Abstract
The present study was carried out to determine interactive and comparative effects of salinity and water stress on growth, proline accumulation, chlorophyll, carotenoid and macro nutrient content and antioxidative enzymes such as superoxide dismutase (SOD), guaiacol peroxidase (POX), and polyphenol oxidase (PPO) in hydroponically grown maize (Zea mays L.cv DKC647) plants. Plants were treated two salt (NaCl) concentrations and polyethylene glycol 6000 (PEG 6000) to create water stress. The results obtained from this experiment show that high salinity reduced growth through decreasing shoot and root dry and fresh weight, chlorophyll, and carotenoid content, but PEG treatment had no significant effect on this parameters. Under NaCl and PEG 6000 treatment, uptake and translocation of mineral nutrients changed drastically. The high presence of Na+ in nutrient solution affected considerably the plant nutritional requirement, especially influencing the uptake of Ca2+ and K+, which were restricted for competition. Proline accumulation, and SOD, POX and PPO activities were increased with the increasing intensity of NaCl stress, but PEG 6000 treatment in addition to NaCl had more significant effect on this enzyme activities. These results suggest that maize plants may be increased proline content to maintain osmotic adjustment and increased the activity of antioxidant enzymes to have a better protection against active oxygen species (AOS) under salt and water stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants are immobile and therefore unable to escape stressful environments. Abiotic stresses such as salt excess (especially NaCl) and drought are among the factors most limiting to plant productivity (Bohnert et al. 1995). In higher plants, exposure to abiotic stresses, e.g. water stress and high salinity, often results in different damages such as oxidative injury (Fadzilla et al. 1997).
Salinity is a major abiotic stress reducing the yield of a wide variety of crops all over the world (Ashraf and Foolad 2007). The deleterious effects of salinity on plant growth are associated with low osmotic potential of soil solution, nutritional imbalance, specific ion effect, or a combination of these factors (Ashraf and Haris 2004). On the other hand, water stress, one of the most common environmental limitations affecting growth and productivity of plants, causes many metabolic and oxidative changes in plants (Kalefetoğlu and Ekmekçi 2005).
Proline accumulation is one of the most frequently reported modifications induced by water and salt stresses in plants and is often considered to be involved in stress resistance mechanisms, although its precise role still remains a controversial subject (Kavi Kishor et al. 1995). Proline accumulation serves as a defense against osmotic challenge by acting as a compatible solute, and proline appears to be a preferred organic osmoticum in many plants. However, the significance of proline accumulation in osmotic adjustment is still debated and varies from species to species (Hoai and Shim 2003).
In addition to ionic and osmotic components, salt and water stress, like other abiotic stresses, also leads to oxidative stress through an increase in active oxygen species (AOS), such as superoxide (O •−2 ), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•) (Mittler 2002; Neill et al. 2002). AOS are highly reactive, and in the absence of any protective mechanism they can seriously disrupt normal metabolism through oxidative damage to lipids, protein, and nucleic acids (Rout and Shaw 2001). Fortunately, plants possess a number of antioxidant enzymes and antioxidants that protect them against the damaging effects of activated oxygen species (Dionisio-Sese and Tobita 1998). In plant cells, one such protective mechanism is an antioxidant system, composed of both non-enzymatic and enzymatic antioxidants (Foyer et al. 1994). Plants have evolved mechanisms to protect cellular and subcellular systems from the effects of these active oxygen radicals by using enzymes such as superoxide dismutase, catalase, peroxidase, glutathione reductase, polyphenol oxidase and non-enzymic ascorbate and glutathione (Agarwal and Pandey 2004).
Superoxide dismutases (SOD, EC 1.15.1.1) since discovered by McCord and Fridovich (1969) attracted the attention of many researchers because they are essential component in an organism’s defense mechanism (Badawi et al. 2004). The SOD (E.C. 1.15.1.1) is the first enzyme involved in the antioxidative process (Rubio et al. 2002). This enzyme converts superoxide radical to hydrogen peroxide (H2O2) and molecular oxygen (O2) (Mhadhbi et al 2004). Hydrogen peroxide can be removed by “non-specific” peroxidases (POD, E.C. 1.11.1.7) which use H2O2 as electron donor to metabolise phenolic compounds. These latter enzymes are ubiquitous and are involved in various processes such as cell growth control and tolerance to environmental stress (Quiroga et al. 2000). Polyphenol oxidase (PPO; E.C.1.10.3.1) is generally used as an indicator enzyme for the adequacy of heat treatment of fruit purees (Williams et al. 1986). However, it is also used as an indicator for the salinity stress. For example, Agarwal and Pandey (2004) in senna and Demir and Kocaliskan (2001) in bean seedlings studied the effect of salinity stress on this enzyme activity.
We hypothesized that increased accumulation of proline maintains osmotic adjustment, and increased activity of antioxidant enzymes, SOD, POX, and PPO, contributes to the protection of maize plants from salt and water stress. Therefore, the aim of this study was to evaluate the effects of salt and water stress on the accumulation level of proline, the activity of antioxidative enzymes; chlorophyll and carotenoid content, protein and macro nutrient content and dry and fresh weight in maize plants, to better understand salt and water stress effects and plant responses in maize.
Materials and methods
Plant material and growth conditions
The experiment was conducted under greenhouse conditions in Mugla (Turkey) with maize (Zea mays L. cv., DK 647 F1). The seeds of maize plants were grown in hydrophonic environment and under greenhouse conditions at 25/20°C, 16/8 h, 75 ± 5%, and 300 μmol m2 s−1 the mean temperature, day/night length, relative humidity, and photosynthetic photon flux density were maintained, respectively. There levels of salt (0) low-rate salt (5 dS m−1 NaCl), and high-rate salt (10 dS m−1) were formed. These three salt treatments were divided into two drought regimes as normal. In order to create water stress, a certain amount of PEG 6000 was used enough to form −1 MPa osmotic potential. For the control treatment, irrigation water and nutrition solution were used. The basic nutrient solution used in this experiment was a modified Hoagland and Arnon formulation. All chemicals used were of analytical grade, and composition of nutrient solution was (mg l−1): 270 N, 31 P, 234 K, 200 Ca, 64 S, 48 Mg, 2.8 Fe, 0.5 Mn, 0.5 B, 0.02 Cu, 0.05 Zn, and 0.01 Mo. The pH of the nutrient solution was adjusted each time to 6.5 with 0.1 mM KOH. Each treatment was replicated three times in a randomised block design and each replicate included six plants (i.e., 18 plants per treatment).
Thirty day after germination, different treatments were initiated. Treatments were: (i) control (C) plant receiving nutrient solution, (ii) low salinity treatment (C + SL): plant receiving nutrient solution plus 5 dS m−1 NaCl, (iii) high salinity treatment (C + SH): plant receiving nutrient solution plus 10 dS m−1 NaCl, (iv) PEG 6000 treatment (C + PEG): plant receiving nutrient solution plus PEG 6000 (a certain amount of PEG6000 is used enough to create −1 MPa osmotic potential), (v) low salinity and PEG treatment (SL + PEG): plant receiving nutrient solution plus 5 dS m−1 NaCl plus PEG 6000 (a certain amount of PEG 6000 was used enough to create −1 MPa osmotic potential), (vi) high salinity and PEG treatment (SH + PEG): plants receiving nutrient solution plus 10 dS m−1 NaCl plus PEG 6000 (a certain amount of PEG 6000 was used enough to create −1 MPa osmotic potential). Each treatment was replicated three times and each replicate included three pots (i.e. nine pots per treatment). Plants were harvested 90 days after seedling emergence.
Dry weight determination and macro nutrient analysis
Three randomly selected plants per replicate were divided into shoots and roots, and dried in a forced air oven at 70ºC for 2 days to determine dry weights. Chemical analyses were carried out on dry weight basis. Total N was determined in samples of 0.1 g dry weight using a Kjeldahl method. The dried samples were ground to powder using a pestle and mortar and stored in polyethylene bottles. Fresh samples were ashed at 550°C for 6 h. The white ash was taken up in 5 ml of 2 M hot HCl, filtered into a 50 ml volumetric flask and made up to 50 ml with distilled water. Na, Ca, K, Mg, and P were determined in these sample solutions. All macro elements were analysed using an ICP (Chapman and Pratt 1982).
Chlorophyll and carotenoid content
One plant per replicate was used for chlorophyll and carotenoid determination. Prior to extraction, fresh leaf samples were cleaned with deionized water to remove any surface contamination. Chlorophyll and carotenoid extractions was carried out on fresh fully expanded leaf material; 1 g leaf sample was ground in 90% acetone using a pestle and mortar. The absorbance was measured with a UV/Visible spectrophotometer (Pye Unicam SP6-550, UK), and chlorophyll concentrations were calculated using the equation proposed by Strain and Svec (1966).
where (A663) and (A645) represent absorbance values read at 663 and 645 nm wavelengths, respectively.
Proline content
Proline was determined according to the method described by Bates et al. (1973). Approximately 0.5 g of fresh plant material was homogenized in 10 ml of 3% aqueous sulfosalicyclic acid and filtered through Whatman’s No. 2 filter paper. Two millilitre of filtrate were mixed with 2 ml acid-ninhydrin and 2 ml of glacial acetic acid in a test tube. The mixture was placed in a water bath for 1 h at 100°C. The reaction mixture was extracted with 4 ml toluene, and the chromophore containing toluene was aspirated, cooled to room temperature, and the absorbance was measured at 520 nm with a Shimadzu UV 1601 spectrometer. Appropriate proline standards were included for calculation of proline in the sample.
Enzyme extraction and enzyme assays
Leaves (0.5 g) were homogenized in 50 mM sodium phosphate buffer (pH 7.0) containing 1% soluble polyvinyl pyrolidine (PVP). The homogenate was centrifuged at 20.000g for 15 min at 4°C and the supernatant used for assays of the activities of POX and SOD. The activity of SOD was assayed by monitoring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) (Beauchamp and Fridovich 1971). One unit of SOD was defined as the amount of enzyme necessary to inhibit the reduction of cytochrome c by 50%. The activity of POX was assayed by adding aliquot of the tissue extract (100 μl) to 3 ml of assay solution, consisting of 3 ml of reaction mixture containing 13 mM guaiacol, 5 mM H2O2, and 50 mM Na-phosphate (pH 6.5) (Chance and Maehly 1955). An increase of the optical density at 470 nm for 1 min at 25°C was recorded using a spectrophotometer. POD activity was expressed as change in absorbance min−1 mg−1 protein. The increase in A470 was measured for 3 min and activity expressed as ΔA470 min−1 mg−1 protein.
Polyphenol oxidase activity (PPO) was assayed with 4-methylcatechol as a substrate according to the method of Zauberman et al. (1991). Half gram of fresh leaf was ground with 10 ml of 0.1 mol/l sodium phosphate buffer (pH 6.8) and 0.2 g of polyvinylpyrrolidone (PVP, insoluble). After centrifugation at 19.000g for 20 min, the supernatant was collected as the crude enzyme extract. The assay of the enzyme activity was performed using 1 ml of 0.1 mol/l sodium phosphate buffer (pH 6.8), 0.5 ml of 100 mmol/l 4-methylcatechol, and 0.5 ml enzyme solution. The increase in absorbance at 410 nm at 25°C was recorded automatically for 5 min. One unit of enzyme activity was defined as an increase of 0.01 in absorbance per min per mg protein. Protein content in the enzyme extracts was determined according to Bradford (1976) using Bovine Serum Albumin (BSA) V as a standard.
Statistical analysis
The experiment was performed twice under the same environmental conditions. Statistical analysis (ANOVA) indicated that there were no significant differences in measurements between the two runs; data presented here are the averages of the two experiments. A two way analysis of variance was performed on all data and the LSD was calculated at P ≤ 0.05.
Results
Plant growth and macro nutrients
In this experiment, dry and fresh weights of both shoot and root were significantly inhibited by salt and water stress (Table 1). Reduction in total plant dry weights in high salinity (C + SH: plants grown in nutrient solution plus 10 dS m−1 NaCl) treatment was 52% compared to the control (plant grown nutrient solution), while it was 56% in high salinity and PEG 6000 (SH + PEG: plants grown nutrient solution plus 10 dS m−1 NaCl plus PEG 6000). Inhibition on plant growth was not significantly affected by PEG 6000 in addition to NaCl treatment, but water stress had been effective on root and shoot dry weights. The high concentrations of NaCl were more harmful than PEG 6000 in maize plants. Total dry weight decreased with increasing concentration of the osmotic agents, with a drastic effect at the highest NaCl concentration.
Effects of the NaCl and PEG 6000 treatments on macro nutrient uptake of maize are shown in Tables 2 and 3. The presence of NaCl in the rooting medium induced an important increase in Na+ concentration in roots and leaves. K+ concentration in the leaves and roots gradually decreased in response to NaCl but, in general, that was not significantly affected by the PEG 6000 treatment. This latter caused an important increase of the Na+ concentration of roots and leaves, with the highest concentrations. The Na+ contents significantly increased, whereas the K+ content decreased by the salt treatments. Potassium concentration of maize leaves was decreased by salinity while it was unchanged in only PEG 6000 treated plants.
The high presence of Na+ in the nutrient solution affected considerably the plant nutritional requirement, especially influencing the uptake of Ca2+, which was restricted for competition. As it can be seen from the Tables 2 and 3, there were significant reductions in NaCl and PEG 6000 treated maize plants calcium contents compared to the control plants. Salinity was more effective than water stress in reduction of calcium uptake.
In our experiment other macro nutrients uptake such as Mg and P decreased when stress conditions increased, indicating that salinity and water stress limited nutrient uptake. It was found that P, K, Mg, and Ca content of both shoots and roots were lowest in 10 dS m−1 NaCl plus PEG 6000 treatment; however, reductions in macro nutrients, except P, were not significant statistically to determine differences between the effects of salt and water stress.
Under non-saline conditions Na+/K+ and Ca2+/Na+ ratios (Table 4) were low and increased significantly with an increase in salinity in both leaves and roots in our research. Ratios of Na+/K+ and Na+/Ca2+ were higher in the salt treatment compared to the control, while water stress had not significant effect on the ion ratios. However, the ratio in leaves and roots significantly increased by the interactive effect of salinity and water stress.
Chlorophyll, carotenoid, and proline contents
The results obtained from this experiment show that high salinity and water stress enhanced proline content and reduced chlorophyll and carotenoid contents of leaves and indicated that the increases and decreases are more significant at 10 dS m−1 NaCl plus PEG 6000 treatment compare to the other treatments (Table 5).
Proline content was increased in the leaves of maize plants grown at high salinity (10 dS m−1 NaCl) and water stress (PEG 6000 treatment) compared to the unstressed control plants. The data in Table 5 show that 5 dS m−1 NaCl treatment caused an increase in proline content in the leaves of maize plants, but the increase was more significant at 10 dS m−1 NaCl plus PEG treatment.
Salinity stress reduced chlorophyll and carotenoid concentration of maize plants in our study. Amounts of photosynthetic pigment substances that we have determined after the NaCl and PEG treatments of the leaves of maize plants are given in Table 5. In the maize plants, the highest amounts of chlorophyll a and chlorophyll b were observed in the control group, while the lowest values were found in the high salinity (10 dS m−1 NaCl), low salinity (5 dS m−1 NaCl) plus PEG and high salinity (10 dS m−1 NaCl) plus PEG treatments. As for the amount of carotenoid are concerned, which is also an antioxidant, the highest values were observed in the control group of the maize plants, while the lowest values were found in 10 dS m−1 NaCl plus PEG treatment groups of maize plants.
Enzyme activities
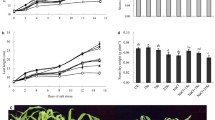
We report in Fig. 1, the effects of various salt concentrations and PEG 6000 on antioxidant enzyme activities such as SOD, POX, and PPO. The activity of SOD, which converts superoxide radical to H2O2, was higher in all leaf samples of plants under stress conditions compared to the control plants. SOD activity was significantly (P ≤ 0.05) higher in high salt and PEG 6000 treated plants than in the only high salt treated plants, and activity was more pronounced in plants under NaCl stress than in the PEG 6000 treated plants. However, high salinity and PEG 6000 treated plants maintained higher (P ≤ 0.05) SOD activity than all other treatments.
Effects of NaCl and PEG 6000 treatments on SOD, POX, and PPO activities of maize plants. Values followed by different letters in each column differ significantly (LSD test, P ≤ 0.05). C: Control, C + PEG: PEG 6000 treatment, C + SL: 5 dS m−1 NaCl treatment, SL + PEG: 5 dS m−1 NaCl treatment plus PEG 6000 treatment, C + SH: 10 dS m−1 NaCl treatment, SH + PEG: 10 dS m−1 NaCl plus PEG 6000 treatment
POX activity, which decomposes the H2O2 produced by SOD, also changed with respect to salinity and water stress. The activity of POX increased with increasing salinity and water stress. However, POX activity was not significantly (P ≤ 0.05) higher in water stress treated plants than in salinity treated. However, maize plants exhibited an increase in POX activity with increasing magnitude of salinity and water stress conditions.
The effect of increasing magnitude of salinity and water stress on PPO activity in the leaves of maize plants is shown in Fig. 1. PPO activity showed an increasing trend with the increase in the NaCl concentrations. The highest PPO activity was found in high salinity and PEG 6000 treatment, whereas the lowest was determined in control plants. As POX activity, salinity was more effective than water stress in increasing PPO activity. However, the interactive effects of salt and water stress found very significant on PPO activity.
In the present study, in general, salinity was more effective on SOD, POX, and PPO than water stress in maize plants. However, it was found that the interactive effects of salinity and water stress induced antioxidant enzymes such as SOD, POX, and PPO. Maize plants under salt and water stress had significantly higher activities of antioxidant enzymes compared to the control and salt or PEG 6000 treated plants.
Discussion
In this study, maize plant growth inhibition was more significant at water stress treatment in addition to NaCl. Similar result was reported in durum wheat plants by Almansouri et al. (1999). However, it seems difficult to determine differences between salt and water stress detrimental effects on plant growth, because PEG 6000 treatment had also been decreased maize plants growth by reduce plant dry and fresh weight. Although the cause of growth inhibition by salinity is still unclear, Munns (1993) suggested that growth is inhibited in two phases. In phase I, growth is reduced by decreased soil water availability, while in phase II, growth is inhibited by ion toxicity. Furthermore, some similar physiological mechanisms have been proposed by Neumann (1997), such as reduction in turgor pressure in expanding tissue, decreased photosynthesis and specific ion toxicity in growing cells.
The relationship between salinity and mineral nutrition of horticultural crops is extremely complex, and a complete understanding of the interactions involved would require the input from a multidisciplinary team of scientists (Grattan and Grieve 1999). Salinity can induce water stress as it increases the osmotic pressure of the soil solution (Greenway and Munns 1980). Under NaCl and PEG 6000 treatment uptake and translocation of macro nutrients changed drastically. The high concentration of Na+ in nutrient solution affected considerably the plant nutritional requirement, especially influencing the uptake of Ca2+ and K+, which were restricted for competition. Antagonistic relations between Na+ and K+ or negative effect of salinity on K+ uptake in different plants were reported by Carjaval et al. (2000) and Grieve and Poss (2000). Similar results were reported in sugar beet cultivars (Ghoulam et al. 2002), in rice (Lutts et al. 1996), and in Sorghum bicolor (Colmer et al. 1996). Under non-saline conditions Na+/K+ and Na+/Ca2+ ratios (Table 4) were low and increased significantly with an increase in salinity in both leaves and roots in our research. The decreases in K+ and Ca2+ concentration with salinity increase contributed to increased Na+/K+ and Na+/Ca2+ ratios (Table 4) and probably had an adverse effect on growth (Levitt 1980).
In this study, interactive effects of salinity and water stress on nutrient uptake of maize plants were investigated. It was observed that macro nutrient contents, such as K, Ca, P, and Mg, of both shoots and roots were decreased with the increasing intensity of stress conditions; however, there is no consensus about the regulation of nutrient uptake in response to salinity and water stress.
Chlorophyll contents have been suggested as one of the parameters of salt tolerance in crop plants (Sairam and Srivastava 2002), but it is also known that the amount of chlorophyll drops in high-concentration NaCl environments, CO2 fixation is inhibited and Hill reaction and electron transport system (E.T.S.) are negatively affected (Hopkins 1995). NaCl reduces chlorophyll content in crop plants such as broad bean (Gadallah 1999), cotton (Boyer 1965), and rice (Sultana et al. 1999). The fact that high NaCl concentrations and water stress have caused a decrease in the amounts of Chl a, Chl b, and carotenoids in our results is consistent with the literature reports.
Proline is known to occur widely in higher plants and normally accumulates in large quantities in response to environmental stresses (Kavi Kishore et al. 2005; Ashraf and Foolad 2007). In response to drought or salinity stress in plants, proline accumulation normally occurs in the cytosol where it contributes substantially to the cytoplasmic osmotic adjustment (Ashraf and Foolad 2007).
Osmotic adjustment through the accumulation of proline was also positively related to PEG concentration (Al-Khayri and Al-Bahrany 2004). In this respect, our research had significant results, because 10 dS m−1 NaCl plus PEG treatment caused higher increase than other treatments in proline content of maize plants. Accumulation of proline under stress in maize plants may be correlated with stress tolerance, because its concentration has been shown to be generally higher in stress-tolerant than in stress-sensitive plants.
Antioxidant enzymes play important roles in adaptation to stress conditions (Misra and Gupta 2006). Therefore, we hypothesized that increased activity of antioxidant enzymes, SOD, POX and PPO, contributes to the protection of maize plants from salt and water stress. As a result of this study, it was found that maize plants under salt and water stress had increased antioxidant enzymes activity such as SOD, POX, and PPO.
The activity of SOD enzyme, which converts superoxide radical to H2O2, was reported to increase under saline conditions in the maize and sunflower seedlings (Rios-Gonzalez et al. 2002), and cotton (Meloni et al. 2003). Many workers found positive correlation between water stress and the abundance of SOD in plants (McKersie et al. 1996; Badawi et al. 2004). Our results are in conformity with these results.
It is known that high NaCl (Meloni et al. 2003) and PEG treatment (Li and Staden 1998) induces POX activity in plants. The results of this study are similar to those reported results. High salinity and PEG 6000 treatment were more effective in increasing POX activity.
Demir and Kocaliskan (2001) found that in bean plants treated with NaCl PPO activity gradually increased as NaCl concentrations increased. Some previous studies have also shown that PPO activity is induced during water stress (Shivishankar 1988; English-Loeb et al. 1997). Similar results were obtained in this study.
In conclusion, the aim of this study was to evaluate the effects of salt and water stress on growth, accumulation of proline, the activity of antioxidative enzymes such as SOD, POX, and PPO, chlorophyll and carotenoid amount, macro nutrient content in maize plants, to better understand interactive effects of stress conditions on plant. The results obtained from this experiment show that high salinity and water stress enhanced proline content, antioxidant enzymes (SOD, POX, and PPO) activity, electrolyte leakage (EC) and reduced chlorophyll and carotenoid contents of leaves and salt stress being more effective than water stress on all these stress parameters. However, interactive effects of salinity and water stress on growth, proline accumulation, chlorophyll and carotenoid amount, macro nutrient content and antioxidative enzymes such as superoxide dismutase (SOD), guaiacol peroxidase (POX), and polyphenol oxidase (PPO) in hydroponically grown plants of maize (Zea mays L.) were more significant than only salt or water stress treatment. Certainly, the present study in Zea mays L. plants about its suffering from PEG 6000 and NaCl stresses is probably not sufficient. Nevertheless, these results suggest that increase in proline content in maize plants may be involved in the maintenance of osmotic adjustment and increased activity of antioxidants enzymes have a better protection against AOS under salt and water stress.
Abbreviations
- AOS:
-
Active oxygen species
- BSA:
-
Bovine serum albumin
- EC:
-
Electrical conductivity
- H2O2 :
-
Hydrogen peroxide
- MPa:
-
Megapascal
- NBT:
-
Nitroblue tetrazolium
- OH• :
-
Hydroxyl radical
- O •−2 :
-
Superoxide radical
- PEG 6000:
-
Polyethylene glycol 6000
- POD:
-
Guaiacol peroxidase
- PPO:
-
Polyphenol oxidase
- PVP:
-
Polyvinylpyrrolidone
- SOD:
-
Superoxide dismutase
References
Agarwal S, Pandey V (2004) Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol Plant 48(4):555–560
Al-Khayri JM, Al-Bahrany AM (2004) Growth, water content, and proline accumulation in drought-stressed callus of date palm. Biol Plant 48(1):105–108
Almansouri M, Kinet JM, Lutts S (1999) Compared effects of sudden and progressive impositions of salt stress in three durum wheat (Triticum durum Desf.) cultivars. J Plant Physiol 154:743–752
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Ku Tanaka, Ki Tanaka (2004) Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166:919–928
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase improved assays and an assay applicable to acrylamide gels. Anal Biochem 444:276–287
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099–1111
Boyer JS (1965) Effects of osmotic water stress on metabolic rates of cotton plants with open stomata. Plant Physiol 40:229–234
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carjaval M, Cerda A, Martinez V (2000) Modification of the response of saline stressed tomato plants by the correction of cation disorders. Plant Growth Reg 30:37–47
Chance B, Maehly C (1955) Assay of catalase and peroxidases. Methods Enzymol 11:764–775
Chapman HD, Pratt PF (1982) Methods of plant analysis. I. Methods of analysis for soils, plants and water. Chapman Publishers, Riverside, CA
Colmer TD, W-M FanT, Higashi RM, Lauchli A (1996) Interactive effects of Ca2+ and NaCl salinity on the ionic relations and proline accumulation in the primary root tip of Sorghum bicolor. Physiol Plant 97:421–424
Demir Y, Kocaliskan I (2001) Effects of NaCl and proline on polyphenol oxidase activity in bean seedlings. Biol Plant 44:607–609
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
English-Loeb G, Stout MJ, Duffey SS (1997) Drought stress in tomatoes: changes in plant chemistry and potential nonlinear consequences for insect herbivores. Oikos 79:456–468
Fadzilla NM, Finch RP, Burdon RH (1997) Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. J Exp Bot 48:325–331
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92:696–717
Gadallah MAA (1999) Effects of prolin and glycinebetaine on Vicia faba responses to salt stress. Biol Plant 42(2):249–257
Ghoulam C, Foursy A, Fores K (2002) Effects of salt stress on growth inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot 47:39–50
Grattan SR, Grieve CM (1999) Salinity-mineral nutrient relations in horticultural crops. Sci Hort 78:127–157
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Ann Rev Plant Physiol 31:149–190
Grieve CM, Poss JA (2000) Wheat response to interactive effects of boron and salinity. J Plant Nutr 23:1217–1226
Hoai NTT, Shim IS (2003) Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Reg 41:159–164
Hopkins WG (1995) Introduction to plant physiology. John Wiley & Sons, Inc., New York, USA, pp 115, 271, 438, 449
Kalefetoğlu T, Ekmekçi Y (2005) The effects of drought on plants and tolerance mechanisms (Review). GU J Sci 18(4):723–740
Kavi Kishor PB, Hong Z, Miao GH, Hu C-AA, Verma DPS (1995) Overexpression of D1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Kavi Kishore PB, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Levitt J (1980) Responses of plants to environmental stresses, vol 11. Academic Press, New York
Li L, Staden JV (1998) Effects of plant growth regulators on the antioxidant system in callus of two maize cultivars subjected to water stress. Plant Growth Reg 24:55–66
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
McCord JM, Fridovich I (1969) Superoxide dismutase, an enzymetic function for erythrocuprein (hemocuprein). J Biol Chem 60:6049–6055
McKersie DB, Stephen RB, Erni H, Oliviier L (1996) Water-deficit tolerance and field performance of transgenic Alfalfa overexpressing superoxide dismutase. Plant Physiol 111:1177–1181
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Mhadhbi H, Jebara M, Limam F, Aouani ME (2004) Rhizobial strain involvement in plant growth, nodule protein composition and antioxidant enzyme activities of chickpea rhizobia symbioses: modulation by salt stress. Plant Physiol Biochem 42:717–722
Misra N, Gupta AK (2006) Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. J Plant Physiol 163:11–18
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Munns R (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16:15–24
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Neumann P (1997) Salinity resistance and plant growth revisted. Plant Cell Environ 20:1193–1198
Quiroga M, Guerrero C, Botella MA, Barcelo AR, Medina MI, Alonso FJ (2000) A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol 122:1119–1127
Rios-Gonzalez K, Erdei L, Lips SH (2002) The activity of antioxidant enzymes in maize and sunflower seedlings as affected by salinity and different nitrogen sources. Plant Sci 162:923–930
Rout NP, Shaw BP (2001) Salt tolerance in aquatic macrophytes: possible involvement of the antioxidative enzymes. Plant Sci 160:415–423
Rubio MC, Gonzalez EM, Minchin FR, Webb KJ, Arrese-Igor C, Ramos J, Becana M (2002) Effects of water stress on antioxidant enzymes of leaves and nodules of transgenic alfalfa over expressing superoxide dismutases. Physiol Plant 115:531–540
Sairam RK, Srivastava GC (2002) Changes in antioxidant activity in subcellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Shivishankar S (1988) Polyphenol oxidase isozymes in coconut genotypes under water stress. Plant Physiol Biochem 15:87–91
Strain HH, Svec WA (1966) Extraction, separation, estimation and isolation of chlorophylls. In: Vernon LP, Seely GR (eds) The chlorophylls. Academic Press, New York, pp 21–66
Sultana N, Ikeda T, Itoh R (1999) Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot 42:211–220
Williams DC, Lim MH, Chen AO, Pangborn RM, Whitaker JR (1986) Blanching of vegetables for freezing—which indicator enzyme to choose. Food Technol 40:130–140
Zauberman G, Ronen R, Akerman M, Weksler A, Rot I, Fuchs Y (1991) Postharvest retention of the red color of litchi fruit pericarp. Sci Hort 47:89–97
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Rapacz.
Rights and permissions
About this article
Cite this article
Köşkeroğlu, S., Tuna, A.L. The investigation on accumulation levels of proline and stress parameters of the maize (Zea mays L.) plants under salt and water stress. Acta Physiol Plant 32, 541–549 (2010). https://doi.org/10.1007/s11738-009-0431-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0431-z