Abstract
Robotic-assisted simple prostatectomy (RASP) has emerged as a safe and effective treatment option for symptomatic patients with lower urinary tract symptoms related to significant benign prostatic enlargement (BPE) above 80 g. The recent release of the da Vinci SP robotic system (Intuitive, Sunnyvale, CA, USA) continues to advance the minimally invasive nature of robotic surgical technology. We now report our institution’s initial experience performing RASP using the da Vinci SP robotic system. An IRB-approved, retrospective chart review was performed of all patients undergoing robotic-assisted simple prostatectomy using the da Vinci SP surgical system in the treatment of benign prostatic enlargement by a single surgeon from March to June 2019. Pre-operative, intraoperative, and post-operative data were collected for descriptive analysis. A total of 10 men, mean age of 69 ± 4 years, with mean prostate volume of 104 ± 11 g underwent surgery. The robotic cannula and a single assistant port were utilized in all cases. No cases required conversion to a multi-port robotic platform or open approach, nor required the placement of additional assistant ports. No intraoperative or immediate post-operative complications were noted. Mean estimated blood loss was 141 ± 98 mL and operative time was 172 ± 19 min. Mean catheter time was 1.9 ± 1.8 days. One patient reported transient de novo stress urinary incontinence. Single-port RASP is a safe and effective intervention for BPE. The smaller surgical footprint from the device appears to make earlier catheter removal possible. Comparative evaluation with multi-port RASP and other modalities is warranted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benign prostatic enlargement (BPE) is a significant problem in aging men and affects more than half of men over 60 years old [1]. BPE is the primary source of lower urinary tract symptoms (LUTS) in men and is associated with significant morbidity [2]. Surgical intervention is appropriate for those with moderate-to-severe LUTS or urinary retention that have failed medical therapy. TURP remains the most common surgical intervention for BPE and is often considered the gold standard; however, its efficacy decreases with increasing gland size [3].
Alternatives to TURP in large glands include open simple prostatectomy (OSP), robotic-assisted simple prostatectomy (RASP), holmium laser enucleation of the prostate (HoLEP), and thulium laser enucleation of the prostate (ThuLEP) [4, 5]. HoLEP has been shown to be a safe and effective treatment modality for large prostate glands [6]. However, the learning curve for this operation is quite steep, and has been estimated to be between 40 and 60 cases [7]. RASP has been described as an excellent surgical option for men with glands over 80 g, with outcomes superior to OSP in terms of blood loss and hospital stay and comparable with HoLEP [8, 9]. The learning curve has also been described as significantly shorter (10–12 cases) in urologists familiar with robotic surgery [10]. However, relative to HoLEP, RASP is more invasive and to date, required longer post-operative catheterizations.
The recent release of the da Vinci SP robotic system (Intuitive, Sunnyvale, CA, USA), a purpose-built single port robotic platform, continues to advance the minimally invasive nature of surgical technology. We report our institution’s initial case series of RASP using the single-port robotic platform.
Materials and methods
Population
After institutional review board approval was obtained, all patients undergoing robotic-assisted simple prostatectomy in the treatment of histologically confirmed benign prostatic hypertrophy using the Da Vinci SP surgical system at a single institution from March to June 2019 were reviewed. All patients had failed medical management of BPE. Prostate size was assessed by pre-operative imaging. In our practice, a cut-off of approximately 80 g is used to offer patients a robotic procedure over an endoscopic approach unless additional pathology is present such as concomitant bladder stones or diverticulum.
Surgical technique
All cases were completed by a single surgeon (JCG). The patient was positioned supine on the operating table and secured to the bed. Patients were placed in minimal Trendelenburg position given the extraperitoneal nature of the surgery. The robot was side docked to the robotic cannula.
A 2.5 cm vertical midline incision was made two fingerbreadths below the umbilicus and carried down to the anterior rectus fascia. Two, 0-polyglactin sutures were placed on either side of midline, the fascia incised sharply, rectus muscle split and the space of Retzius entered. Using digital dissection, a space was created. A GelPOINT mini system (Applied Medical, Rancho Santa Margerita, CA, USA) was placed, through which the robotic cannula was inserted. In the first 3 cases, a balloon dilator (Spacemaker™ Pro, Covidien, Minneapolis, MN, USA) was used to develop the Space of Retzius. In the latter 7 cases, this space was developed bluntly using a finger and the robotic camera after placement of the robotic port. In all cases, a separate 8 or 12 mm assistant port was place in the left lateral extraperitoneal space for suction and suture passage.
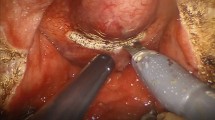
The procedure was started by distending the bladder with saline (Fig. 1a) and making an approximate 4 cm transverse incision over the bladder dome (Fig. 1b). An 0 silk suture on a Keith needle was passed percutaneously into the Space of Retzius just above the pubic bone to hold the cystotomy open (Fig. 1c, d). The trigone and both ureteral orifices were identified (Fig. 2a). The bladder mucosa was incised posteriorly over the median lobe and the dissection plane between the adenoma and the peripheral zone was developed (Fig. 2b). This dissection was carried distally to the apex (Fig. 2c). Dissection then continued in this fashion circumferentially until the only remaining attachment to the adenoma was the urethra. The urethra was sharply incised and the adenoma was placed outside the bladder (Fig. 2d). Running 3-0 barbed sutures were used to achieve capsular hemostasis (Fig. 3a). Subsequently, the bladder trigone was recreated using another 3-0 barbed suture (Fig. 3b). The final catheter was placed prior to closing of the bladder. The bladder was closed in two layers using 3-0 barbed sutures (Fig. 3c). Prior to performing the second layer, the bladder was distended to 300 mL to identify any leaks in the closure. Continuous bladder irrigation was then initiated. A surgical drain was placed through the assistant trocar into the space of Retzius.
Initial procedural steps of a robotic simple prostatectomy using the da Vinci SP robotic system. The bladder is distended with saline (a), after which a transverse incision at the anterior bladder dome is made (b). A silk suture is introduced percutaneously and placed in the anterior portion of the cystotomy (c) to hold it open (d)
Extirpative steps of a robotic simple prostatectomy using the da Vinci SP robotic system. After identification of the trigone and ureteral orifices (a), the posterior bladder mucosa is incised over of the median lobe down to the adenoma (b). This dissection is carried distally and circumferentially (c) until the adenoma is completed free. The adenoma is then placed outside of the bladder (d)
Post-operative care
Continuous bladder irrigation was continued overnight and discontinued on post-operative day one. If the urine remained clear off continuous bladder irrigation on POD1, a voiding trial was performed. Drain output was monitored after catheter removal to assess for a bladder leak. If the drain output did not increase significantly (> 10 ml) after voiding, the catheter was removed and the patient discharged home.
Analyses
Pre-operative demographics and disease characteristics (e.g. body mass index (BMI), prostate specific antigen (PSA), prostate volume), intraoperative data (e.g. operative time, estimated blood loss, hematocrit change), and post-operative data (e.g. hospitalization time, catheterization time, complications, functional outcomes) were collected for descriptive analysis.
Results
Table 1 details the pre-operative patient and disease characteristics. Ten men with mean age 69 ± 4 years old, mean PSA 4.7 ± 2.4 ng/dL and mean prostate volume 104 ± 11 g underwent the procedure. Only one man was catheter dependent prior to surgery. Five patients had undergone prior outlet procedures (two Rezum, two transurethral resection of the prostate, one laser ablation). Mean international prostate symptom score (IPSS) was 20.8 ± 6.4, while mean pre-operative maximum urine flow was 8.6 ± 3.8 mL/s and mean post-void residual (PVR) was 119 ± 98 mL.
Peri-operative and post-operative functional outcomes can be seen in Table 2. Mean operative time was 172 ± 19 min with mean adenoma resection of 65 ± 16 g and tissue yield of 63 ± 14%. Mean estimated blood loss was 141 ± 98 mL. Mean length of stay was 1.1 ± 0.3 days. No intraoperative complications were noted. No patients experienced post-operative complications or required transfusion.
Mean catheter time was 1.9 ± 1.8 days. The first patient was not offered an inpatient voiding trial. The following nine patients underwent a voiding trial on post-operative day (POD) 1, of which 8 (89%) passed. The single patient who failed had a catheter replaced without incident and later passed his voiding trial in the office on POD5. Mean post-operative IPSS was 12.9 ± 7.7 for an average reduction of − 43%. At first post-operative visit, mean maximum flow rate was 11.2 ± 3.6 mL/s with 41 ± 38 mL PVR.
Discussion
This study is the first to describe a clinical series using the da Vinci SP robotic platform to perform RASP. We demonstrate that the procedure is safe, feasible and effective. Further, the majority of patients had their catheters removed on post-operative day 1 without complication.
In men with very large prostate glands, RASP been shown to be safe and effective in relieving outlet obstruction [11,12,13]. Outcomes after RASP have been shown to be comparable to open simple prostatectomy but robotics confers the benefit of reduced blood loss and a shorter hospitalization time [9]. In the largest multi-institutional series to date on minimally invasive simple prostatectomies, Autorino et al. reported the outcomes of 1330 men and found a median operative time of 95 min, median blood loss of 280 cc and median catheter duration of 4 days [11]. Overall complication rate was 7.1%, with the majority (5.6%) being grade I and II. While our series did not approach the operative times reported by Autorino, it is likely as the surgeon and team become more experienced with the SP platform, these will continue to decline.
Our series utilized an extraperitoneal approach for robotic port placement while most RASP series have described a transperitoneal approach [9, 14,15,16,17], likely due to most robotic surgeons experience performing radical prostatectomies. That said, the extraperitoneal approach has been previously reported [18, 19] and was first described by John et al. [18]. In this series, a vertical incision was made at the prostatic-vesical junction and occasionally finger assistance was required to aid with enucleation. Median operative time was 210 min (140 min when enucleation was performed by finger assistance), median blood loss was 500 mL and median catheter time was 6 days. Stolzenburg et al. [19] more recently described their extraperitoneal technique, also using a vesico-capsular incision. In their series of 10 patients, a shorter mean operative time was noted (122.5 min), along with lower mean blood loos (228 mL). Catheter removal was also performed after 6 days. Our results showed similar operative time (175 min) and blood loss (142 mL). Table 3 details the outcomes of multiple series including our own.
One of the major advantages of the SP robotic system remains the small cystotomy required to complete the procedure. This reduction, in addition to the extraperitoneal approach, allows the authors to feel confident about urethral catheter removal on post-operative day one. To date, this has been one of the major criticisms of RASP as compared to endoscopic enucleation of the prostate. In our series, the first patient was discharged with a catheter for 6 days as per our protocol when the procedure was transperitoneal. Of the remaining nine patients who underwent voiding trial on POD1, only one required re-catheterization. This patient successfully passed his voiding trial on POD 5 in clinic.
While RASP has been accepted as an alternative to open surgery, it continues to be criticized for its invasiveness and cost [20,21,22] relative to endoscopic options such as HoLEP. The EAU guidelines strongly recommend HoLEP as an alternative to OSP for men with prostate glands > 80 to 100 g, while still listing RASP as a technique under investigation due to lack of prospective long-term data [23]. Conversely, the AUA guidelines recommend simple prostatectomy for large prostates, leaving the choice of technique to the surgeon [24]. Only two studies to date have compared peri-operative outcomes between RASP and HoLEP [25, 26]. Umari et al. [25] compared 81 men who underwent RASP to 45 who had laser treatment. There was no difference in operative time, transfusion rates or complication rates. However, men who underwent HoLEP had significantly shorter catheterization time (2 vs 3 days) but higher rates of transient incontinence (8.9% vs 1.2%). Zhang et al. [26] compared 600 HoLEP patients to 32 men who were treated with RASP. RASP was found to have a significantly longer operative time (274 min vs 103 min), higher transfusion rate (9.4% vs 1.8%), and longer catheterization time (8 vs 0.7 days). This study was flawed by the significant disproportionate number of patients in each arm, and outcomes reported in the robotic arm not comparable to the other large series in the literature [9, 11, 16].
While the result of multiple endoscopic enucleation has shown good outcomes series, one cannot disregard the disparity in acquiring the skills to perform these procedures. In the hands of an experienced robotic-surgeon, operative time significantly decreased, while tissue yield plateaued and blood loss nadired after only 12 cases [10]. HoLEP has shown much steeper learning curves, requiring 40–60 cases to achieve satisfactory efficiency with good outcomes [7, 27, 28]. While there have been various methods proposed to more quickly acquire laser enucleation skills, particularly in the setting of a fellowship/mentorship [27], the majority of current trainees will already possess the robotic skills needed to perform a RASP. This is supported by the large number of prostatectomies (> 150) that the majority of residents will complete by graduation [29]. That said, it is not clear that the learning curve established for multi-port RASP is readily transferable to SP RASP.
In completing these procedures, there are a few important observations made as part of learning to use the SP system for this particular case. First, the GelPOINT system made achieving the desired distance between the robotic cannula and target anatomy (10–25 cm) easier by allowing the remote center of the robotic cannula to be moved outside of the fascia. Second, the use of all 3 robotic arms simultaneously during enucleation limited the mobility of the camera, in particular when trying to move laterally. Thus, use of two robotics was preferred in our series during the majority of the enucleation. Further, the ‘cobra’ feature of the robotic camera was minimally used given the limitations imposed on both camera and instrument movement. Third, when performing the posterior enucleation, placing the camera in the below position helps to avoid crossing robotic arms, and thus instrument collisions. Fourth, the smaller diameter of the da Vinci SP robot arms (6 mm) compared to other robotic systems (8 mm) has resulted in reduced robotic arm strength, influencing the ability to retract the adenoma. Finally, the presence of an instrument elbow and removal of the EndoWrist® results in instruments that feel like a hybrid of robotic and laparoscopic tools. This is most recognized during suturing but seems to be quickly overcome.
Our study is not devoid of weaknesses. First, our series is limited by the small cohort and short follow up which limits the evaluation of long-term functional outcomes. Second, all procedures were performed by a single, fellowship trained robotic surgeon and thus generalization of these results would need to be evaluated. Finally, our study does not compare outcomes with either open, robotic-simple on another robotic platform or endoscopic procedures. Despite these limitations, this remains the first report to our knowledge to describe a RASP series using the da Vinci SP surgical platform.
Conclusion
RASP using the da Vinci SP robotic surgical system is safe and effective in a small cohort of patients with short term follow-up. The extraperitoneal approach, in conjunction with the small required cystotomy, facilitated catheter removal on the first post-operative day. Further studies in larger cohorts are needed to assess long-term functional outcomes, procedural reproducibility, and comparison to other techniques.
References
Roehrborn CG (2005) Benign prostatic hyperplasia: an overview. Rev Urol 7(Suppl 9):S3
Speakman M, Kirby R, Doyle S, Ioannou C (2015) Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH)—focus on the UK. BJU Int 115(4):508–519. https://doi.org/10.1111/bju.12745
Yang Q, Peters TJ, Donovan JL, Wilt TJ, Abrams P (2001) Transurethral incision compared with transurethral resection of the prostate for bladder outlet obstruction: a systematic review and meta-analysis of randomized controlled trials. J Urol 165(5):1526–1532
McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan SA, Penson DF, Ulchaker JC, Wei JT (2011) Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 185(5):1793–1803. https://doi.org/10.1016/j.juro.2011.01.074
Pariser JJ, Famakinwa OJ, Pearce SM, Chung DE (2014) High-power thulium laser vaporization of the prostate: short-term outcomes of safety and effectiveness. J Endourol 28(11):1357–1362. https://doi.org/10.1089/end.2014.0336
Elzayat EA, Elhilali MM (2007) Holmium laser enucleation of the prostate (HoLEP): long-term results, reoperation rate, and possible impact of the learning curve. Eur Urol 52(5):1465–1471. https://doi.org/10.1016/j.eururo.2007.04.074
Brunckhorst O, Ahmed K, Nehikhare O, Marra G, Challacombe B, Popert R (2015) Evaluation of the learning curve for holmium laser enucleation of the prostate using multiple outcome measures. Urology 86(4):824–829. https://doi.org/10.1016/j.urology.2015.07.021
Sutherland DE, Perez DS, Weeks DC (2011) Robot-assisted simple prostatectomy for severe benign prostatic hyperplasia. J Endourol 25(4):641–644. https://doi.org/10.1089/end.2010.0528
Sorokin I, Sundaram V, Singla N, Walker J, Margulis V, Roehrborn C, Gahan JC (2017) Robot-assisted versus open simple prostatectomy for benign prostatic hyperplasia in large glands: a propensity score-matched comparison of perioperative and short-term outcomes. J Endourol 31(11):1164–1169. https://doi.org/10.1089/end.2017.0489
Johnson B, Sorokin I, Singla N, Roehrborn C, Gahan JC (2018) Determining the learning curve for robot-assisted simple prostatectomy in surgeons familiar with robotic surgery. J Endourol 32(9):865–870
Autorino R, Zargar H, Mariano MB, Sanchez-Salas R, Sotelo RJ, Chlosta PL, Castillo O, Matei DV, Celia A, Koc G, Vora A, Aron M, Parsons JK, Pini G, Jensen JC, Sutherland D, Cathelineau X, Nunez Bragayrac LA, Varkarakis IM, Amparore D, Ferro M, Gallo G, Volpe A, Vuruskan H, Bandi G, Hwang J, Nething J, Muruve N, Chopra S, Patel ND, Derweesh I, Champ Weeks D, Spier R, Kowalczyk K, Lynch J, Harbin A, Verghese M, Samavedi S, Molina WR, Dias E, Ahallal Y, Laydner H, Cherullo E, De Cobelli O, Thiel DD, Lagerkvist M, Haber GP, Kaouk J, Kim FJ, Lima E, Patel V, White W, Mottrie A, Porpiglia F (2015) Perioperative outcomes of robotic and laparoscopic simple prostatectomy: a European-American multi-institutional analysis. Eur Urol 68(1):86–94. https://doi.org/10.1016/j.eururo.2014.11.044
Lucca I, Shariat SF, Hofbauer SL, Klatte T (2015) Outcomes of minimally invasive simple prostatectomy for benign prostatic hyperplasia: a systematic review and meta-analysis. World J Urol 33(4):563–570. https://doi.org/10.1007/s00345-014-1324-3
Shah AA, Gahan JC, Sorokin I (2018) Comparison of robot-assisted versus open simple prostatectomy for benign prostatic hyperplasia. Curr Urol Rep 19(9):71. https://doi.org/10.1007/s11934-018-0820-1
Elsamra SE, Gupta N, Ahmed H, Leavitt D, Kreshover J, Kavoussi L, Richstone L (2014) Robotic assisted laparoscopic simple suprapubic prostatectomy—the Smith Institute for Urology experience with an evolving technique. Asian J Urol 1(1):55–59. https://doi.org/10.1016/j.ajur.2015.04.006
Leslie S, Abreu AL, Chopra S, Ramos P, Park D, Berger AK, Desai MM, Gill IS, Aron M (2014) Transvesical robotic simple prostatectomy: initial clinical experience. Eur Urol 66(2):321–329. https://doi.org/10.1016/j.eururo.2013.12.020
Pokorny M, Novara G, Geurts N, Dovey Z, De Groote R, Ploumidis A, Schatteman P, de Naeyer G, Mottrie A (2015) Robot-assisted simple prostatectomy for treatment of lower urinary tract symptoms secondary to benign prostatic enlargement: surgical technique and outcomes in a high-volume robotic centre. Eur Urol 68(3):451–457. https://doi.org/10.1016/j.eururo.2015.03.003
Sotelo R, Clavijo R, Carmona O, Garcia A, Banda E, Miranda M, Fagin R (2008) Robotic simple prostatectomy. J Urol 179(2):513–515. https://doi.org/10.1016/j.juro.2007.09.065
John H, Bucher C, Engel N, Fischer B, Fehr JL (2009) Preperitoneal robotic prostate adenomectomy. Urology 73(4):811–815. https://doi.org/10.1016/j.urology.2008.09.028
Stolzenburg JU, Kallidonis P, Kyriazis I, Kotsiris D, Ntasiotis P, Liatsikos EN (2018) Robot-assisted simple prostatectomy by an extraperitoneal approach. J Endourol 32(S1):S39–S43. https://doi.org/10.1089/end.2017.0714
Gilling PJ (2017) Editorial comment on: comparison of perioperative outcomes between holmium laser enucleation of the prostate and robot-assisted simple prostatectomy by Zhang et al. (From: Zhang MW, El Tayeb MM, Borofsky MS, et al. J Endourol 2017;31:847-850). J Endourol 31(11):1216. https://doi.org/10.1089/end.2017.0639
Kaplan SA (2016) Re: perioperative outcomes of robotic and laparoscopic simple prostatectomy: a European-American multi-institutional analysis. J Urol 196(4):1218–1221. https://doi.org/10.1016/j.juro.2016.07.031
Montorsi F, Gandaglia G (2015) Re: Riccardo Autorino, Homayoun Zagar, Mirandolino B. Mariano, et al. Perioperative outcomes of robotic and laparoscopic simple prostatectomy: a European-American Multi-institutional Analysis. Eur Urol 2015;68:86–94. Re: Matthew Bultitude, Ben Challacombe. Simple prostatectomy: a step too far for laparoscopy? Eur Urol 2015;68:95–6. European urology 68(1):e7–8. https://doi.org/10.1016/j.eururo.2015.01.022
Gratzke C, Bachmann A, Descazeaud A, Drake MJ, Madersbacher S, Mamoulakis C, Oelke M, Tikkinen KA, Gravas S (2015) EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 67(6):1099–1109
Foster HE, Barry MJ, Dahm P, Gandhi MC, Kaplan SA, Kohler TS, Lerner LB, Lightner DJ, Parsons JK, Roehrborn CG, Welliver C, Wilt TJ, McVary KT (2018) Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. j Urol 200(3):612–619. https://doi.org/10.1016/j.juro.2018.05.048
Umari P, Fossati N, Gandaglia G, Pokorny M, De Groote R, Geurts N, Goossens M, Schatterman P, De Naeyer G, Mottrie A (2017) Robotic assisted simple prostatectomy versus holmium laser enucleation of the prostate for lower urinary tract symptoms in patients with large volume prostate: a comparative analysis from a high volume center. J Urol 197(4):1108–1114. https://doi.org/10.1016/j.juro.2016.08.114
Zhang MW, El Tayeb MM, Borofsky MS, Dauw CA, Wagner KR, Lowry PS, Bird ET, Hudson TC, Lingeman JE (2017) Comparison of perioperative outcomes between holmium laser enucleation of the prostate and robot-assisted simple prostatectomy. J Endourol 31(9):847–850. https://doi.org/10.1089/end.2017.0095
Kampantais S, Dimopoulos P, Tasleem A, Acher P, Gordon K, Young A (2018) Assessing the learning curve of holmium laser enucleation of prostate (HoLEP). A systematic review. Urology 120:9–22. https://doi.org/10.1016/j.urology.2018.06.012
Shigemura K, Yamamichi F, Kitagawa K, Yamashita M, Oka Y, Tanaka H, Fujisawa M (2017) Does surgeon experience affect operative time, adverse events and continence outcomes in holmium laser enucleation of the prostate? A review of more than 1,000 cases. J Urol 198(3):663–670. https://doi.org/10.1016/j.juro.2017.04.087
Hoag NA, Mamut A, Afshar K, Amling C, Mickelson JJ, Macneily AE (2012) Trends in urology resident exposure to minimally invasive surgery for index procedures: a tale of two countries. J Surg Educ 69(5):670–675. https://doi.org/10.1016/j.jsurg.2012.04.007
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Steinberg, N. Passoni, A. Garbens, B. Johnson, and J. Gahan declare they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Steinberg, R.L., Passoni, N., Garbens, A. et al. Initial experience with extraperitoneal robotic-assisted simple prostatectomy using the da Vinci SP surgical system. J Robotic Surg 14, 601–607 (2020). https://doi.org/10.1007/s11701-019-01029-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-019-01029-7