Abstract
Left-sided partial anomalous pulmonary venous connection to the coronary sinus is a rare congenital cardiac defect. Surgical repair is indicated to prevent cardiopulmonary morbidities that may occur in later age. Although the conventional median sternotomy or thoracotomy incisions are used during surgical repair, robotic surgery can be a feasible alternative approach to this pathology. In this case, we report a 14-year-old child, who was diagnosed with left partial anomalous pulmonary venous connection to the coronary sinus. A total endoscopic robotic repair was successfully done via right atriotomy approach. After routing of the pulmonary venous return from the left lung to the left atrium, the interatrial septum was reconstructed with a pericardial patch. We report a successful use of totally endoscopic robotic approach in a patient diagnosed with left-sided partial anomalous pulmonary venous connection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Left-sided partial anomalous pulmonary venous connection (PAPVC) is a rare congenital cardiac defect in childhood [1,2,3]. This condition mostly presents with drainage of the left upper pulmonary veins to the left brachiocephalic vein by way of an anomalous vertical vein [1, 2]. The left pulmonary veins are rarely directly connected to the coronary sinus (CS) or to the right atrium. Left-sided PAPVC may be associated with a partial or complete defect in the roof of CS, atrial septal defect and a left persistent superior vena cava [1]. In such cases, traditional incisions are mostly used, but minimally invasive or totally endoscopic approaches can be preferred as an alternative approach.
Case report
A 14-year-old male was presented to our centre with dyspnea on exertion that has been prolonging for 6 months. The patient weighted 66 kg with a height of 170 cm and a body mass index of 22.8 kg/m2. Physical examination was found to be normal. There was no cyanosis or abnormality of cardiopulmonary system. However, transthoracic echocardiography revealed anomalous drainage of the left pulmonary veins to the CS and to the right atrium with left ventricular ejection fraction of 65% and mean pulmonary artery pressure of 15 mmHg. Transesophageal echocardiography (TEE) showed an enlarged CS and dilated right cardiac chambers with an atrial septal defect of 7 mm in diameter which was located at the orifice of the CS. The right pulmonary veins drained to the left atrium. Thorax computed tomography confirmed the left-sided PAPVC to the CS (Fig. 1) without anomalous systemic venous return.
During operation, the da Vinci SI robotic surgery system (Intuitive Surgical, Inc, Sunnyvale, CA, USA) was used. Following systemic heparinization, venous cannulation was done through right internal jugular vein with right femoral vein and arterial cannulation was done through femoral artery under TEE guidance. Right anterolateral minithoracotomy of 3–4 cm used as the working port was done. A 30° endoscope was inserted into the pleural space through the fourth intercostal space anteriorly to scan the thorax. Two additional instrumental ports were inserted through the third and fifth intercostal space. Atrial retractor was introduced through the fifth intercostal space anteriorly. Carbon dioxide (2 l/min) was insufflated through the trocar in the fifth intercostal space that was used to insert atrial retractor.
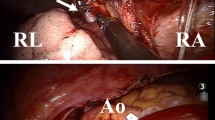
After initiation of cardiopulmonary bypass, the robotic right and left instruments, surgical endoscope, and atrial retractor were inserted into the right hemithorax. Ventilation of the lungs was terminated and a pericardiotomy incision was done 2 cm above and parallel to the right phrenic nerve to expose the aorta, vena cava inferior/superior, and the right atrium. Then, a long-shafted cardioplegia cannula was placed at the right anterolateral side of ascending aorta. This cannula was inserted through the working port, snared, and fixated. A transthoracic aortic cross-clamp was introduced into the right hemithorax through the fourth intercostal space in the mid-axillary line. After clamping the ascending aorta, cardiac arrest was achieved using antegrade blood cardioplegia solution. Both vena cava were occluded using atraumatic vascular bulldog clamps. An oblique right atriotomy incision was done away from the sinus node to avoid iatrogenic injury of the sinus node and its feeding artery. We observed an enlarged orifice of the CS and mild pulmonary venous blood draining the left lung (Fig. 2a) in the right atrium chamber. A small atrial septal defect was located at the terminal portion of the CS. Our plan was to re-route the pulmonary venous blood from the left lung to the left atrium (Fig. 3). First, atrial septostomy was done to create a large communication between the right and left atria, and the roof of the coronary sinus was exposed (Fig. 2b). Then, a “cut back” incision was made to resect a V-shaped tissue along the roof of the CS (Fig. 2b, c). The unroofing of the CS enabled us to create an unrestricted communication between the left pulmonary venous return and the left atrium. Finally, the large interatrial communication was closed using bovine pericardial patch leaving the orifice of the CS and left pulmonary venous return to the left atrium (Fig. 2d). After closure of right atriotomy, the aortic cross-clamp was released, and the heart started beating spontaneously on sinus rhythm. The patient was weaned from cardiopulmonary bypass uneventfully. A 32-F chest tube was placed in the right hemithorax. The robotic instruments and trocars were removed, and the incisions were sutured. Cardiopulmonary bypass and aortic clamping times were 68 and 35 min, respectively.
Illustration of surgical technique after right atriotomy (a) and septostomy (b), the coronary sinus is unroofed and left pulmonary venous drainage was directed to the left atrium (c). Interatrial communication was closed with a patch (d). CS coronary sinus, IVC inferior vena cava, MV mitral valve, SVC superior vena cava, TV tricuspid valve
Postoperative transesophageal echocardiography demonstrated no residual shunt. The patient was discharged on postoperative day 3 and was clinically well after 6 month follow-up period.
Discussion
The repair of PAPVC is traditionally made using median sternotomy and, in some cases, thoracotomy incisions. Robotic surgery is mostly used as an alternative to these incisions in patients with mitral valve pathologies, ischemic heart disease, intracardiac tumors, and atrial septal defects [4,5,6]. However, it can also be a feasible approach in some types of PAPVC of the left and right upper pulmonary veins [2, 3]. In the literature, there are still limited data on the feasibility and safety of minimally invasive robotic repair of this pathology. Recently, total endoscopic robotic repairs of partial left upper PAPVC to the innominate vein and right PAPVC to the right atrium have been reported [2, 3]. In the first case, Pirelli et al. [2] showed minimally invasive, robotically assisted, off-pump technique for correction of left upper PAPVC. The authors used Da Vinci robot for mediastinal dissection and isolation of the distal segment of the left superior anomalous pulmonary vein from the brachiocephalic vein. In this case, anastomosis of the pulmonary vein to the left atrial appendage was performed under direct vision through left thoracotomy incision. In the other case, Onan et al. [3] showed the feasibility of robot-assisted repair of right PAPVC to the right atrium. The authors made a large atrial septectomy and then re-routing of the right anomalous pulmonary venous return to the left atrium using pericardial patch. To the best of our knowledge, this is the only case of robotically assisted repair of a left PAPVC to the CS to have been reported.
Technically, surgical management of left PAPVC to the CS includes re-routing of the left pulmonary venous blood flow to the left atrium. This prevents left-to-right shunting, increased pulmonary blood flow, and systemic desaturation [1]. Following atrial septostomy, a large communication should be created between the roof of the CS and the left atrium (unroofing), which allows an unrestricted return of pulmonary blood flow to the left atrium. A restrictive communication may cause increased pulmonary venous pressure in the left lung and desaturation. The use of a larger patch for closure of interatrial septum also decreases the risk of restricted blood flow from the pulmonary veins. The repair technique is feasible with robotic approach, but the surgeons should exclude additional cardiac pathologies preoperatively to prevent unexpected complications during procedure. Specifically, in adolescents, the thoracic cage should be well developed to make a proper docking and feasibility of robotic procedure.
Chest wall size is an important predictor of the feasibility of robotic cardiac procedures, especially in small patients [7]. The docking procedure or placement of the robotic arms of DaVinci Si endoscopic system needs a relatively large area on the anterolateral chest wall. If all robotic instruments are positioned in a narrow area, the arms of the patient-side unit may collide and affect the feasibility of procedures. Besides, if the intercostal spaces are relatively narrow and the height of the thoracic cage is limited, the placement of the robotic instruments can be difficult or impossible for a cardiac procedure. Moreover, the thickness and size of trocars that are used to insert robotic instruments, endoscope, and retractor into the chest cavity is another issue for robotic set-up in small patients. These are main limitations for the use of robotic system in small patients. In our 5-year experience, the youngest case was 13-year-old female with a weight of 40 kg and a height of 140 cm. In this case, the width of intercostal space was almost 1 cm. In addition, no patient less than 13 year of age was operated robotically due to limitations of set-up procedure in small patients. However, this problem can be eliminated in DaVinci Xi system due to very thin robotic arms and endoscope as well as due to different set-up of the robotic system. However, we still need more specific and advanced robotic systems in the future to be able to perform robotic cardiac procedures in small patients.
In addition, a pediatric robotic surgery program is necessary for cardiac surgeons to address the initial education and training requirements [7]. It has been recommended that the practitioners should have preclinical and clinical education and training programs before application of robotic skills on small patients. With an increasing experience in adults, robotic surgery teams may perform totally endoscopic cardiac operations in small patients. It has been speculated that a satisfactory learning curve needs an initial 20 cases. We have proved this hypothesis in our experience, because all team members including anesthesiologist, perfusionist, technicians, intensivists, and surgeons do a totally different surgical procedure. In each step of operations, each member of the robotic surgery team has a different set-up and approach. In the early period of our experience, operative times for atrial septal defect closure were slightly longer than the conventional procedures. After the learning curve period, cardiopulmonary bypass, aortic clamping, and operation times became comparable to the conventional case, as done in the current case. Hospital stay of a robotic case is also comparable to the conventional cases [3,4,5,6, 8]. In our experience, almost 200 cases of atrial septal defect closure have been performed during the last 5 years. In addition, we perform two or three robotic cases in a week, and therefore, we are ready for repair of suitable cardiac pathologies. We may conclude that the feasibility and surgical strategy of robotic repair should be considered preoperatively, and associated anomalies should be excluded to prevent unexpected complications during operation.
In robotic surgery, skin incisions are very limited, bleeding is minimal and postoperative return to daily life is quicker [3]. The dynamic robotic atrial retractor and endoscopic three-dimensional view greatly enhance the exposure of intracardiac structures such as tricuspid and mitral valve, coronary sinus, pulmonary veins, and cardiac chambers. Despite the advantages of robotic surgery, the lack of tactile feedback is a major disadvantage during manipulations on atrial wall, pericardial patch, and suture needles. The traditional incisions should be preferred in more complicated cases of PAPVC and associated anomalies.
In conclusion, we present a unique application of totally endoscopic robotic surgery in a case of left PAPVC with a favorable outcome. This procedure requires training and maintenance of skills in robotic cardiac surgery.
References
ElBardissi AW, Dearani JA, Suri RM, Danielson GK (2008) Left-sided partial anomalous pulmonary venous connections. Ann Thorac Surg 85:1007–1014
Pirelli L, Kliger CA, Patel NC, Bono M, Ruiz CE, Jelnin V, Fontana GP (2017) Minimally invasive robotically assisted repair of partial anomalous venous connection. Innovations (Phila) 12:71–73
Onan B, Aydin U, Turkvatan A, Bakir I (2016) Robot-assisted repair of right partial anomalous pulmonary venous return. J Card Surg 31:394–397
Onan B, Aydin U, Basgoze S, Bakir I (2016) Totally endoscopic robotic repair of coronary sinus atrial septal defect. Interact Cardiovasc Thorac Surg 23:662–664
Onan B, Kahraman Z, Erturk M, Erkanli K (2017) Robotic resection of giant left ventricular myxoma causing outflow tract obstruction. J Card Surg 32:281–284
Onan B, Kadirogullari E, Guler S, Kahraman Z (2017) Robotic-assisted removal of an Amplatzer atrial septal occluder device for residual shunting, closure of septal defect and simultaneous tricuspid annuloplasty. J Robot Surg. https://doi.org/10.1007/s117010170709
Cundy TP, Mayer EK, Camps JI, Olsen LH, Pelizzo G, Yang GZ, Darzi A, Najmaldin AS (2015) Education and training in pediatric robotic surgery: lessons learned from an inaugural multinational workshop. J Robot Surg 9:57–63
Xiao C, Gao C, Yang M, Wang G, Wu Y, Wang J, Wang R, Yao M (2014) Totally robotic atrial septal defect closure: 7-year single-institution experience and follow-up. Interact Cardiovasc Thorac Surg 19:933–937
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors “Burak Onan”, “Unal Aydin”, “Ersin Kadirogullari”, and “Erkut Ozturk” declare that they have no conflict of interest.
Informed consent
“Written informed consent was obtained from the patient for publication of this Case Report/any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.”
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Onan, B., Aydin, U., Kadirogullari, E. et al. Robotic repair of left-sided partial anomalous pulmonary venous connection to the coronary sinus. J Robotic Surg 13, 319–323 (2019). https://doi.org/10.1007/s11701-018-0825-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-018-0825-2