Abstract
This study analyzed the involvement of nitric oxide (NO) in the root lignification of soybean seedlings. To this end, changes in root cell viability; phenylalanine ammonia-lyase (PAL) and soluble and cell wall bound peroxidase (POD) activities and lignin and hydrogen peroxide (H2O2) contents of soybean roots treated with the NO-donor sodium nitroprusside (SNP) and its relationships with root growth were evaluated. Seedlings were cultivated in a nutrient solution supplemented with 5 to 1,000 μM SNP for 24 h. At an extremely low concentration (5 μM), SNP induced root growth and increased lignification and activities of related enzymes (PAL and cell wall-bound POD). At a high concentration (1,000 μM), SNP reduced root growth and lignification (PAL activity and H2O2 and lignin contents) and caused a loss of cell viability. Application of potassium ferrocyanide (an analog of SNP that cannot release NO) and PTIO (2-phenyl-4,4,5,5,-tetramethylimidazoleline-1-oxyl-3-oxide, a scavenger of NO) revealed that the inhibitory/stimulatory effects on root lignification may be due to NO itself. These results indicate that NO, depending on its concentration, may act as a stress factor, due to its toxic action, or as a signal molecule, inducing soybean root growth and lignification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research on nitric oxide (NO) has recently gained attention for its role in plant growth. As a relatively stable free-radical molecule, and due to its highly lipophilic nature, NO diffuses through membranes and may act as a synchronizing chemical messenger that is involved in many physiological processes in plants. Moreover, NO is generated in various plant species from sources such as nitrate reductase (NR), nitrite:NO-reductase, xanthine oxidoreductase (XOR), Arabidopsis protein AtNOS1, cytochrome P450, plasma membrane-bound enzyme, nitric oxide synthase (NOS), horseradish peroxidase, in addition to nonenzymatic sources. The functions postulated for NO in plants are related to various processes, including growth and development (seed germination, root organogenesis, biosynthesis of biologically active substances, leaf expansion, stomatal closure, senescence and programmed cell death), metabolism of cellular compartments (chlorophyll biosynthesis, effects on cytochrome c oxidase, alternative oxidase, catalase and aconitase), biochemical interactions (iron homeostasis, reactive oxygen species, ethylene, abscisic acid and mitogen-activated protein kinases), and both abiotic (drought, oxidative stress, salinity, high or low temperature and heavy metals) and biotic (hypersensitive reaction and systemic-acquired resistance) stress (Wojtaszek 2000; Arasimowicz and Floryszak-Wieczorek 2007; Neill et al. 2008).
Lignification, the process of sealing a plant cell by lignin deposition, is an important step during root growth. Lignin is a complex cell wall component comprised of phenolic heteropolymers that are covalently bound to both polysaccharides and proteins. It is mainly localized in the impermeable water transport conduits of the xylem and other supporting tissues. Lignin is synthesized by the phenylpropanoid pathway, which is involved in the synthesis of phenolic compounds as well as a wide range of secondary products in plants. The first rate-limiting enzyme of this pathway is phenylalanine ammonia-lyase (PAL), which, in association with other enzymes, leads to the synthesis of p-coumaryl-, coniferyl- and sinapyl alcohols. In the last step of the pathway, peroxidase (POD) catalyzes the monolignol polymerization, leading to the lignin synthesis. As the main structural component of secondarily thickened plant cell walls, lignin contributes to the compression strength of stems, where it imparts mechanical support and allows for the efficient conduction of water and solutes across long distances within the vascular systems (Boerjan et al. 2003).
Previous reports have suggested a differential effect of NO on lignification of Zinnia elegans xylem vessels. Sodium nitroprusside (SNP), a widely used NO-generating compound, inhibited POD activity, but had no effect on H2O2 production (Ferrer and Ros Barceló 1999). Since H2O2 is used by POD for lignification, a possible regulatory role of NO on xylem cell wall lignification has been proposed (Ros Barceló et al. 2002). The effect of NO on cell wall lignification indicates high complexity suggesting that it may be a key factor in mediating programmed cell death and lignification during xylem formation (Gabaldón et al. 2005). Moreover, NO may affect the activity of enzymes related to the lignin biosynthesis, in addition to increasing the transcription of their genes (Ros Barceló et al. 2004). Therefore, the aim of the present report was to investigate whether NO is associated with the lignification process in soybean roots. Measurements of POD and PAL activities, H2O2 levels and lignin content were obtained after treatment of soybean roots with the NO donor, SNP. The experimental conditions used in this study were chosen because growing roots exhibit high metabolic rates and lignification that starts during the early stages of seedling growth.
Materials and methods
General procedures
Soybean (Glycine max L. Merrill, cv BRS-133) seeds, surface-sterilized with 2% sodium hypochlorite for 2 min and rinsed extensively with deionized water, were dark-germinated (at 25°C) on two sheets of moistened filter paper. Twenty-five 3-day-old seedlings of uniform size were supported on an adjustable acrylic plate and dipped into a glass container (10 × 16 cm) filled with 200 ml of pH 6.0 half-strength Hoagland’s solution (Ferrarese et al. 2000a) with or without NO-donor SNP (5 to 1,000 μM). Where indicated, 5 μM of potassium ferrocyanide, an analog of SNP, or 100 μM of 2-phenyl-4,4,5,5,-tetramethylimidazoleline-1-oxyl-3-oxide (PTIO), a scavenger of NO, was added to the nutrient solution, at the same time as the addition of 5 μM SNP. The containers were kept in a growth chamber (25°C, 12/12 h light/dark photoperiod, irradiance of 280 μmol m−2 s−1) for 24 h. Roots were measured before incubation and at the end of the experiment to obtain the difference between them. Fresh root weight was determined immediately after incubation, and the dry weight was estimated after oven-drying the samples at 80°C until it reached a constant weight. SNP and PTIO were purchased from Sigma–Aldrich (St Louis, MO, USA), and all other reagents used were of the purest grade available or rather of the chromatographic grade.
Enzymatic assays
Phenylalanine ammonia-lyase (PAL) was extracted as described by Ferrarese et al. (2000b). Fresh roots (2 g) were ground at 4°C in 0.1 M sodium borate buffer (pH 8.8). Homogenates were centrifuged (2,200×g for 15 min) and the supernatant was used as an enzyme preparation. The reaction mixture (100 μmol sodium borate buffer, pH 8.7, and a suitable amount of enzyme extract in a final volume of 1.5 ml) was incubated (40°C, 5 min) for the PAL activity assay. To start the reaction, 15 μmol of l-phenylalanine was added; and it was stopped after 1 h by adding 50 μl of 5 N HCl. Samples were filtered through a 0.45 μm disposable syringe filter (Hamilton® Co., Nevada, USA) and analyzed (20 μl) with a Shimadzu® Liquid Chromatograph (Tokyo, Japan) equipped with an LC-10AD pump, a Rheodyne® injector, an SPD-10A UV detector, a CBM-101 Communications Bus Module and a Class-CR10 workstation system. A reversed-phase Shimpack® CLC-ODS column (150 × 4.6 mm, 5 μm) was used at 30°C, with an equivalent pre-column (10 × 4.6 mm). The mobile phase was methanol:water (70:30) with a flow rate of 0.5 ml min−1 for an isocratic run of 10 min. Absorption was measured at 275 nm. Data collection and integration were performed with Class-CR10 software (Shimadzu®, Tokyo, Japan). t-Cinnamate, the product of PAL, was identified by comparing its retention time with standard values. Parallel controls without l-phenylalanine or with t-cinnamate (added as an internal standard in the reaction mixture) were performed as previously described (Ferrarese et al. 2000b). PAL activity was expressed as μmol t-cinnamate h−1 g−1 fresh weight.
Peroxidase (POD) was extracted from fresh roots (0.5 g) with 67 mM phosphate buffer (5 ml, pH 7.0). The extract was centrifuged (2,200×g for 5 min at 4°C), and the supernatant was used to determine the activity of soluble POD. For cell wall-bound POD isolation, the pellet was washed with deionized water until no soluble POD activity was detected in the supernatant. The pellet was then incubated in 1 M NaCl (2 ml, 1 h, 4°C), and the homogenate was centrifuged (2,200×g for 5 min). The supernatant contained the cell wall-(ionically)-bound POD. Guaiacol-dependent activities of the soluble and cell wall-bound POD were determined according to dos Santos et al. (2004). The reaction mixture (3 ml) contained 25 mM sodium phosphate buffer, pH 6.8, 2.58 mM guaiacol and 10 mM H2O2. The reaction was started by adding the enzyme extract to the phosphate buffer. The guaiacol oxidation was followed for 5 min at 470 nm, and the enzyme activity was calculated from the extinction coefficient (25.5 mM−1 cm−1) for tetraguaiacol. The blank consisted of a reaction mixture without enzyme extract, and its absorbance was subtracted from the mixture with enzyme extract. POD activities were expressed as μmol tetraguaiacol min−1 g−1 fresh weight.
H2O2 quantification
Fresh roots (1 g) were homogenized in 3 ml of 0.1% trichloroacetic acid (Alexieva et al. 2001). The homogenate was centrifuged at 2,200×g for 20 min. An aliquot (0.5 ml) of supernatant was added to 0.5 ml of 10 mM phosphate buffer (pH 7.0) and 0.2 ml of 5 M potassium iodide. Absorbance was followed for 1 min at 390 nm. The blank consisted of a reaction mixture without potassium iodide, and its absorbance was subtracted from the mixture with H2O2 extract. H2O2 content was calculated using a standard curve prepared with known concentrations of H2O2. Results were expressed as μmol H2O2 g−1 fresh weight.
Lignin quantification
After the incubation period, dry roots (0.3 g) were homogenized in 50 mM potassium phosphate buffer (7 ml, pH 7.0) with a mortar and pestle and transferred into a centrifuge tube (Ferrarese et al. 2002). The pellet was centrifuged (1,400×g for 4 min) and washed by successive stirring and centrifugation as follows: twice with phosphate buffer pH 7.0 (7 ml); three times with 1% (v/v) Triton® X-100 in pH 7.0 buffer (7 ml); twice with 1 M NaCl in pH 7.0 buffer (7 ml); twice with distilled water (7 ml); and twice with acetone (5 ml). The pellet was dried in an oven (60°C for 24 h) and cooled down in a vacuum desiccator. The dry matter obtained was defined as the protein-free cell wall fraction. Next, all dry protein-free tissue was placed into a screw-cap centrifuge tube containing the reaction mixture (1.2 ml of thioglycolic acid plus 6 ml of 2 M HCl) and heated (95°C for 4 h). After cooling at room temperature, the sample was centrifuged (1,400×g for 5 min) and the supernatant was discarded. The pellet contained the complex lignin-thioglycolic acid (LTGA). The pellet was washed three times with distilled water (7 ml) and the LTGA extracted by shaking (30°C for 18 h at 115 oscillations min−1) in 0.5 M NaOH (6 ml). After centrifugation (1,400×g for 5 min), the supernatant was stored. The pellet was washed again with 0.5 M NaOH (3 ml) and mixed with the supernatant obtained earlier. The combined alkali extracts were acidified with concentrated HCl (1.8 ml). After precipitation (0°C for 4 h), LTGA was recovered by centrifugation (1,400×g for 5 min) and washed twice with distilled water (7 ml). The pellet was dried at 60°C, dissolved in 0.5 M NaOH, and diluted to yield an appropriate absorbance for spectrophotometric determination at 280 nm. Lignin was expressed as mg LTGA g−1 dry weight.
Determination of cell viability
After incubation, all seedlings were removed to determine the loss of cell viability by Evans blue (a nonpermeating dye) staining spectrophotometric assay (Baker and Mock 1994 ), with modifications. All freshly harvested roots (treated or untreated with SNP) were incubated for 15 min with 30 ml of 0.25% Evans blue solution. Further, the roots were washed in distilled water for 30 min to remove excess and unbound dye. Excised root tips (3 cm) were soaked in 3 ml of N,N-dimethylformamide for 50 min at room temperature to solubilize the dye bound to dead cells. The absorbance of released Evans blue was measured at 600 nm, using deionized water as a blank. The loss of cell viability was expressed as absorbance at 600 nm of treated roots in relation to untreated roots (control).
Statistical analysis
The experimental design was completely randomized, and each plot was represented by one glass container with 25 seedlings. Data are expressed as the mean of four or six independent experiments ± SE. The one-way variance analysis to test the significance of the observed differences was performed by Sisvar® package (Version 4.6, UFLA, Brazil). Differences between parameters were evaluated by the Scott–Knott test and P values <0.05 were considered to be statistically significant.
Results
Root growth
To evaluate the effects of NO on root growth, soybean seedlings were grown in nutrient solution containing 5–1,000 μM SNP for a short term (24 h) (Table 1). A significant effect on root lengths occurred after SNP treatments (compared to the control) and a dual response was noted. Low SNP concentrations (5 and 10 μM) significantly enhanced root lengths whereas high SNP concentrations (500 and 1,000 μM) inhibited it. Similarly, fresh root weights increased after 5 and 10 μM SNP and decreased after 500 and 1,000 μM SNP treatments. Dry weights increased in 5–100 μM SNP, but decreased after 1,000 μM SNP treatment.
Enzymatic activities
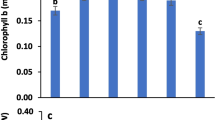
SNP-affected PAL activities were significantly different from those of controls (Fig. 1). At 5 μM, SNP increased the enzymatic activity by 32%, whereas at high concentration (1,000 μM) it reduced the activity by 25%. At 100–1,000 μM, SNP substantially increased (from 40 to 60%) soluble POD activities, relative to the control (Fig. 2a). At 5, 500 and 1,000 μM, SNP increased cell wall-bound POD activities by 24, 16 and 24%, respectively, relative to the control (Fig. 2b).
Lignification and cell viability
SNP-affected H2O2 contents were significantly different from controls (Fig. 3). The H2O2 contents decreased 69 and 60%, using 500 and 1,000 μM SNP, respectively, when compared to control. As a consequence of SNP exposure, lignin content increased by 30% at 5 μM, and decreased 5 and 20% after 500 and 1,000 μM, respectively, in comparison to the control (Fig. 4).
Figure 5 shows the loss of cell viability of roots as measured by Evans blue staining. The most significant amount of Evans blue uptake was detected after 100–1,000 μM of SNP treatments, representing 2.8- to 6.7-fold increases, respectively, relative to control roots. Evans blue uptake was not affected in the 5 or 10 μM SNP treatments.
Confirmation of the NO effects
Potassium ferrocyanide, an analog of SNP that cannot release NO, and PTIO, a known NO scavenger, were used to confirm the action of NO released by SNP. Since the highest concentration (1,000 μM) of SNP was phytotoxic to the roots (Fig. 5), the following experiments were designed to evaluate the effects of 5 μM SNP alone. Because 5 μM SNP, potassium ferrocyanide and PTIO did not change soluble POD activity, H2O2 content and cell viability (data not shown), the effects of SNP were shown alone for root growth, PAL, cell wall-bound POD activities and lignin contents.
Figure 6 reveals that the same concentration of potassium ferrocyanide caused effects opposite to those of the SNP treatments, reducing root length by 15%, PAL activity by 43% and lignin content by 46% (Fig. 6a, b, d). In contrast, the compound increased the cell wall-bound POD activity by 35% (Fig. 6c). Experiments with PTIO are shown in Fig. 7. When applied jointly with SNP, the NO scavenger also caused effects opposite to those of the SNP treatments. PTIO reduced root length, PAL activity and lignin content by 18, 48 and 54% (Fig. 7a, b, d), respectively, but did not change the cell wall-bound POD activity (Fig. 7c).
Changes in the root lengths (a), PAL activity (b), bound POD activity (c) and lignin contents (d) of soybean roots untreated (C control) and treated with 5 μM SNP or 5 μM potassium ferrocyanide (FE). Mean ± SE values (N = 6) followed by different letters are significantly different according to the Scott–Knott test (P < 0.05)
Changes in root lengths (a), PAL activity (b), bound POD activity (c) and lignin contents (d) of soybean roots untreated (C control) and treated with 5 μM SNP or with 5 μM SNP plus 5 μM PTIO. Mean ± SE values (N = 6) followed by different letters are significantly different according to the Scott–Knott test (P < 0.05)
Discussion
The main conclusion that can be drawn from the present study is that the NO-donor SNP has significant and dual effects on soybean root growth (Table 1). These findings are supported by evidences reported previously in various plant species. At extremely low concentrations (0.1 nM–0.1 μM), NO-releasing compounds (including SNP) induce maize root growth in a dose-dependent manner (Gouveia et al. 1997). At 100 μM, SNP inhibits hypocotyl growth in potato, lettuce and Arabidopsis (Beligni and Lamattina 2000). In cucumber, NO (10–100 μM SNP) mediates the auxin response, leading to adventitious root formation, whereas induction is reduced at 1,000 μM (Pagnussat et al. 2002). At 100–500 μM, SNP decreases primary root length and increases lateral root number in tomato seedlings (Correa-Aragunde et al. 2006). Incubation of soybean roots in solutions of SNP at very low concentrations (1–10 μM) promotes growth, whereas greater concentrations inhibit it (Hu et al. 2005). In general, the data in the present study corroborate those obtained in previous studies.
In the present study, SNP not only affected the root growth of soybean seedlings but also changed PAL and POD activities, H2O2 and lignin contents and cell viability (Figs. 1, 2, 3, 4, 5). These findings are of particular interest due to the role of lignification during root growth. Reports have demonstrated that NO changes PAL activity; for example, Guo et al. (2004) noted induction of PAL activity in wheat leaves in 500 and 2,000 μM SNP. Pelargonium leaves treated with 100 μM SNP showed a rapid stimulation of PAL activity (Floryszak-Wieczorek et al. 2006). In soybean cell culture, 500 μM SNP caused a rapid induction of PAL transcripts that was blocked by cPTIO (Delledonne et al. 1998). More recently, París et al. (2007) reported that in wounded potato leaflets, a dose–response was observed with the use of different SNP concentrations, and the maximal level of PAL transcript was detected in 50 and 100 μM SNP. We observed herein a remarkable increase in PAL activity that occurred after 5 μM SNP treatment; whereas a significant reduction was observed at 1,000 μM SNP (Fig. 1). Such dual responses of PAL activity have also been reported for wheat seedlings, i.e., increase in enzyme activity at 200 μM SNP and decrease at 2,000 μM SNP (Tian and Lei 2006). In addition, a low concentration (10 μM) of SNP also stimulated PAL activity in Taxus cells (Wang et al. 2006).
At high concentration (1,000 μM), SNP reduced PAL activity (Fig. 1) and lignin content (Fig. 4), whereas it significantly affected cell viability (Fig. 5) suggesting a cytotoxic effect of SNP. Cytotoxicity and cell death are known actions of NO, especially when it is present in high concentrations (Delledonne et al. 1998; Clarke et al. 2000; Zottini et al. 2002; Víteček et al. 2007; Kolodziejek et al. 2007). Our findings suggest that a concentration of 1,000 μM of SNP induced soybean root cell death, reducing its growth and lignification. Opposite effects were observed with the use of 5 μM SNP.
Plant cells possess highly efficient defense systems for elimination of the detrimental effect of oxidative stress, and guaiacol POD is one of the enzymes with anti-oxidative functions, removing excess H2O2. On its side, cell wall-bound POD plays an important role in cell wall lignification (Passardi et al. 2005). In this study, treatment with SNP significantly increased both soluble and cell wall-bound POD activities (Fig. 2) and reduced H2O2 content (Fig. 3). The anti-oxidative function of the guaiacol POD by removing a possible excess of H2O2, supports these findings. SNP enhanced POD activity in wheat seedlings (Tian and Lei 2006) and decreased H2O2 level in maize (Zea mays) leaves (Sang et al. 2008). In contrast, pure NO (55 μM) or SNP (5,000 μM) inhibited coniferyl alcohol POD activity in the xylem of Zinnia elegans, but had no effect on H2O2 production (Ferrer and Ros Barceló 1999). Similarly, Ros Barceló et al. (2002) verified that 5,000 μM SNP inhibited a basic peroxidase isoenzyme from Z. elegans assayed with syringaldazine. It is well known that plant cells contain multiple isoforms of POD, which are located in different cellular compartments and are expressed by distinct regulatory mechanisms in response to various environmental conditions (Passardi et al. 2005). It is possible that only specific isoforms are affected by NO, thus explaining the increase in POD activities in SNP-treated roots (Fig. 2).
In addition, NO itself appears to serve as an anti-oxidant agent, scavenging excess H2O2 to protect plant cells from toxic effects. NO also may react with superoxide anion (O2 −), leading to formation of the strong oxidant peroxynitrite anion (ONOO−), which is considered to be a cytotoxic agent. Finally, NO also reacts with atmospheric oxygen, forming other nitric oxides, including NO •2 , N2O3, NO3 − and NO2 − (Arasimowicz and Floryszak-Wieczorek 2007). In this way, subsequent deleterious effects may be induced by products of NO metabolism, causing stress, increasing POD activities and reducing root growth. Therefore, it is possible that the findings of our study may reflect the side effects of NO donor SNP. Opposite effects were verified at 5 μM of NO donor SNP, i.e., an increase in the lignification (Fig. 4) associated with similar increases in PAL activity (Fig. 1), cell wall-bound POD (Fig. 2b) and root growth (Table 1), without affecting the cellular viability (Fig. 5).
Girdling experiments evidence that NO may be a key factor in mediating programmed cell death and lignification during xylem formation, since it may affect enzymes related to lignin synthesis (Gabaldón et al. 2005). However, these effects may be rather complex, because NO acts on lignin biosynthetic enzymes by combining its effects on transcriptional regulation and substrate availability or by reacting directly with enzymes (Ferrer and Ros Barceló 1999; Delledonne et al. 2003). Dual (activating or inhibiting) actions of NO on the lignin biosynthetic pathway include interactions with metalloenzymes, such as cinnamate-4-hydroxylase (C4H) and POD, or with signaling enzymes, such as protein kinases and guanylyl cyclase (Ros Barceló et al. 2004; Neill et al. 2008). In fact, NO causes a non-competitive inhibition of C4H, which is a rate limiting step in the lignin synthesis (Enkhardt and Pommer 2000). This inhibition may accumulate cinnamic acid (substrate of C4H), which acts as a component of a regulatory feedback system that operates at the level of phenylpropanoid gene transcription or by inducing PAL inhibition (Bolwell et al. 1986). As noted here, the lignin content decreased (Fig. 4) concomitantly with a reduction in PAL activity (Fig. 1) and root growth (Table 1) at high concentrations of NO donor SNP. Decreases in PAL activity may reduce the production of phenolic acids and additional lignin synthesis of cell walls. A striking fact revealed by Gabaldón et al. (2005) is that the capacity for NO production and cell wall lignification are two inversely related metabolic processes during xylem differentiation of Zinnia elegans; the NO production increases inversely to the lignin content. This fact may explain the dual stimulatory/inhibitory effects of NO donor SNP on lignin content (Fig. 4).
To confirm the hypothesis that 5 μM SNP enhanced lignification and activities of related enzymes, we performed experiments with potassium ferrocyanide or PTIO. Treatment with 5 μM potassium ferrocyanide had opposite effects to SNP on root length, PAL activity and lignin content (Fig. 6). Since ferrocyanide is an analog of SNP that cannot release NO, these findings indicate that the observed effects may be due to NO itself. Interestingly, potassium ferrocyanide stimulated cell wall-bound POD activity in comparison to SNP treatment (Fig. 6c). Structurally, POD contains an iron (III) protoporphyrin IX (protohemin) prosthetic group located at the active site that is essential for the catalytic activity of the enzyme, which involves exchanges of electrons and protons (Veitch 2004). Thus, the simplest explanation for this stimulatory effect may be that ferrocyanide is a substrate for POD (Gazaryan and Lagrimini 1996). PTIO is a potent and specific scavenger of NO, and has been widely used to block NO-responsive events in plants. When PTIO was applied, the effects of SNP on root length, PAL activity and lignin content were abolished (Fig. 7). It is widely accepted that any biological effect of SNP solutions that is reversed by PTIO may be considered as being due to NO itself. Thus, our findings may reflect activity of the NO released by the SNP solutions. In contrast, the biological effect of SNP solutions that are not reversed by PTIO may be ascribed to other nitric oxides, such as N2O3 and NO2 − (Ros Barceló et al. 2004). Thus, the effects of an extremely high concentration (1,000 μM) of NO donor SNP on PAL activity and lignin content were not abolished by PTIO (data not shown).
In conclusion, our results indicate that an exogenous application of the NO donor SNP induces significant changes in soybean root growth and lignification. At high concentration, NO may act as a stress factor that induces reduction of root growth that is associated with cell death and which decreases PAL activity and lignin content. These effects may be attributable to the toxic action of NO itself or its metabolic products. At low concentration, NO induces root growth, lignification and activities of its related enzymes (PAL and cell wall-bound POD) due to its action as a signaling molecule.
Abbreviations
- H2O2 :
-
Hydrogen peroxide
- NO:
-
Nitric oxide
- PAL:
-
Phenylalanine ammonia-lyase
- POD:
-
Peroxidase
- PTIO:
-
2-Phenyl-4, 4, 5, 5,-tetramethylimidazoleline-1-oxyl-3-oxide
- ROS:
-
Reactive oxygen species
- SNP:
-
Sodium nitroprusside
References
Alexieva V, Sergiev I, Mapelli A, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Arasimowicz A, Floryszak-Wieczorek T (2007) Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci 172:876–887
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf discs assays using Evans blue. Plant Cell Tissue Organ Cult 39:7–12
Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210:215–221
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Ann Rev Plant Biol 54:519–546
Bolwell GP, Cramer CL, Lamb CJ, Schuch W, Dixon RA (1986) l-Phenylalanine ammonia-lyase from Phaseolus vulgaris: modulation of the levels of active enzyme by trans-cinnamic acid. Planta 169:97–107
Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ (2000) NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J 24:1–13
Correa-Aragunde N, Graziano M, Chevalier C, Lamattina L (2006) Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J Exp Bot 57:581–588
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394:585–588
Delledonne M, Polverari A, Murgia I (2003) The functions of nitric oxide-mediated signaling and changes in gene expression during the hypersensitive response. Antioxid Redox Signal 5:33–41
dos Santos W, Ferrarese MLL, Finger A, Teixeira ACN, Ferrarese-Filho O (2004) Lignification and related enzymes in Glycine max root growth-inhibition by ferulic acid. J Chem Ecol 30:1199–1208
Enkhardt U, Pommer U (2000) Influence of nitric oxide and nitrite on the activity of cinnamic acid 4-hydroxylase of Zea mays in vitro. J Appl Bot 74:151–154
Ferrarese MLL, Ferrarese-Filho O, Rodrigues JD (2000a) Ferulic acid uptake by soybean root in nutrient culture. Acta Physiol Plant 22:121–124
Ferrarese MLL, Rodrigues JD, Ferrarese-Filho O (2000b) Phenylalanine ammonia-lyase activity in soybean roots extract measured by reverse-phase high performance liquid chromatography. Plant Biol 2:152–153
Ferrarese MLL, Zottis A, Ferrarese-Filho O (2002) Protein-free lignin quantitation in soybean (Glycine max (L.) Merr.) roots. Biologia 57:541–543
Ferrer MA, Ros Barceló A (1999) Differential effects of nitric oxide on peroxidase and H2O2 production by the xylem of Zinnia elegans. Plant Cell Environ 22:891–897
Floryszak-Wieczorek J, Milczarek G, Arasimowicz M, Ciszewski A (2006) Do nitric oxide donors mimic endogenous NO-related response in plants? Planta 224:1363–1372
Gabaldón C, Ros LVG, Pedreño MA, Ros-Barceló A (2005) Nitric oxide production by the differentiating xylem of Zinnia elegans. New Phytol 165:5–7
Gazaryan IG, Lagrimini LM (1996) Purification and unusual kinetic properties of a tobacco anionic peroxidase. Phytochem 41:1029–1034
Gouveia CMCP, Souza IF, Magalhães ACN, Martins IS (1997) NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regul 21:183–187
Guo P, Cao Y, Li Z, Zhao B (2004) Role of endogenous nitric oxide burst in the resistance of wheat to stripe rust. Plant Cell Environ 27:473–477
Hu X, Neill SJ, Tang Z, Cai W (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137:663–670
Kolodziejek I, Koziol-Lipinska J, Waleza M, Korczynski J, Mostowska A (2007) Aspects of programmed cell death during early senescence of barley leaves: possible role of nitric oxide. Protoplasma 232:97–108
Neill SJ, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I (2008) Nitric oxide, stomatal closure and abiotic stress. J Exp Bot 59:165–176
Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129:954–956
París R, Lamattina L, Casalongué CA (2007) Nitric oxide promotes the wound-healing response of potato leaflets. Plant Physiol Biochem 45:80–86
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Ros Barceló A, Pomar F, Ferrer MA, Martinez P, Ballesta MC, Pedreño MA (2002) In situ characterization of a NO-sensitive peroxidase in the lignifying xylem of Zinnia elegans. Physiol Plant 114:33–40
Ros Barceló A, Gabaldón C, Pomar F (2004) Nitric oxide, peroxidase and lignification in higher plants. In: Magalhaes JR, Singh RP, Passos LP (eds) Nitric oxide signaling in higher plants. Studium Press, Houston, pp 277–308
Sang JR, Jiang MY, Lin F, Xu S, Zhang A, Tan M (2008) Nitric oxide reduces hydrogen peroxide accumulation involved in water stress-induced subcellular anti-oxidant defense in maize plants. J Integr Plant Biol 50:231–243
Tian X, Lei Y (2006) Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol Plant 50:775–778
Veitch NC (2004) Horseradish peroxidase: a modern view of a classic enzyme. Phytochem 65:249–259
Víteček J, Wünschová A, Petřek J, Adam V, Kizek R, Havel L (2007) Cell death induced by sodium nitroprusside and hydrogen peroxide in Tobacco By-2 cell suspension. Biol Plant 51:472–479
Wang JW, Zheng LP, Wu JI, Tand RX (2006) Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide 15:351–358
Wojtaszek P (2000) Nitric oxide in plants. To NO or not to NO. Phytochem 54:1–4
Zottini M, Formentin E, Scattolin M, Carimi F, Schiavo FL, Terzi M (2002) Nitric oxide affects plant mitochondrial functionality in vivo. FEBS Lett 515:75–78
Acknowledgments
Supported by the National Council for Scientific and Technological Development (CNPq) and Araucária Foundation (PR), Brazil. O. Ferrarese-Filho and M.L.L. Ferrarese are research fellows of CNPq. F.M.L.Z. Böhm is the recipient of a CNPq fellowship. The authors kindly thank Aparecida M.D. Ramos for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Weidner.
Rights and permissions
About this article
Cite this article
Böhm, F.M.L.Z., Ferrarese, M.L.L., Zanardo, D.I.L. et al. Nitric oxide affecting root growth, lignification and related enzymes in soybean seedlings. Acta Physiol Plant 32, 1039–1046 (2010). https://doi.org/10.1007/s11738-010-0494-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0494-x