Fig. 5). With increasing accelerations, the percentage of cells with transverse microtubules was decreased, while that of cells with longitudinal microtubules was increased in a 5-h treatment (Fig. 5). The percentage of cells with longitudinal or transverse microtubules showed significant correlations with the logarithm of the magnitude of the gravity (R = 0.99 and −0.99). Hypergravity at 30g or more was required to significantly decrease in VaMAP65-1 expression (Fig. 6). The expression of VaMAP65-1 showed a significant correlation with the logarithm of the magnitude of gravity (R = −0.99).
Effects of hypergravity on the orientation of cortical microtubules in azuki bean epicotyls. A 10 mm upper region was marked with India ink, and the seedlings were kept under basipetal hypergravity conditions of different doses for 5 h at 25°C in the dark. The percentage of microtubule orientation was calculated as indicated in Fig. 1. Values are mean ± SE (n = 3)
Effects of hypergravity on the expression of VaMAP65-1 gene in azuki bean epicotyls. Azuki bean seedlings were treated with hypergravity for 5 h as in Fig. 5. The expression of VaMAP65-1 gene was determined as indicated in Fig. 2. The values were expressed as percentage of initial level. Values are mean ± SE (n = 3)
Temporal changes after ACC and hypergravity treatments
The time-course changes in the orientation of cortical microtubules and the transcript levels of VaMAP65-1 in epidermal cells by ACC and hypergravity treatments were examined. The orientation of cortical microtubules did not change during incubation in the control seedlings (Fig. 7). The percentage of cells with transverse microtubules decreased, whereas that with longitudinal microtubules increased in 10−5 M ACC- or 300g-treated seedlings (Fig. 7). The reorientation of microtubules from transverse to longitudinal directions was detected already at 1 h after start of ACC or hypergravity treatment. Figure 8 shows the time-course changes in the transcript levels of VaMAP65-1. In the control seedlings, the expression levels of VaMAP65-1 was almost constant during incubation. By contrast, both ACC and hypergravity decreased the expression levels of VaMAP65-1. A significant decrease in the expression levels of VaMAP65-1 was detected at 2 h after start of ACC and hypergravity treatments.
Time-course changes in the orientation of cortical microtubules in ACC- or hypergravity-treated azuki bean epicotyls. For ACC treatment, azuki bean seedlings with marked epicotyls were grown in the presence of 10−5 M ACC at 25°C in the dark. For hypergravity treatment, the marked seedlings were kept under basipetal hypergravity at 300g at 25°C in the dark. The percentage of microtubule orientation was calculated as indicated in Fig. 1. Values are mean ± SE (n = 3)
Time-course changes in the expression of VaMAP65-1 gene in ACC- or hypergravity-treated azuki bean epicotyls. Azuki bean seedlings were treated with ACC or hypergravity as in Fig. 7. The expression of VaMAP65-1 gene was determined as indicated in Fig. 2. The values were expressed as percentage of initial level. Values are mean ± SE (n = 3)
Discussion
Orientation of cortical microtubules is assumed to be responsible for determination of the direction of cell expansion (Giddings and Staehelin 1991; Shibaoka 1994; Baskin 2001; Wasteneys 2004). It is generally accepted that cortical microtubule arrays are essential for regulation of the orientation of cellulose microfibrils in the innermost layer of the cell wall, although some other mechanisms may also be involved (Baskin 2001; Wasteneys 2004). In 5-day-old azuki bean seedlings, elongation growth occurred only in the upper region of epicotyls. Cells having transverse microtubules were predominant in the upper region of epicotyls, whereas cells having longitudinal microtubules were predominant in the basal region of epicotyls (Fig. 1). It has also been shown that the ratio of transverse microtubules in upper regions of azuki bean epicotyls which have high activity of elongation growth is higher than that in basal regions of epicotyls which have low activity of elongation growth (Sawano et al. 2000). Both ACC and hypergravity suppressed elongation growth and promoted lateral growth in azuki bean epicotyls (Soga et al. 2006, 2010). In the present study, reorientation of cortical microtubules from transverse to longitudinal directions was induced by ACC and hypergravity treatments (Figs. 3, 5, 7). Taken together, these results support the hypothesis that orientation of cortical microtubules is responsible for anisotropic expansion of plant cells.
In the plant cells, microtubules including cortical microtubules in interphase are usually bundled by forming cross-bridges between adjacent microtubules (Sonobe et al. 2001). MAP65 has been biochemically identified as a cross-bridge factor of microtubules from tobacco BY-2 cells (Jiang and Sonobe 1993). It has been shown that the protein contents of MAP65 decreased along epicotyls of azuki bean seedlings (Sawano et al. 2000). The transcript levels of VaMAP65-1 gene decreased along epicotyls of azuki bean seedlings (Fig. 2). Thus, the protein contents of MAP65 are assumed to be regulated at the transcriptional levels of MAP65 gene. Sasabe et al. (2006) showed that activity of microtubule bundling is regulated by phosphorylation of MAP65 during cytokinesis in tobacco cells. These findings suggest that changes in the levels of MAP65 and modification of phosphorylation of MAP65 are cooperatively involved in the regulation of the activity of microtubule bundling in planta.
Sawano et al. (2000) reported that the protein levels of MAP65 showed good parallelism with the orientation of cortical microtubules in azuki bean epicotyls. Namely, the protein levels of MAP65 in rapidly elongating regions of epicotyls which have predominantly transverse cortical microtubules are higher than those in non-elongating regions which have predominantly longitudinal cortical microtubules. Thus, levels of MAP65 may be involved in the regulation of orientation of cortical microtubules, which change direction of cell expansion. In the present study, we revealed that the expression level of VaMAP65-1 in upper region of epicotyls which have predominantly transverse cortical microtubules was higher than that in basal region which have predominantly longitudinal cortical microtubules (Figs. 1, 2). By increasing concentration of ACC, reorientation of cortical microtubules from transverse to longitudinal directions and down-regulation of VaMAP65-1 expression were induced (Figs. 3, 4). Reorientation of cortical microtubules and down-regulation of the expression were also induced by increasing the magnitude of gravity (Figs. 5, 6). These results indicate that the decrease in the transcript levels of VaMAP65-1 is accompanied by reorientation of cortical microtubules from transverse to longitudinal directions. We found high correlations between the percentage of cells with longitudinal or transverse microtubules and the transcript levels of VaMAP65-1 (Fig. 9), when calculating with the whole set of data. However, results of the time-course analysis showed that reorientation of cortical microtubules slightly preceded the decrease in the transcript levels of VaMAP65-1 (Figs. 7, 8). Thus, the down-regulation of VaMAP65-1 transcript may not be directly involved in initiation of reorientation of cortical microtubules. Instead, the decrease in microtubule bundling activity via degradation and/or modification of phosphorylation of MAP65 protein might induce reorientation of cortical microtubules from transverse to longitudinal directions. The expression of MAP65 gene was then down-regulated, and the resultant decrease in levels of MAP65 protein may be involved in the maintenance of longitudinal microtubule orientation. These possibilities need to be examined in the future. Taken together, present results suggest that changes in the protein levels of MAP65 via modification of expression of MAP65 gene are one of the mechanisms for the regulation of orientation of cortical microtubules.
We have analyzed expression profiles of α-and β-tubulin, component of microtubules, and MAPs such as γ-tubulin complex proteins and katanin during reorientation of cortical microtubules. Hypergravity, which induces reorientation of cortical microtubules from transverse to longitudinal directions, increased the transcript levels of α-and β-tubulin genes (Matsumoto et al. 2007). The transcript levels of genes encoding γ-tubulin complex, which is involved in the nucleation of microtubules, were increased transiently under hypergravity conditions (Soga et al. 2008). When cortical microtubules were reoriented by hypergravity, the transcript levels of katanin, microtubule-severing protein, also increased transiently (Soga et al. 2009). ACC, which induces reorientation of cortical microtubules from transverse to longitudinal directions, also increased the transcript levels of γ-tubulin complex and katanin transiently (Soga et al. 2010). In the present study, transcript levels of MAP65 gene decreased during reorientation of cortical microtubules (Figs. 1–8). These findings indicate that transcription of genes encoding tubulin and MAPs is regulated dynamically during reorientation of cortical microtubules. These results also suggest that tubulin genes are inappropriate as a constitutive standard in the study of gene expression, even though they have been often used.
In seedlings treated with ACC, the immediate precursor of ethylene, the production of ethylene is increased resulting in induction of the ethylene responses. Therefore, ACC is widely used in place of ethylene, although the kinetics of the response to ACC is different from that to ethylene (Zhang and Wen 2010). Effects of ethylene are similar to those of hypergravity. Both ethylene (ACC) and hypergravity suppressed elongation growth and promoted lateral growth of azuki bean epicotyls (Soga et al. 2006, 2010). In addition, reorientation of cortical microtubules from transverse to longitudinal directions was induced by ACC and hypergravity (Figs. 3, 5, 7). Similar changes in the transcript levels of MAPs such as MAP65, γ-tubulin complex and katanin were observed in ACC- and hypergravity-treated seedlings (Figs. 4, 6, 8; Soga et al. 2008, 2009, 2010). Thus, ethylene might be involved in the signal transduction of hypergravity-induced responses. However, an ethylene-resistant mutant etr1-1 of Arabidopsis modified growth anisotropy in response to magnitude of gravitational force as in wild-type plants (Soga et al. 2002). These results suggest ethylene and hypergravity act independently in reorientation of cortical microtubules.
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- PBS:
-
Phosphate-buffered saline
- RT-PCR:
-
Reverse transcription-PCR
- VaMAP65-1 :
-
Vigna angularis microtubule-associated protein 65-1
References
Baskin TI (2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215:150–171
Bouquin T, Mattsson O, Naested H, Foster R, Mundy J (2003) The Arabidopsis lue1 mutant defines a katanin p60 ortholog involved in hormonal control of microtubule orientation during cell growth. J Cell Sci 116:791–801
Burk DH, Liu B, Zhong R, Morrison WH, Ye ZH (2001) A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell 13:807–827
Erhardt M, Stoppin-Mellet V, Campagne S, Canaday J, Mutterer J, Fabian T, Sauter M, Muller T, Peter C, Lambert AM, Schmit AC (2002) The plant Spc98p homologue colocalizes with γ-tubulin at microtubule nucleation sites and is required for microtubule nucleation. J Cell Sci 115:2423–2431
Giddings TH, Staehelin LA (1991) Microtubule-mediated control of microfibril deposition: a re-examination of the hypothesis. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 85–99
Jiang CJ, Sonobe S (1993) Identification and preliminary characterization of a 65 kDa higher-plant microtubule-associated protein. J Cell Sci 105:891–901
Matsumoto S, Saito Y, Kumasaki S, Soga K, Wakabayashi K, Hoson T (2007) Up-regulation of expression of tubulin genes and roles of microtubules in hypergravity-induced growth modification in Arabidopsis hypocotyls. Adv Space Res 39:1176–1181
Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, Horio T, Hasebe M (2005) Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nat Cell Biol 7:961–968
Pastuglia M, Azimzadeh J, Goussot M, Camilleri C, Belcram K, Evrard JL, Schmit AC, Guerche P, Bouchez D (2006) γ-Tubulin is essential for microtubule organization and development in Arabidopsis. Plant Cell 18:1412–1425
Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, Hidaka M, Machida Y (2006) Phosphorylation of NtMAP65–1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev 20:1004–1014
Sawano M, Shimmen T, Sonobe S (2000) Possible involvement of 65 kDa MAP in elongation growth of azuki bean epicotyls. Plant Cell Physiol 41:968–976
Shibaoka H (1974) Involvement of wall microtubules in gibberellin promotion and kinetin inhibition of stem elongation. Plant Cell Physiol 15:255–263
Shibaoka H (1994) Plant hormone-induced changes in the orientation of cortical microtubules: Alterations in the cross-linking between microtubules and the plasma membrane. Annu Rev Plant Physiol Plant Mol Biol 45:527–544
Soga K, Wakabayashi K, Kamisaka S, Hoson T (2002) Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta 215:1040–1046
Soga K, Wakabayashi K, Kamisaka S, Hoson T (2006) Hypergravity induces reorientation of cortical microtubules and modifies growth anisotropy in azuki bean epicotyls. Planta 224:1485–1494
Soga K, Kotake T, Wakabayashi K, Kamisaka S, Hoson T (2008) Transient increase in the transcript levels of γ-tubulin complex genes during reorientation of cortical microtubules by gravity in azuki bean (Vigna angularis) epicotyls. J Plant Res 121:493–498
Soga K, Kotake T, Wakabayashi K, Kamisaka S, Hoson T (2009) The transcript level of katanin gene is increased transiently in response to changes in gravitational conditions in azuki bean epicotyls. Biol Sci Space 23:23–28
Soga K, Yamaguchi A, Kotake T, Wakabayashi K, Hoson T (2010) 1-Aminocyclopropane-1-carboxylic acid (ACC)-induced reorientation of cortical microtubules is accompanied by a transient increase in the transcript levels of γ-tubulin complex and katanin genes in azuki bean epicotyls. J Plant Physiol 167:1165–1171
Sonobe S, Yamamoto S, Motomura M, Shimmen T (2001) Isolation of cortical MTs from tobacco BY-2 cells. Plant Cell Physiol 42:162–169
Steen DA, Chadwick AV (1981) Ethylene effects in pea stem tissue. Evidence of microtubule mediation. Plant Physiol 67:460–466
Wasteneys GO (2004) Progress in understanding the role of microtubules in plant cells. Curr Opin Plant Biol 7:651–660
Zhang W, Wen CK (2010) Preparation of ethylene gas and comparison of ethylene responses induced by ethylene, ACC, and ethephon. Plant Physiol Biochem 48:45–53
Acknowledgments
The present study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, a Grant for Ground-based Research for Space Utilization from Japan Space Forum, and by Sasakawa Scientific Research Grant from the Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Wang.
Rights and permissions
About this article
Cite this article
Soga, K., Kotake, T., Wakabayashi, K. et al. Changes in the transcript levels of microtubule-associated protein MAP65-1 during reorientation of cortical microtubules in azuki bean epicotyls. Acta Physiol Plant 34, 533–540 (2012). https://doi.org/10.1007/s11738-011-0850-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0850-5