Abstract
Credentialing processes for surgeons seeking robotic thoracic surgical privileges are not evidence-based, and the learning curve has not been reported. The goal of this study is to review our experience with robotic lobectomies and provide evidence for the development of a more uniform credentialing process. We performed a retrospective review of the first 272 consecutive robotic lobectomies performed between 2011 and 2017 by a single surgeon with prior video-assisted thoracoscopic (VATS) experience. Primary outcomes were operative duration, blood loss, chest tube duration, length of hospital stay, intraoperative complication, and conversion to thoracotomy. The patients were subdivided by surgical date into two cohorts of 120 consecutive patients to compare differences in outcomes, thereby illustrating the learning curve. Between 2011 and 2017, 272 patients (median age 67.5 years) underwent a robotic lobectomy by a single surgeon. The majority of patients (157/272) had early stage (T1N0) adenocarcinoma. For the entire cohort, median operative time was 160 min (83–317 min). The median blood loss was 75 mL (10–4000 mL). Median chest tube duration was 2 days (1–23 days) and median hospital stay was 3 days (1–25 days). Intraoperative complications occurred in seven patients. Only six patients required conversion to thoracotomy. Using multivariable logistic regression, it was found that the age, gender, and stage do not factor into conversion to thoracotomy, but BMI was found to be a significant covariate (p 0.043). As the surgeon performs more surgeries, there is a significantly shorter operative time (p < 0.001), decreased blood loss (p < 0.001), and shorter hospital stay (p < 0.014). When the first 120 and last 120 surgeries were compared, there was significantly less blood loss (234.6 vs 78.69 cc, p < 0.001), shorter operative time (181.9 vs 147.4 min, p < 0.001), shorter tube duration (3.49 vs 3.11 days, p 0.007), and shorter length of stay (4.03 vs 3.48 days, p < 0.001), respectively. More intraoperative complications were observed during the first 120 surgeries (6/120) compared to the last 120 surgeries (0/120; Fischer exact p = 0.029). Regression model plots did not show any apparent and significant change points, but rather a steady improvement. The more cases the surgeon does, the better is the outcome in terms of operative duration, blood loss, post-operative length of stay and intraoperative complications. The learning curve for robotic surgery for a surgeon with prior VATS experience is that of a continuous improvement with experience instead of a particular change point. Since most thoracic surgeons who perform robotic-assisted surgery have already gotten past their VATS learning curves, they no longer have a definable learning curve for robotic surgery. Hence, if a surgeon is already proficient and credentialed to perform VATS lung resections, he or she is no longer faced with a significant learning curve for robotic lung resections, and should be credentialed to do so once he or she has undergone the appropriate training with the equipment and technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Minimally invasive lobectomy has now been accepted as the standard of care for early stage non-small cell lung cancer. As compared to open thoracotomy, minimally invasive lobectomy has been proven to offer equivalent long-term oncologic results yet is associated with shorter recovery and length of stay, decreased blood loss, and atrial fibrillation [1]. However, despite these proven advantages, in 2008, according to the Society of Thoracic Surgeons (STS) database, only 30% of lobectomies were being performed using a minimally invasive approach [2]. Nationwide, this proportion was even lower. Of these minimally invasive lobectomies, only a minority were performed robotically. The majority were being done by thoracoscopy (VATS). In a similar analysis based on the European Society of Thoracic Surgeons database published by Falcoz et al. the proportion of minimally invasive lobectomies steadily increased from 1% in 2007 to 23% in 2013 [3].
Indeed, utilization of robotics in pulmonary resection remains in its infancy, but its adoption is now steadily increasing due to its many technological advantages. Compared to thoracoscopy (VATS), robotic surgery offers the advantages of three-dimensional visualization, superior visual optics, improved maneuverability in a confined space and more dexterous instrumentation. Although oncologic equivalence or superiority of robotic vs thoracoscopic surgery has not been proven, according to a study published by Oh et al., the use of robotic technology when performing a lobectomy was associated with lower blood loss, conversion rates, and post-operative complications as compared to VATS [4]. The use of robotics in lobectomy increased from 8 to 18% between 2011 and 2015 as per the Premier Healthcare Database. As robotic technology evolves and improves, its use for pulmonary resection will inevitably increase.
As with the previous introduction of other surgical techniques or tools, such as laparoscopy, there is necessarily a learning curve associated with this new technique. In the US, there has been recent litigious publicity about peri-operative complications associated with robotic surgery. To optimize patient safety, hospital administrators and surgical departments have created credentialing parameters for thoracic surgeons requesting robotic surgical privileges. However, these credentialing processes are widely variable across hospitals and are not evidence-based. The credentialing process to obtain robotic thoracic surgical privileges at our institution consists of the surgeon’s completion of a 4-h dry lab, a 1-day cadaveric lab, a live case observation and performing satisfactorily two cases under the supervision of an experienced proctoring surgeon.
The learning curve for robotic lobectomy has previously been published from our institution; however, it was a small case series of 20 patients [5]. This study reviews the first 272 consecutive robotic pulmonary lobectomies performed by a single surgeon (JPF) after his completion of the aforementioned credentialing process. The purpose of this study was to define the actual learning curve, if any, provide further insight and analysis on the learning curve, operative technique, and peri-operative complications associated with robotic pulmonary resections. This study would also validate our institution’s credentialing process.
Methods
Data collection
IRB approval was obtained to perform a retrospective analysis of a database from a single surgeon (JPF) at a single institution which was compiled from February 2011 to October 2017. The analysis consisted of his first 272 consecutive patients who underwent robotic lobectomy. This was selected from a larger cohort of patients who underwent lobectomy using other techniques. Pulmonary wedge resections and other robotic thoracic procedures were excluded. Robotic pulmonary lobectomies comprised over 80% of his robotic procedures during this time period. JPF had a recorded experience of approximately 120 thoracoscopic (VATS) lobectomies prior to performing robotic lobectomies.
Preoperative evaluation included a history and physical examination, chest computed tomography scans, positron emission tomography scans, brain magnetic resonance imaging (MRI) if indicated, as well as assessment of functional status and physiologic reserve. Age, gender, tumor stage, lobe resected, estimated blood loss, operative time, conversions to open thoracotomy, intraoperative complications, chest tube duration, hospital length of stay and 30-day mortality were all recorded and entered into a database.
Operative technique
All the lobectomies were performed using the Intuitive daVinci robotic surgical system—most using the Si model as the Xi model only became available at our institution in 2017. After double lumen endotracheal intubation, the patient was placed in lateral decubitus position with the bed flexed to place the hips below the chest wall thus decreasing hindering the movement of the camera arm. Urinary catheters are avoided and the use of peri-operative fluids is restricted.
Various port placements were trialed for the first few robotic lobectomy cases by the surgeon, but the port placement and technique was then standardized and applied consistently for the subsequent cases. This standardized port placement and technique does not vary with the laterality or lobe anatomy and is identical to the port placement used for thoracoscopic lobectomy by the same surgeon. Thus, a thoracoscopic or robotic approach can be interchangeably used with an identical four port/incision placement.
First, an 8-mm trocar is placed in line with the tip of the scapula at about the 9th intercostal space as low as possible in the pleural cavity to provide the most panoramic view of the entire pleural cavity. The remaining three ports/incisions are then placed under thoracoscopic guidance based on internal anatomy. The 8-mm 30° robotic camera is used. Warm humidified CO2 is temporarily insufflated to a maximum pressure of 8 mmHg to aid in the collapse of the lung and to improve visualization of the surgical field. A multilevel intercostal nerve block with 0.5% bupivicaine/epinephrine is performed under thoracoscopic guidance for pre-emptive analgesia in lieu of the use of a thoracic epidural.
Next, a 12-mm trocar is placed in the same 9th intercostal space 8–10 cm posterior to the camera port. This is followed by an 8-mm trocar placed in the anterior axillary line at the level of the superior pulmonary vein, and lastly a 35-mm utility incision in the anterior axillary in line with the major fissure. A 12-mm trocar is placed into this 35-mm utility incision. The robot is docked over the patient’s head and targeted 15° posterior to the midline. All four robotic arms are used. The bedside-assistant suctions retrieve specimens through the 35-mm incision. Robotic stapling is performed through either the anterior or posterior 12-mm trocars. A retraction stitch is sometimes temporarily placed on the dome of the diaphragm if necessary to optimize visualization of the surgical field (Fig. 1).
The dissection is standardized and mobilization of the lung is minimized. A “no touch” technique is used by avoiding picking up or manipulating the lobe not being resected. After division of the inferior pulmonary ligament, the posterior hilum is dissected including a lymphadenectomy of stations 7, 8, 9, and 11 (right side) and stations 7, 9, 10, and 11 (left side). An anterior hilar dissection is then performed with sequential division of the vein, artery, and bronchus in addition to lymph node stations 10 and 11. The sequence of division of the hilar structures may vary depending on the lobe resected. Mediastinal lymphadenectomy is completed of stations 2 and 4 on the right and stations 5 and 6 on the left. A single 28 French chest tube is placed.
Post-operative care is also standardized using our institutional thoracic pathway or ERAS protocol. Patients get transferred from the recovery room to the thoracic surgical ward with telemetry monitoring. Patients are ambulated within 8 h after surgery and undergo a standardized respiratory care protocol with bronchodilators every 6 h until discharged. Analgesia consists of ketorolac and acetaminophen every 6 h with supplemental oral and intravenous hydromorphone as needed. Chest tubes are placed to water seal the morning after surgery and removed once the air leak resolves and the output is serous and less than 500 mL/day. The routine use of urinary catheters is avoided. Laboratory analysis and chest radiographs are routinely obtained on post-operative day #1 and only if clinically necessary afterwards.
Statistical analysis
Continuous variables were reported as mean, median and standard deviation. Categorical variables were reported as frequency and proportion. In terms of the outcomes, operative time was analyzed in the original scale, while blood loss, chest tube duration, and post-operative length of stay were transformed logarithmically for parametric analysis. Univariable and multivariable regression models were used for continuous outcomes. The Fischer exact test was used to compare categorical variables. Regression model plots were composed. A p value of 0.05 was used to declare statistical significance.
Results
From February 2011 until October 2017, a total of 272 patients underwent robotic anatomic pulmonary resections (165 women and 107 men) by JPF. Median age was 67.5 years (22–88 years). Formal pulmonary function tests (FEV1) were inconsistently obtained pre-operatively, as clinical functional status assessment was deemed sufficient for many patients (Table 1).
Right upper lobectomies were the most frequent, comprising 104 of the 272 lobectomies. This was followed by left upper lobectomy in 51, left lower lobectomy in 42, right lower lobectomy in 41, and finally right middle lobectomy in 28 patients. Bilobectomies were done in 6 patients.
Per the American Joint Committee on Cancer (AJCC) staging, 157 patients undergoing resection for lung cancer were deemed early stage (stage I). The remaining 115 patients were advanced stage lung cancers, or metastases from a different primary tumor. All patients underwent hilar and mediastinal lymphadenectomy. The average number of lymph nodes resected was 13, with an average of 4 thoracic lymph node stations sampled (Table 2).
Median operative time for 272 patients undergoing robotic-assisted lobectomy was 160 min, ranging from 83 to 317 min. For the entire cohort, median blood loss was 75 mL (10–4000 mL). Median chest tube duration was 2 days (1–23 days) and median hospital stay was 3 days (1–25 days). There was one death, for a 30 days mortality rate of 0.4%. This patient expired of respiratory failure from pneumonia 28 days after surgery. There were no deaths attributable to the robotic technique.
Intraoperative complications occurred in seven patients. Six patients required conversion to thoracotomy, four of which were for pulmonary arterial bleeding, one for a difficult fissure and one due to significant adhesions from prior coronary bypass surgery. Using multivariable logistic regression, it was found that the age, gender, and stage do not factor in to conversion to thoracotomy, but BMI was found to be a significant covariate (p 0.043). None of these covariates (age, gender, BMI, and stage) were found to be associated with intraoperative complications (Tables 3, 4).
As the surgeon performs more surgeries, there is a significantly shorter operative time (p < 0.001), decreased blood loss (p < 0.001), and shorter hospital stay (p < 0.014). When the first 120 and last 120 surgeries were compared, there was significantly less blood loss (234.6 vs 78.69 cc, p < 0.001), shorter operative time (181.9 vs 147.4 min, p < 0.001), shorter tube duration (3.49 vs 3.11 days, p 0.007), and shorter length of stay (4.03 vs 3.48 days, p < 0.001), respectively (Tables 5, 6).
More intraoperative complications were observed during the first 120 surgeries (6/120) compared to the last 120 surgeries (0/120; Fischer exact p = 0.029). In fact, none of the intraoperative complications occurred in the last 120 patients. The most common intraoperative complication was an iatrogenic injury to the pulmonary artery PA causing pulmonary artery PA bleeding which occurred in five patients. The two other complications were a tear in the pulmonary vein and breast implant injury.
In contrast, there was no difference in conversion to thoracotomy when the first 120 and last 120 surgeries were compared.
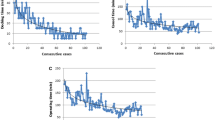
Regression model plots did not show any apparent and significant change points, but rather a steady improvement. The figures below show these regression model plots for operative time, blood loss, and tube duration.

Discussion
Since the introduction of robotics in the 1990s, this technique has been routinely implemented in many other surgical subspecialties including gynecology and urology; however, robotics in thoracic surgery remains limited and without a defined learning curve. Despite the existing evidence, there remains concern regarding safety and efficacy with regard to oncologic resection for pulmonary resection. However, this retrospective analysis demonstrates robotic lung resection as a safe and feasible option for patients.
There have been various papers published that defined a particular learning curve for VATS lobectomy. In 2014, Li et al. reported that a surgeon requires between 100 and 200 cases to be efficient at VATS lobectomy [6]. There has been conflicting evidence so far published for learning curves for robotic lobectomy. Meyer published in 2012 that the learning curve for robotic lobectomy could be possibly completed in 15 operations [7], while Toker in 2016 reported that the learning curve is completed in 14 operations [8]. Meyer looked at operative time, morbidity, mortality, conversion rate, length of stay, and surgeon comfort, and defined learning curves for each parameter, which was the change in the slope of the curve, right, where the plateau of the curve begins. Toker reported the learning curve in terms of operative time, complication, and mortality rates. In our study, however, the regression model plots did not show any significant change point or abrupt decrease in terms of operative time, blood loss, chest tube duration, and length of stay, but rather a steady improvement with more and more cases in all of these variables. Thus, we believe that there is no definite learning curve for robotic lobectomy in contrast with VATS lobectomy, the reason being that the surgeon has already gotten past his VATS lobectomy learning curve, and is already proficient at it.
As shown in Table 7, there was no correlation with conversion to thoracotomy with the number of cases. However, there was correlation with intraoperative complications. This finding may seem illogical and discordant, because intraoperative complications usually lead to conversions. It is worthwhile to note, however, that of the seven intraoperative complications, two were iatrogenic vessel injuries that were controlled robotically with pressure application, and one was a breast implant injury. These three definitely did not require conversion to thoracotomy. Three iatrogenic injuries due to PA bleeding required conversion, and these happened in the first 120 cases. There were also conversions in the last 120 cases, but these were due to severe postop adhesions from CABG and a difficult fissure. Our study also validated the previous findings that a higher BMI poses a patient at higher risk for conversion. Although BMI is not predictive, it may help especially the younger surgeons in selecting their patients early on in their practice or training. Furthermore, the results of our paper validate the fact that iatrogenic injuries remain to be the most common cause for conversions to open thoracotomy from minimally invasive approaches.
There was only one post-operative death in this series, resulting in a 0.4% mortality rate, further substantiating data that have been previously published in papers of Park [9], Meyer and Gharagozloo [10], that robotic surgery is a safe approach to pulmonary lobectomy for lung cancers. One limitation, however, is the lack of lymph node analysis. It would be interesting to analyze whether or not there is a difference or an improvement in the number of lymph node stations harvested with time and more experience.
Conclusion
Our study demonstrated a steady improvement in terms of operative time, blood loss, and post-operative length of stay with time. This may be related to experience with VATS, which may prove to be of benefit to surgeons wanting to learn robotic surgery. Since most thoracic surgeons who perform robotic lobectomies went through VATS first and have gotten past their VATS learning curves, they no longer have a definable curve for robotic surgery. Hence, if a surgeon is already proficient and credentialed to do VATS resections, he/she is no longer faced with a significant learning curve for robotic lobectomy, and should be credentialed to do so. However, this study does not address the issue of credentialing surgeons who do not have prior VATS lobectomy experience or who perform annually a small volume of lung resections. Further studies are recommended to specifically analyze the learning curves for surgeons without extensive VATS experience who are transitioning from open to robotic lobectomy.
References
Yang CJ, Sun Z, Speicher PJ, Saud SM, Gulack BC, Hartwig MG, Harpole DH Jr, Onaitis MC, Tong BC, D’Amico TA, Berry MF (2016) Use and outcomes of minimally invasive lobectomy for stage a non-small cell lung cancer in the national cancer data base. Ann Thorac Surg 101:1037–1042
Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD (2008) Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 135(2):253–254
Falcoz PE et al (2016) Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 49(2):602–609
Oh DS, Reddy RM, Gorrepati ML, Mehendale S, Reed MF (2017) Robotic-assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent premier data. Ann Thorac Surg 104:1733–1740
Hernandez JM, Humphries LA, Keeling WB, Golkar F, Dimou F, Garrett J, Sommers KE (2012) Robotic lobectomy: flattening the learning curve. J Robot Surg 6(1):41–45
Li X, Wang J, Ferguson MK (2014) Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 147:1150–1154
Meyer M, Gharagozloo F, Tempesta B et al (2012) The learning curve of robotic lobectomy. Int J Med Robot 8:448–452
Toker A, Ozyurtkan MO, Kaba E et al (2016) Robotic anatomic lung resection: the inial expereice and description of learning in 102 cases. Surg Endosc 30:676–683
Park BJ, Flores RM, Rusch VW (2006) Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 131:54–59
Gharagozloo F, Margolis M, Tempesta B (2009) Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 88:380–384
Funding
None of the authors received outside funding for the production of this original manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Baldonado, M. Amaral, J. Garrett, C. Moodie, L. Robinson, R. Keenan R, and E. M. Toloza declare that they have no conflict of interest. J. P. Fontaine has received speaking honorarium from Intuitive Surgical Inc.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5).
Rights and permissions
About this article
Cite this article
Baldonado, J.J.A.R., Amaral, M., Garrett, J. et al. Credentialing for robotic lobectomy: what is the learning curve? A retrospective analysis of 272 consecutive cases by a single surgeon. J Robotic Surg 13, 663–669 (2019). https://doi.org/10.1007/s11701-018-00902-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-018-00902-1