Abstract
Suspension cultures have been established from embryogenic tissues of Pinus nigra initiated from immature zygotic embryos. The growth of tissues in liquid medium has been influenced by initial tissue weight used for the establishment of the cultures as well as by genotype. In most of the cases initial tissue weight 0.5 g was insufficient and the cultures showed poor growth and later degeneration. Higher amount of initial tissues (1 or 2.5 g) was more efficient for the establishment and proliferation of somatic embryos in liquid medium. The growth of suspension cultures was also cell line dependent. Somatic embryo maturation in liquid medium was very limited and no plantlet regeneration occurred. Cotyledonary somatic embryos developed and produced emblings when the suspension was plated on filter paper discs and cultured on solid maturation medium. Based on our experiments we can state that the embryogenic tissues are able to grow and proliferate in liquid medium but somatic embryo maturation and plantlet regeneration occur only on solid medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic embryogenesis appears to be a promising technique for mass propagation of conifers. Initiation of somatic embryogenesis has been reported for many conifer species (Jain et al. 1995, 1999, 2005). For the initiation, immature and mature zygotic embryos as well as cotyledons dissected from emblings or seedlings have been used as explants. Culture of mentioned explants resulted in proliferation of embryogenic tissue containing bipolar early somatic embryos. For the majority of conifer species for the long-term cultivation solid media have been used.
The embryogenic tissues of conifers are capable of growth in liquid suspension cultures and also provide a reliable experimental model for the study of growth and development of somatic embryos (Krogstrup 1990). For conifers embryogenic suspension cultures have been established for several species, among other for Picea abies (Boulay et al. 1988; Find et al. 1998), Picea glauca (Hakman and Fowke 1987; Hakman and von Arnold 1988; Dong and Dunstan 1994), Picea sitchensis (Krogstrup 1990), Picea glauca-engelmannii and Picea mariana (Lulsdorf et al. 1992; Klimaszewska 1995), Pinus pinaster (Miguel et al. 2001), Pinus taeda (Silveira et al. 2003), Pinus strobus (Finer et al. 1989), Pseudotsuga menziesii (Gupta and Timmis 2005), Abies nordmanniana (Nørgaard et al. 1992).
The suspension liquid culture system allows the study of different physiological and biochemical characteristics such as growth parameters, protein and DNA synthesis (Dong and Dunstan 1994), plant growth regulators effect (Silveira et al. 2003), nutrient uptake and maturation capacity (Find et al. 1998), somatic embryo morphology and extracellular proteins (Mo et al. 1996). Recently suspension cultures have also been involved in genetic transformation studies (Wenck et al. 1999; Ishii 2002).
The establishment of suspension cultures and study of mentioned parameters in liquid culture system are important for large scale production of somatic embryos in bioreactors that would allow the automation of somatic embryo production and their commercial use in forest industry. Liquid cultures offer a number of technical advantages over solid cultures. Cultures grown in liquid medium have shown a faster rate of growth. Cultures are bathed in nutrients, which allows rapid uptake of nutrients by cells and speedy nutrient replacement at the cell surface by diffusion and movement from outlying liquid (Gupta and Timmis 2005).
Although establishment and growth of suspension cultures have been reported for many conifer species, somatic embryo maturation in liquid cultures is limited and plantlet production is less frequent than on solid media. Production of high quality mature somatic embryos in bioreactor was reported for Picea glauca (Attree et al. 1994). These somatic embryos were of excellent appearance with well-developed cotyledons and possessed high level of storage material and produced plantlets. Gorbatenko and Hakman (2001) also achieved Picea abies somatic embryo maturation in liquid medium. The somatic embryos obtained in their system could withstand high degree of desiccation and produce plantlets.
For P. nigra, embryogenic tissues have been initiated from immature zygotic embryos and maintained on solid media with capacity of maturation and plantlet regeneration (Salajova et al. 1999).
The aim of present study was the establishment and characterization of embryogenic suspension cultures, the study of growth parameters, somatic embryo morphology as well as maturation ability in liquid culture system. In detail we have studied the effect of initial tissue weight (inoculum) for the growth of suspension cultures of selected cell lines. The aim was also to test the maturation ability in liquid as well as on solid medium.
Materials and methods
Plant material
The embryogenic tissues have been initiated from immature zygotic embryos on DCR medium (Gupta and Durzan 1985) containing 2,4-D (9 μM), BA (2.2 μM), l-glutamine (50 mg l−1), enzymatic caseine hydrolysate (500 mg l−1). The medium was solidified with gelrite (0.3%) and also used for the long-term maintenance of embryogenic cultures. The cultures were kept in dark at 25°C in culture room. Transfer to new media occurred regularly in 2–3 weeks intervals. For the experiments, 15 cell lines have been selected (E 138, E 140, E 141, E 142, E 144, E 146, E 153, E 156, E 157, E 160, E 166, E 167, E 184, E 193, E 196).
Experiment 1
In this experiment we have focused on the investigation of the growth capacity of suspension cultures in dependence on the initial tissue weight (inoculum) used for the establishment of the cultures. Well-growing embryogenic tissues (0.5, 1.0, and 2.5 g) 7 days after subculture were resuspended in 25 ml of liquid DCR medium (the same composition as for initiation and long-term culture on solid media) and poured into 100 ml Erlenmeyer flasks. The suspensions have been cultured on rotary shaker (100–110 rpm) at 25°C in the dark. After 1 week of culture the first medium change occurred and in the following period fresh media were supplemented in 2 weeks intervals.
Measurement of sedimented cell volume (SCV)
The content of Erlenmeyer flasks was poured into 25 ml measuring cylinders and allowed to sediment for 30 min. The resulting SCV was used as non-destructive quantitative measurement of growth. The medium changes occurred by pipetting 3 ml of SCV into 22 ml of fresh liquid medium.
Experiment 2
This experiment aimed to investigate the effect of cell density on the growth of embryogenic suspension cultures. SCV of 3 and 5 ml were compared. The medium composition was the same as in experiment 1. Medium changes occurred in two weeks intervals by pipetting 5 ml of SCV to 20 ml of liquid medium to reach the final volume 25 ml.
The experiments 1 and 2 were repeated twice with 5–6 flasks for each cell line and treatment. For most of cell lines final evaluation of growth (SCV) has been done after 7 weeks of culture in liquid medium. However, in case of 0.5 g inoculum the evaluation was done after 3 weeks (cell lines E 166, E 167) or after 5 weeks of culture (E 146, E 160), respectively.
Morphological observation of somatic embryos
Two ml of the suspension was pipetted into Petri plate (6 cm in diameter) and investigated under inverted microscope Nikon TMS-F.
Experiment 3
The growth kinetic was followed in two cell lines (E 156, E 196). The SCV (initial 3 ml) was measured every second day for cell line E 196 and every third day for cell line E 156 during the period of 16 (E 196) or 27 (E 156) days. The measurements were repeated with 10–15 flasks for both cell lines at each day concerned.
Maturation of somatic embryos
For maturation experiments cell lines containing well organized somatic embryos (Salajova and Salaj 2005) have been selected (cell lines E 140, E 146, E 157, E 184, E193, E 196). Before transfer to maturation medium the suspension was washed three times by DCR medium containing half-strength macro- and microelements. After washing the suspension was allowed to sediment and 3 ml of SCV was pipetted into liquid maturation medium. The maturation medium was DCR (Gupta and Durzan 1985) basal medium containing abscisic acid (ABA, 94.69 μM), maltose (9%), enzymatic casein hydrolysate (500 mg l−1) and l-glutamine (50 mg l−1). The suspensions were cultured in darkness at 25°C on rotary shaker (100–110 rpm).
The maturation capacity of somatic embryos from liquid cultures was tested also on solid media. In this case the cells were allowed to sediment, the liquid was discarded and the sediment was washed three times with PGR (plant growth regulators)-free DCR medium with half concentration of macro- and microelements. After last wash the cells were allowed to sediment again and 2 ml SCV was pipetted to stacked filter paper discs to remove the liquid and the upper disc with cells was placed on solid medium. Two different maturation media were used. DCR medium with the same composition as the liquid medium but solidified with 0.4% gelrite was used for cell lines E 140, E 146, E 157. For cell lines E 184, E 193, E 196 the maturation medium was slightly modified as follows: the concentration of maltose was reduced from 9 to 6% and 1% gelrite was used. The experiments were repeated three times with 4–5 petri plates for each treatment and cell line.
Results
Experiment 1
The growth of embryogenic tissues of P. nigra in liquid medium is affected by the inoculum weight used for the establishment of cultures. Initial tissue weight 0.5 g was insufficient for further growth (proliferation) of tissue and many of cell lines failed to proliferate or showed minimum increase of SCV. Cell lines E 166 and E 167 terminated their growth after 3 weeks of culture in liquid medium and cell lines E 146 and E 160 after 5 weeks of cultivation. Better growth parameters have been obtained using 1 or 2.5 g tissue as inoculum, but among cell lines still relatively big differences have been observed (Table 1). Some cell lines showed intensive proliferation irrespective of the initial tissue weight, e.g. SCV increase in cell line E 142 is 639% during two weeks of sub-cultivation using 0.5 g inoculum. The differences between SCV increase at 0.5 g initial tissue weight and 1 or 2.5 g were statistically significant in most of cell lines.
Experiment 2
Cell lines that proliferated extremely slowly or failed to grow in liquid medium used for sub-cultivation of 3 ml SCV, were selected for the experiment 2 with the aim to improve their growth by increasing the initial SCV used for subculture to 5 ml. The cell density increase resulted in significant improvement of proliferation and almost all these “slowly” growing cell lines grew rapidly reaching higher SCV in comparison to initial cell density 3 ml (Table 2). Except the cell lines E 160, the differences in final SCV volume were statistically significant at level P 0.05.
Experiment 3
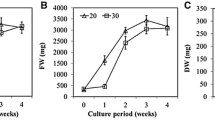
The growth kinetics has been followed in 27 days period fast growing cell line E 156. The SCV gradually increased and at day 18 reached the maximum volume 23.36 ml representing 778.66% form 3 ml SCV at the beginning of growth. This was followed by growth decline in later period and the cultures turned rapidly brown (Fig. 1). The growth in the cell line E 196 was even faster, which was measured every second day. This cell line showed a linear growth until the day 14. After this period the SCV decreased (Fig. 2). At the day 14, the SCV reached 26.82 ml, which represents 894% of the initial 3 ml SCV.
The structure of somatic embryos has not been profoundly affected by culture in liquid medium. Somatic embryos in liquid medium showed similar morphology as in tissues cultured on solidified media. Mostly bipolar structures reaching different organization of the “head” and suspensor cells among cell lines have been observed in suspension cultures (Fig. 3a–c).
Somatic embryo proliferation and maturation. a Well organized somatic embryo in liquid medium (cell line E 157). b Somatic embryo of cell line E 156 is characterized by less organized embryonal part as well as suspensor (liquid medium). c Poorly organized bipolar structures from suspension culture of cell line E 138. d Developing somatic embryo in liquid maturation medium; in this stage the somatic embryos stopped their development. e Cotyledonary somatic embryos matured on solid medium (cell line E 184). f Regenerated plantlets from cotyledonary embryos matured on solid medium (cell line E 184). Bars a–d 100 μm, e 500 mm, f 1 cm
Somatic embryo maturation
For the maturation experiments cell lines E 140, E 146, E 157, E 184, E 193, E 196 have been selected. These cell lines were characterized by the presence of somatic embryos with the ability to regenerate plantlets (maintenance and maturation on solid media).
Somatic embryo maturation of these cell lines in liquid medium was very limited. After 4 weeks of culture in liquid maturation medium the embryo “head” enlarged as a result of cell division and the suspensor cells were reduced in number (Fig. 3d). In this stage the somatic embryos stopped their development and necrotized. Fresh medium change has not contributed to further maturation of somatic embryos.
The maturation of somatic embryos, cultured in liquid medium, occurred on solid media. Cotyledonary somatic embryos developed in cell lines E 140, E 157, E 184, E 193 and E 196. Cell line E 146 produced no cotyledonary embryos, although previously plantlet regeneration occurred in this cell line on solid media (Salaj T, in preparation). Cell line E 157 produced few cotyledonary somatic embryos that developed no plantlets. Plantlet regeneration occurred in cell lines E 140, E 184, E 193 and E 196 (Table 3, Fig. 3e, f).
Discussion
Embryogenic suspension cultures of conifers, as a rule, are established by resuspending of vigorously growing tissue on solid medium. The initial tissue weight used for culture establishment varied among species and experimental design. It reaches from 100 mg for Picea sitchensis (Krogstrup 1990) to 400 mg for Picea glauca (Hakman and Fowke 1987), or 2 g for Pinus pinaster (Miguel et al. 2001), 2–3 g for Pseudotsuga menziesii (Gupta and Timmis 2005). In our experiments we were interested to test the inoculum weight in order to achieve vigorous growth of suspension cultures of Pinus nigra. Inoculum 0.5 g seems to be less suitable and better growth parameters have been achieved by increasing inoculum weight. For many species referred one or few cell lines are involved in experiments and experimental testing. We have included 12 cell lines to test the variable responses among them. This effect has been confirmed and P. nigra cell lines greatly differ in ability to grow in liquid medium. The growth parameters are also affected by initial cell density, since increase of SCV from 3 ml (in total volume 25 ml) to 5 ml (in total volume 25 ml) led to intensive proliferation and higher growth rate in “slowly” growing cell lines.
The embryogenic tissues of P. nigra belong to different cell lines and proliferate on solid media contain bipolar somatic embryos that differ in morphology and maturation capacity (Salajova and Salaj 2005). In suspension cultures very similar structures occurred and no remarkable differences in somatic embryo morphology have been observed in comparison with solid medium. Harvengt et al. (1997) referred more structured somatic embryos on solid media than in liquid suspension cultures. The growth of suspension cultures depends also on the structure of somatic embryos. Find et al. (1998) investigated the growth and maturation capacity of two different cell lines belonging to A and B type (Jalonen and von Arnold 1991). This investigation revealed major differences regarding growth and maturation in both cultures. The growth in term of SCV was equal for A and B type during the first 11 days of culture. After 11 days the A type continued to grow at the same rate while the B type entered the stationary phase without further increase of SCV.
The maturation of Pinus nigra somatic embryos in liquid medium was limited to the very early developmental stage without cotyledonary somatic embryo formation and plantlet regeneration. Although the proliferation of embryogenic tissues occurs in liquid media, the maturation is less frequent. Similarly as in Pinus nigra, Gösslova (2000) obtained only precotyledonary somatic embryos in liquid cultures of Picea abies. In the same species Gorbatenko and Hakman (2001) referred somatic embryo maturation and plantlet regeneration from suspension cultures.
Successful plantlet regeneration has been referred for some species when proliferation occurred in liquid medium and the maturation on solid media. By this cultivation method in Picea sitchensis large amount of plantlets have been produced and transferred to soil but the somatic embryo maturation was strongly dependent on the plating densities (Krogstrup 1990). Similarly, in Picea glauca plantlet regeneration from suspension cultures occurred on solid media (Hakman and von Arnold 1988). In our experiments cotyledonary somatic embryos developed only on solid maturation medium. The maturation was genotype dependent and out of tested six cell lines plantlet regeneration occurred only in four cell lines.
These results suggest that no general and repeatable methods for conifer somatic embryo production in liquid media have been elaborated so far. The experimental design includes proliferation in liquid medium and maturation as well as plantlet regeneration on solid medium. In several conifer species successful somatic embryo maturation occurred when some supports were used for embryogenic tissues cultivation. For Picea abies culture of embryogenic tissue on polypropylene membrane rafts resulted in synchronization of somatic embryo development and improved maturation (Vagner et al. 2005). For immobilization of embryogenic tissues polyuretan layers (Paques et al. 1995), cheese clothes (Boulay et al. 1988) or absorbent pads (Gupta and Timmis 2005) have also been used with success.
References
Attree SM, Pomeroy MK, Fowke LC (1994) Production of vigorous, desiccation tolerant white spruce (Picea glauca [Moench.] Voss.) synthetic seeds in a bioreactor. Plant Cell Rep 13:601–606
Boulay MP, Gupta PK, Krogstrup P, Durzan DJ (1988) Development of somatic embryos from cell suspension cultures of Norway spruce (Picea abies Karst.). Plant Cell Rep 7:134–137
Dong JZ, Dunstan D (1994) Growth parameters, protein and DNA synthesis of an embryogenic suspension culture of white spruce (Picea glauca). J Plant Physiol 144:201–208
Find J, Nørgaard JV, Krogstrup P (1998) Growth parameters, nutrient uptake and maturation capacity of two cell-lines of Norway spruce (Picea abies) in suspension culture. J Plant Physiol 152:510–517
Finer JJ, Kriebel HB, Becwar MR (1989) Initiation of embryogenic callus and suspension cultures of eastern white pine (Pinus strobus L.). Plant Cell Rep 8:203–206
Gorbatenko O, Hakman I (2001) Desiccation-tolerant somatic embryos of Norway spruce (Picea abies) can be produced in liquid cultures and regenerated into plantlets. Int J Plant Sci 162:1211–1218
Gösslova M (2000) Norway spruce (Picea abies L. Karst.): Somatic embryogenesis performed in liquid media and zygotic embryogenesis [in Czech]. Master Thesis, Faculty of Science, Charles University, Prag, pp 1–138
Gupta PK, Durzan DJ (1985) Shoot multiplication from mature trees of Douglas fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep 4:177–179
Gupta PK, Timmis R (2005) Mass propagation of conifer trees in liquid cultures—progress towards commercialization. In: Hvoslef-Eide AK, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, Dordrecht pp 389–402
Hakman I, Fowke LC (1987) An embryogenic cell suspension culture of Picea glauca (white spruce). Plant Cell Rep 6:20–22
Hakman I, von Arnold S (1988) Somatic embryogenesis and plant regeneration from suspension cultures of Picea glauca (white spruce). Physiol Plant 72:579–587
Harvengt L, Bercetche J, Paques M (1997) Somatic embryogenesis to amplify Pinus pinaster selected for traits with low heritability level. In: Joint meeting of the IUFRO working parties 2.04-7 and 2.04-06: somatic cell genetics and molecular genetics of trees, August 12–16, Quebec City, Canada 64–64
Ishii K (2002) Liquid culture and transformation of Hinoki Cypress (Chamaecyparis obtusa Sieb. Et Zucc.). J For Res 7:99–104
Jain MS, Gupta PK, Newton RJ (1995) Somatic embryogenesis in woody plants. In: Gymnosperms. vol 3. Kluwer, The Netherlands, pp 388
Jain MS, Gupta PK, Newton RJ (1999) Somatic embryogenesis in woody plants, vol 4. Kluwer, The Netherlands, pp 547
Jain MS, Gupta PK (2005) Protocol for somatic embryogenesis in woody plants. Springer, The Netherlands, pp 585
Jalonen P, von Arnold S (1991) Characterisation of embryogenic celllines of Picea abies in relation to their competence for maturation. Plant Cell Rep 10:384–387
Klimaszewska K (1995) Somatic embryogenesis in Picea mariana (Mill.). In: Jain MS, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants. Gymnosperms, vol 3. Kluwer, The Netherlands, pp 67–79
Krogstrup P (1990) Effect of culture densities on cell proliferation and regeneration from embryogenic cell suspensions of Picea sitchensis. Plant Sci 72:115–123
Lulsdorf MM, Tautorus TE, Kikcio SI, Dunstan DI (1992) Growth parameters of embryogenic suspension cultures of interior spruce (Picea glauca engelmannii complex) and black spruce (Picea mariana Mill.). Plant Sci 82:227–234
Miguel C, Tereso S, Marum L, Goncalves S, Oliveira M (2001) Embryogenic liquid cultures of maritime pine. In: COST 843-WG 2 Meeting, 22–25 September, Thessaloniki, pp 25–26
Mo LH, Egertsdotter U, von Arnold S (1996) Secretion of extracellular proteins by somatic embryos of Picea abies is dependent on embryo morphology. Ann Bot 77:143–152
Nørgaard JV, Baldursson S, Krogstrup P (1992) Establishment of embryogenic suspension cultures from embryogenic cultures of Abies nordmanniana, osmotic effects. In: Cost 87 Meeting—regeneration from suspension cultures, September 24–27, 1992, Drøbak, Norge 67–69
Paques M, Bercetche J, Palada M (1995) In: Jain MS, Gupta PK, Newton RJ (eds) History, molecular and biochemical aspects and application, vol 1. Kluwer, The Netherlands pp 399–414
Salajova T, Salaj J (2005) Somatic embryogenesis in Pinus nigra: embryogenic tissue initiation, maturation and regeneration ability of established cell lines. Biol Plant 49:333–339
Salajova T, Salaj J, Kormutak A (1999) Initiation of embryogenic tissues and plantlet regeneration from somatic embryos of Pinus nigra Arn. Plant Sci 145:33–40
Silveira V, Floh EIS, Handro W, Guerra MP (2003) Effect of plant growth regulators on the cellular growth and levels of intracellular protein, starch and polyamines in embryogenic suspension cultures of Pinus taeda. Plant Cell Tiss Org Cult 76:53–60
Wenck AR, Quinn M, Whetten RW, Pullman G, Sederoff R (1999) High-efficiency Agrobacterium mediated transformation of Norway spruce (Picea abies) and loblolly pine (Pinus taeda). Plant Mol Biol 39:407–416
Vágner M, Vondráková Z, Fischerová L, Opatrná J (2005) Norway spruce somatic embryogenesis: membrane rafts as a compromise between liquid and solidified media. In: Hvoslef Eide AK, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, Dordrecht, pp 295–302
Acknowledgments
The project was supported by the Slovak Grant Agency VEGA, Proj. No. 2-5022-25. The authors are grateful to Mrs. Margita Pavčírová for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Lojkowska.
Rights and permissions
About this article
Cite this article
Salaj, T., Blehová, A. & Salaj, J. Embryogenic suspension cultures of Pinus nigra Arn.: growth parameters and maturation ability. Acta Physiol Plant 29, 225–231 (2007). https://doi.org/10.1007/s11738-007-0028-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-007-0028-3