Abstract
Robot-assisted laparoscopic radical prostatectomy (RALP) relies heavily on the bedside assistant (BA). Currently, the relationship between BA experience and surgical outcomes in robotic surgery is not clear. We examined whether bedside assistant experience can significantly affect positive margin rate and peri-operative outcomes for RALP for surgeons within their learning curve. A retrospective cohort study of a single surgeon’s peri-operative outcomes during RALP was examined and compared with and without an experienced bedside assistant. Patient demographic data and peri-operative data, margin rate, and length of stay (LOS), were collected and analyzed. Univariate and multivariable analyses were performed to determine if expert BA was a predictor of post-operative outcomes. In total, 170 consecutive cases over three years were analyzed. 111 (65%) were performed without an expert BA. The two groups were not significantly different with regards patient demographics (p > 0.05). On univariate analysis, having an expert BA was associated with a significantly lower LOS (31 h ± 21 vs. 42 h ± 26, p = 0.004), EBL (296 ml ± 180 vs. 441 ml ± 305, p < 0.0001) and positive margin rate (20% vs. 37%, p = 0.03). Other surgical outcomes were comparable between groups. On multivariable analysis, expert BA remained a predictor of, EBL (B stat = − 146, 95% CI − 240 to − 52, p = 0.003) and positive margin rate (OR 0.4, 95% CI 0.2–0.96, p = 0.04). Our results demonstrate that the use of an expert BA may result in improved patient outcomes early in the learning curve of RALP, most notably, positive margin rate and estimated blood loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its introduction in 2001, robot-assisted laparoscopic radical prostatectomy (RALP) has become the most common approach for surgical treatment of localized prostate cancer, with over 80% of radical prostatectomies in the United States performed using the robotic approach [1, 2]. Robotic surgery is unique when compared to open surgery in that the surgeon and assistant are physically separated: the surgeon is at the robotic console while the surgical assistant (often called the bedside assistant) remains sterile at the bedside. A skillful bedside assistant (BA) must be adept in laparoscopy, possess good visual spatial skills, and understand the anatomy and steps of the operation. In addition to simply passing suture, clips and exchanging instruments, the BA assistant can help retract tissue and suction blood, thus optimizing visualization of the surgical field. In addition, due to the separation of the surgeon and bedside assistant, the operating surgeon cannot physically correct the bedside assistant, making the operating surgeon more reliant on the BA’s intuition and initiative to allow for rapid and fluid adjustments.
The relationship between increased surgical volume and improved outcomes has been well studied and reported in radical prostatectomy [3]. The improvement in outcomes that occurs with increased case volume has been termed the learning curve, or the time it takes to master a surgery. While the learning curve for RALP varies from surgeon to surgeon, it has been extensively reported and has been proposed to exist between 150 and 300 cases [3,4,5]. This is quite high considering that more than 80% of urologists in the USA perform less than 10 prostatectomies per year. These urologists also account for approximately 40% of the total number of prostatectomies performed [6]. Therefore, for the majority of urologists, many years will be spent in their learning curve. Interestingly, the majority of studies have shown no benefit in terms of the quality/experience of the bedside assistant as they pertain to surgical outcomes [7, 8], although a few showed an improvement in operative time [9, 10]. However, these studies examined surgeons who were well beyond their learning curve.
We sought to examine the relationship between bedside assistant experience and perioperative outcomes in patients who underwent RALP by a surgeon still in their learning curve, starting with the first case performed in clinical practice.
Methods
Participant selection
Approval was gained from the Veterans Affairs Hospital for a retrospective review of all RALPs performed by a single surgeon from July 2013 to November 2015. The series began with the first RALP performed by the surgeon after fellowship and culminated after 3 years (2015). All RALP were completed in a trans-peritoneal posterior approach and had a bilateral lymph node dissection performed when clinically indicated. Patients were included if clinical staging patients were cT3b or less. The bedside assistants utilized at our institution were divided into two groups: a non-expert group which included resident trainees in their second and third years of training or physician assistants without formal laparoscopic or bedside assistant training. Expert assistants included physician assistants who had completed a formal training program (UTSW Urology PA residency) or cRNFAs from a contracted group who completed a formal hands-on and didactic bedside assistant program followed by a period of apprenticeship. The decision of when an expert BA was utilized for a case was based on the expert BA’s availability and not at the discretion of the operating surgeon. Expert BAs became more widely available in years 2014 and 2015.
Data collection
Patient demographic data, case number, date of surgery, body mass index (BMI), race and preoperative tumor characteristics including preoperative PSA, prostate size and biopsy-grade group were collected.
Perioperative outcomes including estimated blood loss (EBL), length of stay in hours (LOS) and console time (defined as from the time the robot was docked to until it was undocked) were collected. Pathologic stage, grade group and positive margin status were also collected. The assignment of bedside assistant to a case was dependent on the availability of assistants and not under the direct control of the operating surgeon.
Statistical analysis
The primary outcome was positive margin status. Secondary outcomes included console time, EBL and LOS. The cohort was stratified based on expert bedside assistant (yes/no) present at case. Descriptive statistics were performed on patient demographic, pre-, peri- and post-operative variables. Comparisons between groups were made using Mann–Whitney U test, Analysis of variance (ANOVA), independent T test and chi-squared test were performed as appropriate. Multivariable regression analysis was performed for primary and secondary outcomes using case number (expressed as quartiles), expert assistant, pre-operative PSA, prostate size, race, patient BMI, RP specimen grade group and final pathological stage as predictors in the model. Logistic regression was used for binary outcomes (positive margin status) and linear regression for continuous variables (EBL, LOS and console time). Statistical analyses were performed using SPSS v.25 (IBM, NY, USA). The p value for significance was set to < 0.05. Tests were two-tailed unless otherwise stated.
Results
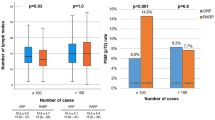
In total, 170 cases were performed during the study period, of which 111 (65%) were performed with a novice bedside assistant and 59 (35%) were performed with an expert bedside assistant. When stratified by expert bedside assistant, a greater percentage of surgeries performed in 2013 had non-expert bedside assistants compared to expert assistants (20% vs. 8%, p = 0.007). Otherwise the two groups were similar with regard to BMI, race, pre-operative PSA, prostate size and TRUS biopsy-grade group (Table 1, p > 0.05 for all). The mean pre-operative PSA values were 10.4 (± 8.8) and 10.6 (± 11.5) for non-expert and expert assistants, respectively (p = 0.92). Mean prostate size was 44.9 g (± 21.2) and 43.5 g (± 22.0) for non-expert and expert assistants, respectively (p = 0.67). Grade group 2 was the most common biopsy pathology with 51/111 (46%) and 29/59 (50%) for non-expert and expert assistants, respectively (p = 0.96). All baseline demographics are reported in Table 1.
Univariate analysis of peri- and post-operative outcomes for RALP, stratified by expert group is reported in Table 2. Mean robotic console time was 226 (± 70.7) min and 210 (± 46.8) min for non-expert and expert groups, respectively (p = 0.1). Surgeries that had an experienced assistant had significantly lower percentage of positive margins 12/59 (20%) vs. 41/111 (37%) (p = 0.03), estimated blood loss 296 ml (± 180) vs. 441 ml (± 305) (p < 0.0001) and shorter length of stay 31 h (± 21) vs. 42 h (± 26) (p = 0.004). There were no significant differences in final ISUP-grade group (p = 0.87) or final tumor stage (p = 0.82) between expert and non-expert assistants (Table 2).
Results of the multivariate analysis predicting surgical outcomes are presented in Tables 3, 4, 5, 6. An expert assistant was not a significant predictor of console time (Table 3). However, BMI and increasing case number were significant predictors of console time, with each 1 point increase in BMI being associated with almost 3 min more of console time (p = 0.002). Second, third and fourth quartile case numbers all had significantly shorter console times than cases completed in the first quartile (p < 0.0001 for all, Table 3). Surgeries performed with expert assistants had significantly less blood loss (B stat = − 146.3, 95% CI − 240.3, − 52.3, p = 0.003) compared to surgeries with non-expert assistants (Table 4). Case number was not a significant predictor of EBL (Table 4, p > 0.05 for all). Using an expert assistant was not a significant predictor of hospital LOS (B stat = − 5.2, 95% CI − 12.7, 2.3, p = 0.2, Table 5). Increasing case number was significantly associated with decreased length of stay (p < 0.05 for all, Table 5).
The use of an expert assistant was a significant predictor of positive margin status (Table 6). The use of an expert assistant was associated with a 60% decrease in positive margin rates than when surgery was performed with a non-expert assistant (OR 0.4, 95% CI 0.2–0.96, p = 0.04, Table 6). PSA at diagnosis and prostate size were also significant predictors of positive margin rate (Table 6). Increasing surgeon case number was not associated with decreased positive margin rate.
Discussion
This study is the first to show that the bedside assistant’s level of experience can affect the oncologic and perioperative outcomes for surgeons still in their learning for RALP. We found that using an expert assistant resulted in 60% decrease in positive margins (Table 6) and almost 150 ml less blood loss per case (Table 4) on multivariable analyses compared to using a non-expert bedside assistant. The implications for this are significant.
It has been well established that surgical margins after RALP are one of the biggest risk factors for recurrence and, therefore, may have significant downstream affects to the patient. Patients with positive margins are more likely to go on to radiation treatment, incurring further morbidity, possible secondary malignancies and lead to significant increases in cost [11]. Furthermore, salvage radiation can also lead to worsening erectile dysfunction and incontinence, negatively affecting the patient’s quality of life [11]. Given that majority of practicing urologists perform 10 or less RALPs per year [1, 6, 12], most urologists remain in their learning curve for the first 10 years of practice, with some lower volume surgeons never moving beyond their learning curve. This number is not insignificant, representing thousands of urologists within the United States [13]. Therefore, these findings may be applicable to the majority of practicing urologists performing RALPs in the United States.
While the Da Vinci robotic system has allowed for improved visualization, motion scaling (fine motor movements) and increased dexterity, robotic surgeons remain reliant on the bedside assistant to maintain an appropriate visual field during surgery. We hypothesize the expert assistant contributed to the improved perioperative outcomes in 3 important ways. First, because there is no tactile feedback, visual feedback is essential for robotic surgeons [14]. During radical prostatectomy, significant bleeding can occur that may obscure visualization of important structures, resulting in sub-optimal identification of tissue planes. The expert BA can optimize visualization, resulting in lower surgical margin rates. Second, the expert assistants likely provide more optimal retraction of tissue allowing for improved visualization and dissection of correct tissue planes. As they are familiar with the steps of the procedure, they recognize the appropriate structures requiring visualization and can make appropriate adjustments [15]. Novice assistants, on the other hand, would not yet have acquired this knowledge, and may retract the wrong areas or retract with too little or too much force, resulting in sub-optimal visualization, bleeding and/or injury to tissue. Third, the expert beside assistant may be able to offer advice and/or tips to the less experienced surgeon to help them through difficult or uncertain portions of the surgery. Thiel et al. [16] discussed the importance of bedside assistant’s performance on patient outcomes. Indeed, studies have reported complications directly caused by the BA, such as aortic injuries [17] and lost needles, during minimally invasive surgery [18], which can lead to significant cost and morbidity to the patient. Despite this hypothesized benefits of having a skilled BA, most studies to date have failed to show statistically significant differences between experienced and non-experienced assistants for outcomes other than operative time; however, these studies had 2 important differences: (1) the surgeons were past their learning curve and (2) the definition of expert BA used would not have met our criteria.
Nayyar et al. [10] performed a retrospective analysis of 222 urologic robotic procedures performed at a single tertiary care center by two experienced robotic surgeons and two bedside assistants. However, the definition of expert and novice differed from ours in that they assumed that the bedside assistants were novices in the first half of cases and experienced for the second half of cases. This study was also limited by having two different surgeons performing 5 different types of surgeries and the assumption that the bedside assistant was inexperienced at the beginning of the study, without any formal training to define the difference between and novice and expert. Despite this, they found that OR time was significantly reduced by 13.7 min (p = 0.001) and there was trend for decreased blood loss with expertise (p = 0.057) [10]. Furthermore, details of the bedside assistants prior training are not given, it is simply stated that they are usually second- or final-year residents.
Mitsinikos et al. published a retrospective study examining the relationship between bedside assistant experience and operative outcomes in robotic partial nephrectomy [9]. They included 18 surgeons from teaching and non-teaching hospitals. Bedsides, assistants were either residents (teaching) or attending physicians (non-teaching). They also reported a decrease in hospital length of stay in surgeries that had an experienced bedside assistant (1 day shorter, p = 0.001) and decreased operative time by 16 min (p = 0.011) [9]. They hypothesized the difference in length of stay was likely due to different post-operative pathways as the surgeries were completed by different surgeons at different hospitals. This differs from our study, which was performed at the same hospital with the same post-operative pathway and the same surgeon. Two additional studies examining the relationship between bedside assistant experience and surgical outcomes have been looked at and did not find any significant difference in surgical margin rates or operative time in robotic partial nephrectomy [8] or laparoscopic radical prostatectomy [7]. However, it is important to note that these are very different surgeries from RALP, and the role of the bedside assistant in each is significantly different.
Most similar to our study, Cimen et al. [19] examined the relationship between bedside assistant experience and perioperative and oncologic outcomes for surgeons at the early part of the learning curve in RALP. In this study, the first 20 cases of two different surgeons at the beginning of their robotic experience were compared. One surgeon used a novice bedside assistant and the second surgeon used an experienced bedside assistant. They found no significant difference in surgical margin rates, EBL or complication rates. However, they did find shorter operative time with the use of an experienced bedside assistant [19]. This study was small, with only 36 patients included in final analysis. In addition, as this study used two different surgeons, and baseline differences in performance between the two surgeons were not presented and could have influenced oncological and perioperative outcomes that may have masked the effects of the bedside assistant.
There are several limitations worth discussing in this study. First, as we only examined a single surgeon during the early phase of their learning curve, it is unknown whether these results could be applied to other surgeons also in the early phase of their learning curve. However, utilizing a single surgeon’s experience allowed for us to control for the variability of surgeon skill that was a limitation in many other studies. It is likely that an expert BA could provide benefit given the reasons hypothesized previously. It is further unknown whether our results could be applied to experienced surgeons no longer in their learning curve. Experienced surgeons may be better at directing novice assistants how to help them retract tissue and may likely not need their input regarding tissue plains, thereby negating some of the benefits of an experienced bedside assistant. Indeed, the previous discussed studies have alluded to this hypothesis [7,8,9]. We also did not examine longer-term outcomes, such as erectile dysfunction and incontinence rates, which could be affected by bedside assistant experience as well. The ability for centers to provide “expert” assistance is also not universal. In many academic centers, the junior residents provide this assistance and based on our definition, would not be considered experts. However, given our results, one might advocate that centers provide more experienced residents, fellows or physician assistants to assist junior surgeons who are early in their learning curve. Finally, we did not assess costs in our study. Hiring an expert BA could result in higher health care costs than utilizing residents or other non-expert assistants, but this would be easily offset by the downstream treatment needed given a significantly higher positive margin rate.
While not the focus of this study, these results may also support the idea that a standardized evaluation criteria designed to identify the expertise of a BA could be beneficial. However, few training curricula have been reported in the literature to date [16, 20], and training is not standardized. With much emphasis placed on the skill of the operating surgeon [21], it makes sense that the bedside assistant in robotic surgery also be the focus of improvement training.
Conclusion
The use of an experienced bedside assistant during RALP may be associated with a lower rate of positive margins and decreased blood loss for surgeons still in their learning curve. Because the majority of surgeons performing RALP in the United States will continue in their learning curve for many years, institutions should consider the potential benefits of providing expert BA for these surgeons. Larger studies are needed to determine if these findings are broadly applicable to other surgeons and other robotic-assisted surgeries.
References
Lowrance WT, Eastham JA, Savage C et al (2012) Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol 187(6):2087–2093. https://doi.org/10.1016/j.juro.2012.01.061
Tsui C, Klein R, Garabrant M (2013) Minimally invasive surgery: National trends in adoption and future directions for hospital strategy. Surg Endosc 27(7):2253–2257. https://doi.org/10.1007/s00464-013-2973-9
Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS (2003) Role of surgeon volume in radical prostatectomy outcomes. J Clin Oncol 21(3):401–405. https://doi.org/10.1200/JCO.2003.05.169
Abboudi H, Khan MS, Guru KA et al (2014) Learning curves for urological procedures: a systematic review. BJU Int 114(4):617–629. https://doi.org/10.1111/bju.12315
Sharma NL, Papadopoulos A, Lee D et al (2011) First 500 cases of robotic-assisted laparoscopic radical prostatectomy from a single UK centre: learning curves of two surgeons. BJU Int. https://doi.org/10.1111/j.1464-410X.2010.09941.x
Savage CJ, Vickers AJ (2009) Low annual caseloads of united states surgeons conducting radical prostatectomy. J Urol 182(6):2677–2681. https://doi.org/10.1016/j.juro.2009.08.034
Abu-Ghanem Y, Erlich T, Ramon J, Dotan Z, Zilberman DE (2017) Robot assisted laparoscopic radical prostatectomy: assistant’s seniority has no influence on perioperative course. J Robot Surg 11(3):305–309. https://doi.org/10.1007/s11701-016-0655-z
Potretzke AM, Knight BA, Brockman JA et al (2016) The role of the assistant during robot-assisted partial nephrectomy: does experience matter? J Robot Surg 10(2):129–134. https://doi.org/10.1007/s11701-016-0582-z
Mitsinikos E, Abdelsayed GA, Bider Z et al (2017) Does the level of assistant experience impact operative outcomes for robot-assisted partial nephrectomy? J Endourol 31(1):38–42. https://doi.org/10.1089/end.2016.0508
Nayyar R, Yadav S, Singh P, Dogra PN (2016) Impact of assistant surgeon on outcomes in robotic surgery. Indian J Urol 32(3):204–209. https://doi.org/10.4103/0970-1591.185095
Pisansky TM, Thompson IM, Valicenti RK, D'Amico AV, Selvarajah S (2019) Adjuvant and salvage radiotherapy after prostatectomy: ASTRO/AUA guideline amendment 2018–2019. J Urol 202(3):533–538. https://doi.org/10.1097/JU.0000000000000295
Vickers AJ, Savage CJ, Hruza M et al (2009) The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncol 10(5):475–480. https://doi.org/10.1016/S1470-2045(09)70079-8
Oberlin DT, Flum AS, Lai J, Meeks JJ (2016) The impact of minimally invasive prostatectomy on practice patterns of American urologists. Urol Oncol 34(6):1–13. https://doi.org/10.1016/j.urolonc.2016.01.008.The
Hagen ME, Meehan JJ, Inan I, Morel P (2008) Visual clues act as a substitute for haptic feedback in robotic surgery. Surg Endosc 22(6):1505–1508. https://doi.org/10.1007/s00464-007-9683-0
Kumar R, Hemal AK (2006) The “scrubbed surgeon” in robotic surgery. World J Urol 24(2):144–147. https://doi.org/10.1007/s00345-006-0068-0
Thiel DD, Lannen A, Richie E, Dove J, Gajarawala NM, Igel TC (2013) Simulation-based training for bedside assistants can benefit experienced robotic prostatectomy teams. J Endourol 27(2):230–237. https://doi.org/10.1089/end.2012.0382
Gibson B, Abaza R (2011) Robotic repair of access-related aortic injuries: unexpected complication of robot-assisted prostatectomy. J Endourol 25(2):235–238. https://doi.org/10.1089/end.2010.0367
Omar MA, Davidson A, Karim OMA (2012) Lost needle: a dilemma in robotic-assisted laparoscopic surgery. J Robot Surg 6(1):73–75. https://doi.org/10.1007/s11701-011-0321-4
Cimen HI, Atik YT, Altinova S, Adsan O, Derya-Balbay M (2018) Does the experience of the bedside assistant effect the results of robotic surgeons in the learning curve of robot assisted radical prostatectomy? Am Hosp 3(1):54–60. https://doi.org/10.1590/S1677-5538.IBJU.2018.0184
Sur RL, Wagner AA, Albala DM, Su LM (2008) Critical role of the assistant in laparoscopic and robot-assisted radical prostatectomy. J Endourol 22(4):587–589. https://doi.org/10.1089/end.2007.9837
Birkmeyer JD, Finks JF, O’Reilly A et al (2013) Surgical skill and complication rates after bariatric surgery. N Engl J Med 369(15):1434–1442. https://doi.org/10.1056/NEJMsa1300625
Funding
We, the authors have no funding sources to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We, the authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garbens, A., Lay, A.H., Steinberg, R.L. et al. Experienced bedside-assistants improve operative outcomes for surgeons early in their learning curve for robot assisted laparoscopic radical prostatectomy. J Robotic Surg 15, 619–626 (2021). https://doi.org/10.1007/s11701-020-01146-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-020-01146-8