Abstract
Seedlings of Trigonella foenum-graecum were treated with four heavy metal salts (CdCl2, CoCl2, K2Cr2O7 and NiCl2) to study the effect of heavy metals on growth and diosgenin production. It was found that CdCl2 increased diosgenin production up to 40-fold and CoCl2 increased diosgenin production up to 41-fold at concentrations which did not affect growth significantly. But K2Cr2O7 and NiCl2 were toxic to growth and inhibited diosgenin production. Effect of exogenously applied methyl jasmonate (MeJa) and calcium (Ca2+) on diosgenin production in seedlings of T. foenum-graecum was also investigated. MeJa enhanced the production of diosgenin. Maximum increase (10.5-fold) was found at 100 µL L−l concentration of MeJa. To study the role of Ca2+ on diosgenin production, seedlings of T. foenum-graecum were treated with a promoter of Ca2+ influx (calcium ionophore A23187), calcium depleted medium, Ca2+ channel blocker (verapamil) and antagonist (LaCl3), a divalent cation chelator (EGTA) and modulator of calcium release (caffeine). All the treatments were compared with a control containing 220 mg L−l concentration of CaCl2. The results suggest that the increase in cytosolic Ca2+ has an inhibitory role on diosgenin production. However, a calcium chelator or Ca2+ channel inhibitors could be used to elicit diosgenin production in this plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elicitation can be an important strategy towards improved production of plant secondary metabolites. A wide variety of substances (biotic and abiotic) are able to act as elicitors which can trigger the production of many secondary metabolites in plants. As the elicitation process is mediated through different signal transduction pathways, many workers have also used the signaling molecules as elicitors (De 2001). Methyl jasmonate (MeJa) and calcium (Ca2+) are the two well-known compounds that affect the signal transduction involved in the elicitation process. Diosgenin is an important steroidal metabolite used as a starting material for the synthesis of steroidal drugs (Evans 1996). It has an estrogenic effect on mammary gland (Aradhana et al. 1992) and plays an important role in the control of cholesterol metabolism (Roman et al. 1995; Sauvaire et al. 1991). Methyl protodioscin, a potent agent with antitumour activity, has been synthesized from diosgenin (Cheng et al. 2003). Trigonella foenum-graecum is one of the several plant sources that produce diosgenin (Evans 1996). Seedlings of the plant are also reported to produce diosgenin (Hardman and Fazli 1972; Bhavsar et al. 1980; Ortuno et al. 1999). Previously we have studied the role of ethylene on diosgenin production in T. foenum-graecum seedlings (De and De 2003) and in Dioscorea floribunda (De and De 2005). In the present work, different concentrations of salts of heavy metals such as Cd, Co, Cr, Ni were used to study their effect on growth and diosgenin production in the seedlings of T. foenum-graecum. In an attempt to elicit diosgenin production we have also studied, in this paper, the effect of exogenously applied MeJa on diosgenin production and the role of Ca2+ on the production of the same in T. foenum-graecum seedlings.
Materials and methods
Treatment of seedlings
Seeds of T. foenum-graecum were surface sterilized and were placed in sterile lidded glass vessels (12 × 6 cm) on the filter paper soaked with sterile water for germination. On day 2 the germinated seeds were treated with different concentrations of heavy metal salts such as CdCl2, CoCl2, K2Cr2O7 and NiCl2 after dissolving the compound in MS salt solution (1/2 strength) (Murashige and Skoog 1962). Thirty seedlings (2 days old) were placed on two layers of filter paper soaked with 6–7 ml of salt solution containing different concentrations of above-mentioned substances in each sterile lidded glass vessel and kept for another 5 days at 15–18°C under fluorescent lamp for 8/16 h light/dark period. Controls were exposed to 1/2 strength MS salt solution. After 5 days of treatment, treated and control seedlings were harvested for analysis.
For studying the role of calcium, the seedlings were treated with different concentrations of calcium salt, promoter (calcium ionophore A23187), inhibitor [verapamil, LaCl3, Ethylene glycol bis (β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA)] and modulator (caffeine) of Ca2+ influx. Except methyl jasmonate, all other treatments were applied after dissolving the compounds in 1/2 strength MS salt solution as described above. In case of MeJa treatment, germinated seeds were exposed to MeJa vapour by placing MeJa (which is a liquid at room temperature) dissolved in 100% ethanol onto the tip of a cotton swab. The cotton swab was kept in the glass vessel, but not in direct contact with the seedlings. Control set received 100% ethanol only. MeJa was added at concentrations of 50–1,000 µl L−1 (of glass vessel volume). After 5 days of treatment, treated and control seedlings were harvested for analysis. Every experiment was repeated in triplicate.
Growth measurement
Seven-day-old seedlings treated with metal salts were harvested and seed coats were removed. Fresh weight and seedling length were measured. The seedlings were then dried at 45°C for 48 h and dry weights were taken.
Analysis of diosgenin
Diosgenin was extracted following the method of Asolkar and Chadha (1979). Powdered dried seedling, 3 N HCl and hexane were refluxed in a glass apparatus on a magnetic stirrer with a hot plate for 2 h at 90–96°C. The mixture was allowed to cool and the aqueous phase was extracted three times with 25 ml hexane each time. The combined organic phase was washed with 1% NaHCO3 solution and subsequently with distilled water and then evaporated to dryness. The extract was then analyzed by HPLC following the method of Indrayanta et al. (1994) optimized for our work conditions (De and De 2003).
Results and discussion
Effect of metal salt solutions
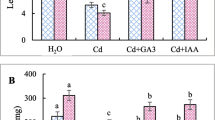
There are several reports where heavy metals elicited different secondary metabolite formation in some plant cell culture system. Manganese induced cardenolide production in Digitalis thapsi (Crochete et al. 1991) and D. lanata (Ohlsson and Berglund 1989). Copper induced berberine production in Thallictrum rugosum suspension culture (Kim et al. 1991), total indole alkaloid production in Catharanthus roseus cell culture (Backer-Royer et al. 1990), reserpine production in Rauwolfia serpentina culture (Solichatun and Anggarwulan 2008). Gaisser and Heide (1996) reported induction of acetyl shikonin and rosemarinic acid production by arsenate in Lithospermum erythrorhizon cell culture. Eu3+ increased total anthraquinone content in Cassia obtusifolia hairy root culture (Guo et al. 1998). Cobalt chloride induced l-Dopa production in Stizolobium hassjoo (Sung and Huang 2000). During the present study, toxic effect on growth of seedlings was observed 5 days after treatment with 500 µM concentration of CdCl2. At this concentration dry and fresh weight remained unaffected, but the length of seedlings decreased significantly (Table 1). At 100 and 300 µM concentrations of CdCl2 growth was unaffected. So these two concentrations showed no toxic effect on growth. In response to different concentrations of CdCl2, diosgenin production increased. At 300 µM concentration of CdCl2, maximum increase of diosgenin (40-fold) (P < 0.001) was observed (Fig. 1) without affecting the growth. In 100 and 500 µM concentrations of CdCl2 there were about 14-fold and 7-fold increases of diosgenin (P < 0.001), respectively. At 5 days after treatment of the seedlings, 400 and 800 μM concentrations of CoCl2 showed stimulatory effect only on seedling length, and at all of the concentrations tested fresh weight and dry weight decreased slightly although the decrease was not statistically significant (Table 2). So, it may be said that the treatment with all the three concentrations had no toxic effect on growth. In all the concentrations of CoCl2 diosgenin production increased significantly (Fig. 2). Maximum increase (about 41-fold) was found at 200 μM concentration (P < 0.001) without affecting the growth of the seedlings. In 400 and 800 μM concentrations the content increased about 25-fold (P < 0.001) and 10-fold (P < 0.05), respectively. But all of the concentrations of K2Cr2O7 (300 and 500 μM) and NiCl2 (200 and 400 μM) had inhibitory effect (P < 0.01) on diosgenin production (Figs. 3, 4).
Effect of methyl jasmonate
The production of diosgenin was elicited after treatment with methyl jasmonate (Fig. 5). A maximum increase (10.5-fold) (P < 0.001) was found at 100 µL L−1 concentration of MeJa. Then the diosgenin content decreased up to 1,000 µL L−1 concentration MeJa after which no significant change in diosgenin content was observed. MeJa has an integral role in the cascade of events that occur in the elicitation process, causing the activation of the genes for secondary metabolism either directly or indirectly. MeJa induces secondary metabolites formation in plants (De 2001). MeJa has been observed to increase anthocyanin accumulation in germinating soybean seedlings (Franceschi and Grimes 1991), Vitis vinifera suspension cultures (Curtin et al. 2003), Vaccinium pahalae cell cultures (Fang et al. 1999), Daucus carota cell culture (Sudha and Ravishankar 2003a, b), MeJa induced glycyrrhizin production in cultured Glycyrrhiza glabra cells (Shabani et al. 2009), production of shikonin in Onosma paniculatum cells (Ding et al. 2004), silymarin secretion in Silybum marianum cultures (Madrid and Corchete 2010), and benzophenanthridine alkaloid accumulation in Eschscholtzia californica suspension cultures (Cho et al. 2008). The positive effect of MeJa on ginsenoside production from ginseng cell suspension, hairy root and adventitious root cultures has been documented (Lu et al. 2001; Palazo’n et al. 2003; Choi et al. 2005; Bae et al. 2006; Kim et al. 2009). In addition, external application of MeJa enhanced accumulation of monomeric alkaloids in seedlings of Catharanthus roseus (Aerts et al. 1996), triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants (Mangas et al. 2006). The result of the present study shows that MeJa can be used as an elicitor for the induced production of diosgenin in the seedlings of T. foenum-graecum.
Effect of calcium
The process of elicitation activates various Ca2+- and calmodulin-dependent protein kinases by increasing the level of free Ca2+ in the cytoplasm and somehow triggers the cellular responses, which may include alterations in gene expression. The multiple Ca2+ mobilization pathways and release sites go some way in explaining how stimulus-specific Ca2+ signals may be generated. But there is still further complexity: different cell types may have different types of Ca2+ channels (Sudha and Ravishankar 2002). The effect of calcium on diosgenin production in seedlings of T. foenum-graecum is shown in Table 3. Treatment of seedlings with calcium depleted medium increased diosgenin production by 60% (P < 0.01). Twofold increase in the concentration of calcium in the medium (440 mg L−1) resulted in 54% (P < 0.01) decrease in diosgenin production. Addition of the calcium ionophore A23187, a compound known to increase the concentration of cytosolic Ca2+ (Reed and Lardy 1972) decreased diosgenin production in T. foenum-graecum seedlings, as compared to the control. It is reported that EGTA is a Ca2+ specific ligand, capable of reducing the availability of extracellular calcium (Mahady and Beecher 1994). Addition of EGTA, a calcium chelator, to the medium resulted in 80% (P < 0.01) increase in diosgenin production by the seedlings. To further test the role of extracellular Ca2+, varapamil and LaCl3 which are Ca2+ channel inhibitors which inhibit the flow of Ca2+ across the plasma membrane (Knight et al. 1992) were used. Treatment with all the concentrations of verapamil and LaCl3 resulted in increased production of diosgenin. Maximum increases in diosgenin production during verapamil and LaCl3 treatment were 159% (about 2.5-fold) (P < 0.001) and 602% (7-fold) (P < 0.001), respectively, which were seen at 3 mM concentration. Caffeine, a modulator of calcium release (Bolwell et al. 1991) had an inhibitory role on diosgenin production. Calcium ions seem to play a role in the control of the production of secondary metabolites but in a different manner depending on the species. It has stimulatory as well as inhibitory role in production of the secondary metabolites in plants. Calcium stimulated anthocyanin formation in Vitis vinifera cells (Vitrac et al. 2000), and Daucus carota cells (Sudha and Ravishankar 2003a, b). Production of shikonin from cell suspension cultures of Onosma paniculatum was promoted by a fungal elicitor extracted from Aspergillus oryzae. This was accompanied by a decrease in the intracellular concentration of Ca2+. Treatments which induce Ca2+-influx were found to suppress the elicitor promoted shikonin formation and agents that decrease the intracellular-free Ca2+ level were found to enhance shikonin biosynthesis even in the absence of elicitor (Ning et al. 1998). Calcium acted on Digitalis thapsi cultures by inhibiting cardenolide accumulation (Cacho et al. 1995). Calcium deprivation markedly enhanced guggulsterone accumulation in cell cultures of Commiphora wightii (Dass and Ramawat 2009). A similar effect has been reported for hecogenin production in callus cultures of Agave amaniensis (Indrayanta et al. 1996), and alkaloid production in cell suspensions cultures of Tabernaemontana divaricata (Sierra et al. 1991). The results of the present study suggest that production of diosgenin in T. foenum-graecum seedlings was inhibited by calcium. However, treatment with calcium chelator or Ca2+ channel inhibitors could be used to elicit diosgenin production in this plant.
References
Aerts RJ, Schafer A, Hesse M, Baumann TW, Slusarenk A (1996) Signalling molecules and the synthesis of alkaloids in Catharanthus roseus seedling. Phytochemistry 42:417–422
Aradhana M, Rao AC, Kale RK (1992) Diosgenin a growth stimulator of mammary gland of ovariectomized mouse. Indian J Exp Biol 30:367–370
Asolkar IV, Chadha YR (1979) Diosginin and other steroid drug precursors. Publication & Information Directorate, CSIR, New Delhi
Bae KH, Choi YE, Shin CG, Kim YY, Kim YS (2006) Enhanced ginsenoside productivity by combination of ethephon and methyl jasmonate in ginseng (Panax ginseng C.A. Meyer) adventitious root cultures. Biotechnol Lett 28:1163–1166
Bhavsar GC, Kapadia NS, Patel NM (1980) Studies of Trigonella foenum-graecum (Linn). Indian J Pharm sci 42:39–40
Bolwell GP, Coulson V, Rodgers MW, Murphy DL, Jones D (1991) Modulation of the elicitation response in cultred fresh bean cells and its implication for the mechanism of signal transduction. Phytochmistry 30:397–405
Cacho M, Moran M, Tarrago JF, Crochete P (1995) Calcium restriction induces cardenolide accumulation in cell suspension cultures of Digitalis thapsi L. Plant Cell Rep 14:786–789
Cheng MS, Wang QL, Tian Q, Song HY, Lin YX, Li Q, Xu X, Miao HD, Yao XS, Yang Z (2003) Total synthesis of methyl protodioscin: a potent agent with antitumour activity. J Org Chem 68:3658–3662
Cho HY, Son SY, Rhee HS, Yoon SH, Lee-Parsons CWT, Park JM (2008) Synergistic effects of sequential treatment with methyl jasmonate, salicylic acid and yeast extract on benzophenanthridine alkaloid accumulation and protein expression in Eschscholtzia californica suspension cultures. J Biotechnol 135:117–122
Choi DW, Jung JD, Ha YI, Park HW, In DS, Chung HJ, Liu JR (2005) Analysis of transcripts in methyl jasmonate-treated ginseng hairy roots to identify genes involved in the biosynthesis of ginsenosides and other secondary metabolites. Plant Cell Rep 23:557–566
Crochete MP, Jimenez MA, Moran M, Cacho M, Fernandez-Tarrago J (1991) Effect of calcium, manganese and lithium on growth and cardenolide content in cell suspension cultures of Digitalis thapsi. Plant Cell Rep 10:394–396
Curtin C, Zhang W, Franco C (2003) Manipulating anthocyanin composition in Vitis vinifera suspension cultures by elicitation with jasmonic acid and light irradiation. Biotechnol Lett 25:1131–1135
Dass S, Ramawat KG (2009) Calcium deprivation markedly enhances guggulsterone accumulation in cell cultures of Commiphora wightii. Curr Sci 96:1022–1024
De B (2001) Cell signalling and elicitator induced plant metabolite production. J Med Aromat plants 23:413–428
De Backer-Royer C, Vannereau A, Duret S, Villavigues R, Cosson L (1990) Action de metaux Lourds Cu et cd surdes cellules de Catharanthus Roseus cultivees in vitro adaptation et production alcaloidiqu. Les Colloq de I’INRA 51:247–249
De D, De B (2003) Effect of ethephon on antioxidant enzymes and diosgenin production in seedlings of Trigonella foenum-graecum. Food Chem 82:211–216
De D, De B (2005) Elicitation of diosgenin production in Dioscorea floribunda by ethylene generating agent. Fitoterapia 76:153–156
Ding J, Shi S, Jiang B, Yang Y, Huang J, Shen S, Xia K, Zhang J, Jiang X (2004) Effects of methyl jasmonate with indole-3-acetic acid and 6-benzylaminopurine on the secondary metabolism of cultured Onosoma paniculatum cells. In Vitro Cell Dev Biol—Plant 40:581–585
Evans WC (1996) Trease and evans pharmacognosy, 14th edn. WB Saunders, Philadalphia
Fang Y, Smith MAL, Pépin MF (1999) Effects of exogenous methyl jasmonate in elicited anthocyanin-producing cell cultures of ohelo (Vaccinium phalae). In Vitro Cell Dev Biol—Plant 35:106–113
Franceschi VR, Grimes HD (1991) Induction of soybean vegetative proteins and anthocyanins by low level atmospheric methyl jasmonate. Proc Natl Acad Sci USA 88:6745–6749
Gaisser S, Heide L (1996) Inhibition and regulation of shikonin biosynthesis in suspension cultures of Lithospermum. Phytochemistry 41:1065–1072
Guo H, Chang Z, Yang R, Gou D, Zeng J (1998) Anthraquinones from hairy root cultures of Cassia obtusifolia. Phytochemistry 49:1623–1625
Hardman R, Fazli FRY (1972) Labelled steroidal sapogenins and hydrocarbons from Trigonella foenum-graecum by acetate, mevalonate and cholesterol feeds to seeds. Planta Med 21:188–195
Indrayanta G, Utani M, Santosa MH, Syarani A (1994) In: Florey K (ed) Analytical profiles of drug substances, vol 23. Academic Press, New York
Indrayanta G, Utami W, Syahrani A (1996) Agave amaniensis Trel. and Nowell: in vitro culture and the production of phytosteroids. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 37, Medicinal and aromatic plants IX, Springer, pp 1–15
Kim D, Pederson H, Chin C (1991) Stimulation of berberine production of Thalictrum rugosum suspension cultures in response to addition of cupric sulfate. Biotechnol Lett 13:213–216
Kim OT, Bang KH, Kim YC, Hyun DY, Kim MY, Cha SW (2009) Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tissue Organ Cul 98:25–33
Knight MR, Smith SM, Trewavas AJ (1992) Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci 89:4962–4971
Lu MB, Wong HL, Teng WL (2001) Effects of elicitation on the production of saponin in cell culture of Panax ginseng. Plant Cell Rep 20:647–677
Madrid E, Corchete P (2010) Purificación Silymarin secretion and its elicitation by methyl jasmonate in cell cultures of Silybum marianum is mediated by phospholipase D-phosphatidic acid. J Exp Bot 61:747–754
Mahady GB, Beecher CWW (1994) Elicitor stimulated benzophenanthridine alkaloid biosynthesis in blood root suspension cultures is mediated by calcium. Phytochemistry 37:415–419
Mangas S, Bonfill M, Osuna L, Moyano E, Tortoriello J, Cusido RM M, Piñol T, Palazón J (2006) The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry 67:2041–2049
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissu culture. Physiol Plant 15:473–497
Ning W, Wang J-Xi, Liu Y-M, Li N, Cao R-Q (1998) The effects of CaSUP2+/SUP during the elicitation of shikonin derivatives in Onosma Paniculatum cells. In Vitro Cell Dev Biol–Plant 34:261–265
Ohlsson AB, Berglund T (1989) Effects of high MnSO4 levels on cardenolide accumulation by Digitalis lanata tissue cultures in light and darkness. J Plant Physiol 135:505–507
Ortuno A, Oncina R, Botia JM, Del Rio JA (1999) Regulation of the diosgenin expression in Trigonella foenum-graceum plants by different plant growth regulators. Food Chem 65:227–232
Palazo′n J, Cusido′ RM, Bonfill M, Mallol A, Moyano E, Morales C, Pin˜ol MT (2003) Elicitation of different Panax ginseng transformed root phenotypes for an improved ginsenoside production. Plant Physiol Biochem 41:1019–1025
Reed PW, Lardy HA (1972) A23187: a divalent cation ionophore. J Biol Chem 247:6970–6977
Roman ID, Thewles A, Coleman R (1995) Fractionation of livers following diosgenin treatment to elevate biliary cholesterol. Biochem Biophys Acta 1255:77–81
Sauvaire Y, Ribes G, Bacccou JC (1991) Implication of steroid saponins and sapogenins in the hypocholesterolemic effect of fenugreek. Lipids 26:191–197
Shabani L, Ehsanpour AA, Asghari G, Emami J (2009) Glycyrrhizin production by in vitro cultured Glycyrrhiza glabra elicited by methyl Jasmonate and salicylic acid Russian. J Plant Physiol 56:621–626
Sierra MI, Dagnino D, Van der Heijden R, Verpoorte R (1991) Influence of Ca on peroxidase activity and alkaloid formation in Tabernaemontana divaricata cell suspension cultures. In: Lobarzewski J, Greppin H, Panel C, Th Gaspar (eds) Biochemical molecular and physiological aspects of plant peroxidases. University of Geneva, Geneva, pp 295–304
Solichatun NN, Anggarwulan E (2008) The reserpine production and callus growth of indian snake root (Rauvolfia serpentina (L.) Benth. Ex Kurz) culture by addition of Cu2+. Biodiversitas 9:177–179
Sudha G, Ravishankar GA (2002) Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tissue Organ Cult 71:181–212
Sudha G, Ravishankar GA (2003a) The role of calcium channels in anthocyanin production in callus cultures of Daucus carota. Plant growth Regul 40:163–169
Sudha G, Ravishankar GA (2003b) Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta Physiol Plant 25:249–256
Sung SL, Huang YS (2000) Headspace ethylene accumulation on Stizolobium hassjoo hairy root culture producing 1–3, 4 dihydroxyphenylalanine. Biotechnol Lett 22:875–878
Vitrac X, Larronde F, Krisa S, Decendit A, Deffieux G, Merillon JM (2000) Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanin. Phytochemistry 53:659–665
Acknowledgments
The authors gratefully acknowledge financial assistance by Department of Science and Technology, Government of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Hajduch.
Rights and permissions
About this article
Cite this article
De, D., De, B. Elicitation of diosgenin production in Trigonella foenum-graecum L. seedlings by heavy metals and signaling molecules. Acta Physiol Plant 33, 1585–1590 (2011). https://doi.org/10.1007/s11738-010-0691-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0691-7