Abstract
To evaluate trends in contemporary robotic surgery across multiple organ sites as they relate to robotic prostatectomy volume. We queried the National Cancer Database for patients who underwent surgery from 2010 to 2013 for prostate, kidney, bladder, corpus uteri, uterus, cervix, colon, sigmoid, rectum, lung and bronchus. The trend between volumes of robotic surgery for each organ site was analyzed using the Cochran–Armitage test. Multivariable models were then created to determine independent predictors of robotic surgery within each organ site by calculating the odds ratio with 95% CI. Among the 566,399 surgical cases analyzed, 35.1% were performed using robot assistance. Institutions whose robotic prostatectomy volume was in the top 75 percentile compared to the bottom 25 percentile performed a larger percentage of robotic surgery on the following sites: kidney 32.6 vs. 28.8%, bladder 23.6 vs. 18.6%, uterus 52.5 vs. 47.7%, cervix 43.5 vs. 39.2%, colon 3.2 vs. 2.9%, rectum 10.7 vs. 8.9%, and lung 7.3 vs. 6.8% (all p < 0.0001). It appears that increased trends toward robotic surgery in urology have lead to increased robotic utilization within other surgical fields. Future analysis in benign utilizations of robotic surgery as well as outcome data comparing robotic to open approaches are needed to better understand the ever-evolving nature of minimally invasive surgery within the United States.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the United States Food and Drug Administration approved the da Vinci surgical robot (Intuitive Surgical, Sunnyvale, CA) for clinical use in 2000, its adoption has been rapid and transformative, but not without controversy. The costs to acquire and maintain this surgical platform are among the most debated topics. These include an initial purchase price estimated to be between U.S.$1 and 2.5 million dollars, as well as per-case and annual maintenance contract costs. Indeed, the da Vinci system remains the only robotic surgical platform available for commercial use in the United States, and in a 2007 analysis, it was single handedly estimated to increase U.S. health care costs by 13% (approximately U.S.$2 billion) [1].
Another hotly debated question was whether there was any meaningful clinical benefit from using this robotic system in surgery. Urological surgeons were some of the earliest adopters of this technology and prostate cancer surgery, in particular, was uniquely positioned to answer this question. First, prostate cancer is the most common non-skin malignancy in U.S. males, which creates a large cohort for study that has been featured in peer-reviewed literature since at least 2001 [2]. Second, the three dimensional vision and multiple degrees of freedom of motion afforded by the robot made for technically easier cavernous nerve (erectile) and sphincteric preservation deep in narrow male pelvis. Third, the laparoscopic approach with CO2 insufflation dramatically decreased intraoperative blood loss and reduced postoperative pain, leading to quicker patient recovery.
In prostate cancer, the evidence of superior outcomes of robotic prostatectomy compared to the traditional open approach are mature and well-documented [4,5,6,6]. Furthermore, population-based data even suggests reductions in positive surgical margins, decreased need for postoperative radiation therapy, and decreased 30-day mortality compared to open radical prostatectomy [7]. Consequently, 67–85% of recertifying urologists reported performing radical prostatectomy with robotic assistance [8]. While the advantage of robotic surgery is actively being investigated in other organ sites, aggressive direct-to-consumer marketing by hospitals and the surgical robot manufacturer may be factors increasing utilization ahead of evidence [9]. For many organ sites, especially gynecology, the majority of data which argues for robotic surgery has been criticized as retrospective and prone to selection bias [10].
While the literature regarding robotic surgery utilization is often reported in a single organ or operation directed fashion, robotic systems do not exist in a vacuum. Robotic operating-room time is shared amongst surgeons of different disciplines at a given hospital. The time-consuming training of operating-room staff may alter staffing and workflow across disciplines. Hospital administrators, care-team specialization, equipment availability, and economic incentives would be expected to drive robotic utilization across disciplines once a robot is purchased by a hospital [11]. Hence in this exploratory and novel study using a large national cancer database, our objectives were:
-
1)
To assess contemporary trends in robotic surgical approach across a multitude of organ systems for oncologic surgery;
-
2)
To explore the frequency of and identify predictors for robotic surgery at particular medical centers;
-
3)
To address if a higher volume of robotic prostatectomy in a hospital system predicts for higher utilization of robotics in other disciplines.
Methods
Database
The National Cancer Database (NCDB) is a hospital-based cancer registry capturing 70% of all cancer diagnoses in the United States from more than 1400 hospitals and is recognized as the largest clinical registry in the world [12]. Data comes from hospitals located in 49 US states and Puerto Rico. It includes patient demographics, socioeconomic status, clinical and pathologic staging, treatment course, comorbidities, and survival status based on patient records and death registries. Patients are associated with facility site codes, which are insufficient to identify specific hospitals, but do allow for comparison of treatment patterns for different cancers within a single site. Additionally, data regarding the hospital setting, type, and location relative to each patient is provided. The disease course and therapy of each patient are coded and reported based on the American College of Surgeons׳ Facility Oncology Registry Data Standards (http://www.facs.org/cancer/coc/fordsmanual.html) for each organ site. Our institutional review board approved this study for exemption given no personal patient data was examined during the course of investigation.
Population
We identified all patients in NCDB with cancer in the following organ sites: prostate, kidney, bladder, corpus uteri, uterus, cervix, colon, sigmoid, rectum, lung and bronchus. For the purposes of analysis, cancer of the sigmoid and rectum were combined into a single category rectum. Non-small cell carcinoma, small cell carcinoma and other cancers of the lungs and bronchus were combined into lung. Cancers of the corpus uteri and uterus were combined into uterus. Patients were included only if their hospital of diagnosis matched the site of treatment to maximize the integrity of the data. They were included if they presented with non-metastatic disease as defined by the American Joint Committee on Cancer (AJCC) category cM0 for each cancer type. Patients were excluded if they had a history of more than one non-cutaneous malignancy.
The NCDB began including the surgical approach code (open, laparoscopic, or robotic) in 2010, thus, our study cohort was limited to 2010–2013. For each organ system, codes representing surgery of the primary site were reviewed and surgical treatment was defined as at least a segmental resection or greater. In other words, local tumor excision alone was excluded. It was unlikely such cases would be approached in a robotic fashion; moreover, in these organ sites, this approach would not be considered oncologically appropriate for definitive surgical management of cancer. For example, in terms of bladder cancer, local excision is typically a biopsy performed via transurethral resection of bladder tumor and this would have been appropriately excluded, whereas a partial cystectomy would have been included. Similarly, polypectomy for colon cancer was excluded, whereas partial colectomy was included.
Analysis
All eligible patients’ baseline demographic and clinical characteristics including age, race, facility type, and cancer characteristics were collected. Deyo-Charlson Comorbidity index (CCI) was calculated from 6 preexisting comorbidities. The facility type is defined by the NCDB as “community program” if more than 100 but fewer than 500 patients with cancer are treated in that setting per year. “Comprehensive programs” treat more than 500 cancer cases per year. “Academic programs” treat over 500 cancer cases and have at least 4 specialties involved in education. “Integrated Network Cancer Programs” represent joint ventures with multiple facilities providing integrated cancer care, may or may not have residents, and does not have any volume-associated definition, but offers comprehensive services. Each facility site’s overall surgical volume for each organ site and utilization of the robotic approach were calculated and used to stratify into quartiles. The patient’s clinical stage was derived according to the AJCC Cancer Staging Manual edition in use during the year in which each cancer was diagnosed.
Trends in robotic use over time were assessed using the Cochran-Armitage test for trend. Rates of robotic approach were compared between facility and patient characteristics using the Chi square test for independence. A multivariable logistic regression model of predictors of a robotic approach was created for each organ site. For all tests of significance, the 2-sided p < 0.05 was considered statistically significant. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
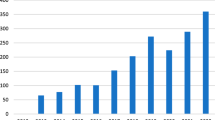
In total, 566,399 surgical cases met inclusion criteria and 35.1% were performed using robot assistance. Of these 199,076 robotic cases, 74.4% were performed for urological cancers, confirming that urological surgeons are the most frequent utilizers of this platform. From 2010 to 2013, utilization of robotics increased significantly for other organ sites suggesting increased expertise with robotics and adoption by other surgical fields (Fig. 1). For example, lung surgery experienced a 266% relative percentage increase in the use of robotics during this time period. Interestingly, if a facility performed robotic gynecologic, colorectal, or thoracic surgery in over 90% of cases, at least one robotic prostatectomy was also done there. (Table 1) Only 9 (0.71%) facilities were in the top quartile for robotic volume for all surgery sites.
The adoption of robotics in different surgical fields was variable. Each organ site had substantial differences in the number of cases needed for inclusion in the top 25 and 5% of robotic volume for that respective organ. For example, a center could be characterized as top 25% in colon robotic volume by performing only 6 surgeries over 4 years vs. 30 for robotic kidney (Table 1). Only 8% of colon cancer patients at the highest quartile robotic colon centers underwent a robotic procedure, compared to 58.4% for robotic hysterectomy at the facilities in the highest quartile for robotic uterine cancers (Table 2). Similarly, adoption was slow in thoracic cancer surgery. Another observation was that if a facility were in a lower volume robotic prostate cancer center, they were also less commonly using the robotic approach for other organ sites. The overall correlation between robotic approaches for prostate and the other organ sites (pooling all facilities) is represented by the radar plot (Fig. 2).
In terms of demographic factors influencing the use of robotic oncological surgery, Supplementary Table 2 summarizes our findings. Interestingly, we found that in the urologic and gynecologic oncology fields, surgery at a rural setting increased the patient’s chances of undergoing robotic surgery, while the opposite was true in thoracic and colorectal surgery. In each field, lower clinical stage at presentation was associated with increased likelihood of undergoing robotic surgery. For example, 4.6% of cT4 patients undergoing uterine cancer surgery underwent the robotic approach vs. 49.3% of cT1. (p < 0.0001). Except for lung cancer, older patients were less likely to undergo a robotic approach and lower Charlson comorbidity index was associated with greater robotic surgery use. Patients with a lower prostate specific antigen (PSA) or Gleason Score from diagnostic prostate biopsy were more likely to undergo robotic surgery.
Independent predictors for robotic surgery were assessed using multivariate analysis for each organ site (Supplementary Table 2). In general, patients with a higher clinical stage were significantly less likely to undergo robotic surgery. In kidney, uterus and cervical cancer, African–Americans were significantly less likely to have a robotic surgery, despite controlling for potential confounders. Patients with either low income or low levels of education suffering from kidney, bladder, uterus, cervix, or colon cancer had slightly less utilization of robotics. For all organ sites, community cancer programs were most likely to offer robotic surgery. The strongest predictor of a patient undergoing robotic surgery was presenting to a facility in the top quartile of robotic volume for that organ site. While this statement seems self-evident, a more nuanced view is that a patient presenting to a high volume robotic surgery center is more likely to undergo the robotic approach, regardless of age, comorbidity, race, education level, and disease characteristics.
Finally, comparing patient level characteristics of all-comers treated at facilities in the bottom 25 vs. top 25% of robotic volume for each site revealed a slightly different patient mix. In terms of clinical stage and Charlson comorbidity, high volume robotic surgery centers have access to slightly healthier patients. Additionally, high volume centers tend to have patients with higher education levels, income, and younger patients. Most striking were racial disparities. For example, 20.3 vs. 12.0% of the patients were African Americans, at centers in the bottom and top quartiles for robotic prostate volume, respectively (p < 0.001).
Discussion
In contrast to recent literature on the utilization of robotic surgery limited to single organ systems, our findings demonstrate gains in robotic utilization across organ sites in the cancer population. Prostate cancer is treated robotically upwards of 67% of the time in the literature and 76% of the time in our dataset [8]. Robot use for colon and rectum was reported as 2.8% nationally as of 2009–2010 [13]. Similarly, estimated national use of robotic surgery for lung cancer was 3.4% in 2010 [14]. In our study, we found robotic lung surgery increasing from 2.5% in 2010 to 9.3% by 2013. Among 406 members of the Society of Gynecologic Oncology (SGO), 75% reported using robotic hysterectomy for cervical cancer, which is much greater than 36.9% of robotic cases we captured in NCDB [15]. This suggests that non-SGO surgeons who operate for cervical cancer use the robotic approach much less frequently compared to their SGO colleagues.
The impetus for this analysis was our hypothesis that robotic prostatectomy and its demonstrated benefits would have increased the adoption of robotics for cancer surgery in other organ systems. In absolute numbers, there were 3.4 times as many robotic prostate cases as the next most prevalent, uterus. In over 90% of cases, at least one robotic prostatectomy was done at a facility in which other organs were removed via a robotic approach suggesting that urological surgeons’ demand for access to the robotic platform allowed other surgeons at their institution to explore its use in their cancer patients. Certainly, based on timing of implementation and high percent utilization it could be suggested a hospital purchase of a robotic system for prostate surgery served as an entry point for other disciplines to gain access to the robot. Applying innovation theory, robotic prostatectomy is in a late assessment stage and is quickly becoming standard of care, whereas robotic lung surgery is still being performed by early adopters and is still in the development stage [16]. At most, we found only 10.5% of centers were performing robotic surgery in the absence of robotic prostate surgery. Technical considerations may prevent a widespread transition away from pure laparoscopic approaches in particular fields. Robotics is arguably most helpful with suturing and reconstruction, and there are stapling devices used in colorectal and thoracic extirpative surgery, potentially reducing perceived advantages of this technology.
Disparities in patients’ access to robotic surgery were also evident in our data. This mirrors data from the state of California, where Hispanic, Medicare and Medicaid patients were more likely to be treated at hospitals without robotics. Even within hospitals which performed robotic approaches, Medicaid patients had 58% lower probability of receiving a robotic prostatectomy [17]. Similarly, nationally representative data suggest that African–American and Hispanic patients, or those insured by Medicaid, were less likely to receive a robotic prostatectomy [18]. These findings are not unique to urology, as striking socioeconomic and even regional disparities have been documented for minimally invasive approaches for endometrial, uterine, cervical cancer and colorectal disease [20,21,21].
The hospital at which patients present for treatment greatly informs the surgical approach the patient eventually undergoes. In uterine cancer, for example, 7.3% of the patients presenting to the center with the lowest quartile of robotic case volume received a robotic surgery compared to 58.4% of those who were at a hospital at the highest quartile. This approach disparity may represent learning curve, comfort with robotics, limited available robotic operating-room time, or widely different patient selection criteria. Data suggests experience matters in terms of improved patient outcomes no matter the surgical approach [23,24,24] (additional refs available). Physicians at low-volume centers may recognize this, restricting the robotic approach to only those candidates they view as ideal to safely perform, while they are still on the learning curve. As we found patients at lower volume robotic centers were less healthy overall, there simply may be a smaller pool from which to recruit robotic candidates. It has been suggested that operating-room efficiency has the largest impact on profitability of a robotic case, and so, from a hospital administration viewpoint, more complex cases may be incentivized towards an alternate approach [25].
How facilities transition from a low-volume to higher volume robotic center is an interesting question, but not one we could explore fully with only 4 years of data. High volume robotic centers overall served healthier, younger, and a less diverse patient mix independent of the ultimate surgical approach. It is unknown if access to such patients leads to the development of higher volume robotics or if higher volume robotic centers attract these types of patients outright.
Limitations
Robotic surgery performed for benign disease could not be addressed as the NCDB is limited to cancer cases. Moreover, data herein should not be considered nationally representative and may not be generalizable to centers not captured by the database. Unmeasured confounding factors may also affect the selection of surgical approach for any particular patient. Furthermore, by the nature of this work we have combined multiple cancer types into organ categories; each pathology may have specific treatment guidelines which could promote or discourage robotic utilization.
Conclusion
Robotic cancer surgery utilization dramatically increased from 2010 to 2013. High volume robotic centers are significantly more likely to pursue the robotic approach, lending credence to the idea of ‘feeding the robot.’ This work is hypothesis generating in terms of disparities in robotic access for economic, racial, age or educational reasons. Finally, higher volumes of robotic prostatectomy trend with higher robotic utilization for other organ sites, in particular other urologic and gynecologic surgery.
References
Barbash GI, Glied SA (2010) New technology and health care costs—the case of robot-assisted surgery. N Engl J Med 363:701–704. https://doi.org/10.1056/NEJMp1006602
Menon M, Tewari A, Peabody JO et al (2004) Vattikuti Institute prostatectomy, a technique of robotic radical prostatectomy for management of localized carcinoma of the prostate: experience of over 1100 cases. Urol Clin North Am 31:701–717. https://doi.org/10.1016/j.ucl.2004.06.011
Beauval J-B, Roumiguié M, Ouali M et al (2015) A prospective trial comparing consecutive series of open retropubic and robot-assisted laparoscopic radical prostatectomy in a centre: Oncologic and functional outcomes. Prog En Urol J Assoc Fr Urol Société Fr Urol 25:370–378. https://doi.org/10.1016/j.purol.2015.03.007
Alemozaffar M, Sanda M, Yecies D et al (2015) Benchmarks for operative outcomes of robotic and open radical prostatectomy: results from the Health Professionals Follow-up Study. Eur Urol 67:432–438. https://doi.org/10.1016/j.eururo.2014.01.039
Haglind E, Carlsson S, Stranne J et al (2015) Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol 68:216–225. https://doi.org/10.1016/j.eururo.2015.02.029
Caras RJ, Lustik MB, Kern SQ et al (2014) Laparoscopic radical prostatectomy demonstrates less morbidity than open radical prostatectomy: an analysis of the American College of Surgeons-National Surgical Quality Improvement Program database with a focus on surgical trainee involvement. J Endourol Endourol Soc 28:298–305. https://doi.org/10.1089/end.2013.0475
Pearce SM, Pariser JJ, Karrison T et al (2016) Comparison of perioperative and early oncologic outcomes between open and robotic assisted laparoscopic prostatectomy in a contemporary population based cohort. J Urol. https://doi.org/10.1016/j.juro.2016.01.105
Lowrance WT, Eastham JA, Savage C et al (2012) Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol 187:2087–2092. https://doi.org/10.1016/j.juro.2012.01.061
Lowrance WT, Parekh DJ (2012) The rapid uptake of robotic prostatectomy and its collateral effects. Cancer 118:4–7. https://doi.org/10.1002/cncr.26275
Tapper A-M, Hannola M, Zeitlin R et al (2014) A systematic review and cost analysis of robot-assisted hysterectomy in malignant and benign conditions. Eur J Obstet Gynecol Reprod Biol 177:1–10. https://doi.org/10.1016/j.ejogrb.2014.03.010
Yafchak R (2000) A longitudinal study of economies of scale in the hospital industry. J Health Care Finance 27:67–89
Bilimoria KY, Stewart AK, Winchester DP, Ko CY (2008) The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 15:683–690. https://doi.org/10.1245/s10434-007-9747-3
Halabi WJ, Kang CY, Jafari MD et al (2013) Robotic-assisted colorectal surgery in the United States: a nationwide analysis of trends and outcomes. World J Surg 37:2782–2790. https://doi.org/10.1007/s00268-013-2024-7
Kent M, Wang T, Whyte R et al (2014) Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 97:236–242. https://doi.org/10.1016/j.athoracsur.2013.07.117
Conrad LB, Ramirez PT, Burke W et al (2015) Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the society of gynecologic oncology. Int Gynecol Cancer Soc 25:1121–1127. https://doi.org/10.1097/IGC.0000000000000450
Barkun JS, Aronson JK, Feldman LS et al (2009) Evaluation and stages of surgical innovations. Lancet Lond Engl 374:1089–1096. https://doi.org/10.1016/S0140-6736(09)61083-7
Kim J, ElRayes W, Wilson F et al (2015) Disparities in the receipt of robot-assisted radical prostatectomy: between-hospital and within-hospital analysis using 2009–2011 California inpatient data. BMJ Open 5:e007409. https://doi.org/10.1136/bmjopen-2014-007409
Kim SP, Boorjian SA, Shah ND et al (2013) Disparities in access to hospitals with robotic surgery for patients with prostate cancer undergoing radical prostatectomy. J Urol 189:514–520. https://doi.org/10.1016/j.juro.2012.09.033
Blake EA, Sheeder J, Behbakht K et al (2016) Factors impacting use of robotic surgery for treatment of endometrial cancer in the United States. Ann Surg Oncol. https://doi.org/10.1245/s10434-016-5252-x
Esselen KM, Vitonis A, Einarsson J et al (2015) Health care disparities in hysterectomy for gynecologic cancers: data from the 2012 national inpatient sample. Obstet Gynecol 126:1029–1039. https://doi.org/10.1097/AOG.0000000000001088
Robinson CN, Balentine CJ, Sansgiry S, Berger DH (2012) Disparities in the use of minimally invasive surgery for colorectal disease. J Gastrointest Surg 16:897–903. https://doi.org/10.1007/s11605-012-1844-3
Siemens DR, Mackillop WJ, Peng Y et al (2014) Processes of care and the impact of surgical volumes on cancer-specific survival: a population-based study in bladder cancer. Urology 84:1049–1057. https://doi.org/10.1016/j.urology.2014.06.070
Halm EA, Lee C, Chassin MR (2002) Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med 137:511–520
Keller DS, Hashemi L, Lu M, Delaney CP (2013) Short-term outcomes for robotic colorectal surgery by provider volume. J Am Coll Surg 217:1063–1069.e1. https://doi.org/10.1016/j.jamcollsurg.2013.07.390
Geller EJ, Matthews CA (2013) Impact of robotic operative efficiency on profitability. Am J Obstet Gynecol 209:20.e1-5. https://doi.org/10.1016/j.ajog.2013.03.030
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Richard J. Fantus, Andrew Cohen, Christopher B. Riedinger, Kristine Kuchta, Chi H. Wang, Katharine Yao, Sangtae Park declare they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fantus, R.J., Cohen, A., Riedinger, C.B. et al. Facility-level analysis of robot utilization across disciplines in the National Cancer Database. J Robotic Surg 13, 293–299 (2019). https://doi.org/10.1007/s11701-018-0855-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-018-0855-9