Abstract
We present preliminary results of a case series on refractory bladder neck contracture (BNC) treated with robot-assisted laparoscopic Y-V plasty (RAYV). Between 01/2013 and 02/2016, 12 consecutive adult male patients underwent RAYV in our hospital. BNC developed after transurethral procedures (n = 9), simple prostatectomy (n = 2) and HIFU therapy of the prostate (n = 1). Each patient had had multiple unsuccessful previous endoscopic treatments. All RAYV procedures were performed using a transperitoneal six-port approach (four-arm robotic setting). There were no intraoperative or major postoperative complications. During a median follow-up of 23.2 months two cases of refractory BNC were observed. In both cases a postoperative International Prostate Symptom Score (IPSS) of 20 and 25 was reported, respectively. In contrast, amongst the patients without evidence of refractory BNC the median IPSS was 6.5 reflecting an only mildly impaired voiding function in most cases, thus, suggesting a treatment success in 83.3% of patients. To the best of our knowledge, this is the first report on RAYV for refractory BNC. In our series RAYV was feasible in all patients, and only two cases of refractory BNC were reported during a median follow-up of almost 2 years. At the same time, no intraoperative or major postoperative complications were observed. More clinical data with a longer follow-up are needed in this promising field to reveal the actual efficacy and relevance of RAYV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the 2014 International Consultation on Urological Diseases (ICUD) consensus on urethral strictures, the correct term for narrowing of the bladder neck when the prostate is still in situ is “bladder neck stenosis” rather than “bladder neck contracture” or “bladder neck stricture” [1]. However, since the vast majority of publications in this field use the term “bladder neck contracture” (BNC) we decided to still use this term in our article. Furthermore, the ICUD consensus group proposes that genuine BNC should be clearly differentiated from cases of “vesico-urethral anastomotic stenosis” in patients after radical prostatectomy. Thus, in the present case series we report on the treatment of refractory real BNC.
BNC in the adult male patient is iatrogenic in the majority of cases. A frequent cause is a transurethral procedure for lower urinary tract symptoms resulting from benign prostatic obstruction. A very recent review and meta-analysis reports rates of 5.2, 4.6 and 2.6% following bipolar resection, monopolar resection and photo vaporization of the prostate, respectively [2]. Similar rates of up to 5% had already been observed in an open simple prostatectomy series by Helfand et al. [3]. Another patient population with a high risk of developing such a stenosis of the bladder neck is prostate cancer patients who undergo local therapy such as HIFU therapy, percutaneous pelvic radiation therapy or brachytherapy [4, 5].
In the case of primary BNC there is general agreement on the initial treatment comprising endoscopic procedures such as bladder neck incision, bladder neck resection and balloon dilation [5]. However, recurrent contracture of some degree is reported to develop in 40–50% of cases following initial treatment [6] requiring at least one repeat intervention in about 42% of patients [5]. A considerable proportion of about 11.5% of patients finally develop complex refractory BNC that makes further treatment cycles necessary [5]. Nevertheless, there is little evidence on the management of such complex refractory BNC [5]. We agree with Sokoloff et al. and it is the policy of our hospital that if a patient experiences at least two unsuccessful transurethral procedures, surgical repair in the form of a Y-V plasty should be recommended [6]. The rationale for this procedure is to prevent repeated scarring of the widened bladder neck by transposition of a well-vascularized bladder wall flap into the completely transected anterior aspect of the bladder neck.
We present preliminary results of a feasibility study on 12 patients with refractory BNC treated with robot-assisted laparoscopic Y-V plasty (RAYV) and described in detail the operative technique used. To the best of our knowledge this is the first report worldwide on such a minimally invasive robot-assisted approach to Y-V plasty.
Patients and methods
Between 01/2013 and 02/2016, 12 consecutive male patients underwent RAYV due to refractory BNC in our hospital (Table 1). In seven cases BNC developed after transurethral resection of the prostate, in two cases after simple retropubic prostatectomy and in one case each after transurethral resection of the bladder, prostatic laser vaporization and HIFU therapy. Each patient had had multiple (≥2) unsuccessful previous endoscopic treatments. In one case RAYV was accompanied by a bladder diverticulectomy that was necessary due to a large dorsolateral bladder diverticulum which most probably developed on the basis of an obstructive prostatic enlargement and initially became evident at the time of the first BNC treatment (patient no. 1). In a further case, bilateral extravesical ureteral reimplantation (in the technique by Roehl-Dreikorn) due to benign ureteral strictures was performed simultaneously (patient no. 5). The ureteral strictures developed on the level of the ureteric ostia following transurethral resection of the interureteric fold. In another case of a patient with a combination of a refractory BNC and a recurrent penobulbar urethral stricture a concurrent urethral reconstruction with bladder mucosa was necessary (patient no. 12). In this case, the use of bladder mucosa seemed to be the most favorable option since in a first stage procedure a urethral plate had already been built using buccal mucosa.
All data concerning the patient history, surgical treatment, postoperative course and follow-up were collected retrospectively using the patients’ charts and questionnaires sent to the patients. At the time of the latest follow-up round the questionnaires also comprised the validated International Prostate Symptom Score (IPSS) which is identical with the American Urological Association symptom index [7]. All patients provided written informed consent. For documentation of postoperative complications we used the Clavien–Dindo classification [8]. In lieu of a formal ethics committee, the principles of the Helsinki Declaration were followed.
Surgical technique
All procedures were performed by two experienced robotic surgeons with the daVinci SI® (Intuitive Surgical, Inc., Sunnyvale, CA) robotic system using a transperitoneal six-port approach (four-arm robotic setting as for transperitoneal robotic radical prostatectomy) with the patients in a steep Trendelenburg position (Fig. 1).
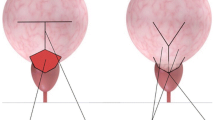
Our RAYV technique resembles the classical open approach. First, cystoscopy is performed to determine the relationship between bladder neck and urethral sphincter (Fig. 2). The prevesical space is approached for radical prostatectomy. After removal of the fatty tissue, the bladder neck is identified. Then the course of the Y-shaped incision is outlined with monopolar hot scissors. To avoid possible thermal damage to the V-shaped bladder flap the Y-incision is performed through all tissue layers of the bladder neck with a cold scissor or knife blade held by a needle driver, creating a well-vascularized anterior bladder wall flap (Fig. 3). The caudal extension of the incision is performed under direct vision up to the seminal colliculus (Fig. 4). Thereafter, interrupted sutures (3-0 polyglactin suture armed with a 5/8 needle) are placed in such a way that the apex of the V-flap is brought to the base of the Y-incision, thus accomplishing a wide bladder neck. Importantly, the first stitch is always taken through the apex of the V and the urethra at the base of the Y to keep the symmetry of the tissues (Fig. 5). At the end of the procedure a suprapubic bladder catheter and a pelvic drain are placed.
Results
The patient characteristics and perioperative results are shown in Table 1. There were no intraoperative or major postoperative complications. Six patients experienced minor complications. The postoperative hospital stay ranged from 5 to 14 days. Follow-up comprised six patients with a follow-up period of more than 2 years and four patients with a follow-up period of more than 1 year. During a median follow-up of 23.2 months two cases (16.7%) of refractory BNC were observed (patients nos. 3 and 7).
In one patient with a HIFU therapy induced BNC and a history of 12 transurethral procedures a de novo stress urinary incontinence grade 3 became apparent immediately after RAYV, which, however, resolved completely in the further course without any surgical or medical treatment, finally, leaving the patient with a good voiding function (IPSS 2) (patient no. 8). In another patient who had a urethral reconstruction with bladder mucosa at the same time as RAYV a recurrent short membranous urethral stricture developed while the bladder neck remained wide (patient no. 12). Nevertheless, following a single dilation of the re-strictured urethra the patient regained an unimpaired voiding function (IPSS 1). Patient no. 2 had to undergo a successful transobturator male sling procedure 4 months after RAYV due to a pre-existing stress urinary incontinence grade 3 that had developed after simple retropubic prostatectomy. The latter patient came from a foreign country and was lost to follow-up after discharge from our hospital following the male sling procedure (patient no. 2). Patient no. 4 was lost to follow-up after 10.1 months when he answered the last questionnaire. Thus, in both patients (nos. 2 and 4) no IPSS was available as it was only assessed in the very last follow-up round. Based on the answers of the remaining ten patients, the median IPSS was 8 reflecting a mildly to moderately impaired voiding function in most cases. Only the two patients with a recurrent BNC after RAYV reported severe voiding symptoms with an IPSS of 25 and 20, respectively (patients nos. 3 and 7). Amongst the patients without evidence of a recurrent BNC the median IPSS was 6.5. However, it is important to mention that all patients but patient no. 3 experienced an improvement in their voiding function following RAYV, and, in the light of their current situation, would retrospectively choose to undergo RAYV again. An example of a persistent wide bladder neck at 28 months after RAYV is presented in Fig. 6.
Discussion
The occurrence of BNC following open surgical or transurethral treatment for benign prostatic obstruction is a well-known fact. However, the absolute number of patients who experience this kind of complication is frequently underestimated. According to the German Federal Bureau of Statistics, 74,980 transurethral resections of the prostate were performed in Germany in 2012 and 4918 open simple prostatectomies in 2013. Thus, with BNC occurring in roughly 5% of all these patients, nearly 4000 adult men will be newly diagnosed with this complication per year in Germany [2, 3]. In the further course 40–50% of these patients (i.e. 1600–2000 men) will eventually develop refractory BNC [6]. The few publications available that discuss the treatment options in refractory BNC are in agreement about the usefulness of open surgical reconstruction procedures such as abdominoperineal bladder neck excision with anastomosis or Y-V plasty of the bladder neck in complex refractory cases [5, 6].
We and Sokoloff et al. prefer surgical repair in the form of a Y-V plasty in patients in whom the refractory BNC is the result of a benign condition [6]. However, with an open surgical approach a relatively large and morbid median laparotomy is necessary to achieve adequate exposure of the surgical field. Furthermore, optical magnification is strongly recommended to ensure precise dissection of the bladder neck and avoid damage to the urethral sphincter. In the light of the aforesaid, we believe that a laparoscopic approach using the robotic system has clear advantages compared with an open approach. On the one hand, the patient benefits from the minimal invasiveness of this laparoscopic approach. On the other hand, the accurate dexterity of the robotic surgical instruments combined with three-dimensional depth perception and magnification, enables the surgeon to perfectly mimic the open surgical technique. Additionally, it has been reported that suturing and tissue handling in the limited space of the pelvis can be more easily performed with the robot compared with conventional laparoscopy [9, 10].
The results of our case series demonstrate that RAYV is feasible and can be performed safely in patients with BNC. Especially the minimally invasive character of RAYV is corroborated since no intraoperative or major postoperative complications were observed. Furthermore, a median hospital stay of 9.5 days in patients with postoperative transurethral and suprapubic catheters, as well as an easy-flow abdominal drain in place for several days can be regarded quite short in the German Health Care System where patients are very reluctant to leave the hospital with any kind of catheter or drain in situ. During a median follow-up of 23.2 months BNC recurrence was only reported in 2 patients suggesting a success rate of RAYV of more than 80%. However, it must be considered that not all patients received urethrocystoscopy in the postoperative course so that a masked BNC recurrence cannot be entirely excluded. Nevertheless, a median IPSS of 6.5 (excluding the two patients with a recurrent BNC) most likely corroborates a treatment success in most patients. Accordingly, in the light of their current situation almost all patients would decide to undergo RAYV again in retrospect.
In general, the preliminary follow-up results of our RAYV series suggest that the robot-assisted laparoscopic approach will probably be as effective in the treatment of BNC as its open surgical ancestor that has already passed the test of time [11, 12].
Conclusions
To the best of our knowledge, this is the first report on RAYV for refractory BNC. In our case series RAYV was feasible in all patients. At the same time, no intraoperative or major postoperative complications were observed. Certainly, more clinical data with a longer follow-up are needed in this promising field to reveal the actual efficacy and relevance of RAYV. Nevertheless, authors believe that RAYV has the potential to become established as a standard procedure in patients with refractory BNC.
References
Latini JM, McAninch JW, Brandes SB, Chung JY, Rosenstein D (2014) SIU/ICUD Consultation On Urethral Strictures: epidemiology, etiology, anatomy, and nomenclature of urethral stenoses, strictures, and pelvic fracture urethral disruption injuries. Urology 83(3 Suppl):S1–S7
Cornu JN, Ahyai S, Bachmann A, de la Rosette J, Gilling P, Gratzke C, McVary K, Novara G, Woo H, Madersbacher S (2015) A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: an update. Eur Urol 67(6):1066–1096
Helfand B, Mouli S, Dedhia R, McVary KT (2006) Management of lower urinary tract symptoms secondary to benign prostatic hyperplasia with open prostatectomy: results of a contemporary series. J Urol 176(6 Pt 1):2557–2561 (discussion 2561)
Rebillard X, Soulié M, Chartier-Kastler E, Davin JL, Mignard JP, Moreau JL, Coulange C, d’Urologie Association Francaise (2008) High-intensity focused ultrasound in prostate cancer; a systematic literature review of the French Association of Urology. BJU Int 101(10):1205–1213
Ramirez D, Simhan J, Hudak SJ, Morey AF (2013) Standardized approach for the treatment of refractory bladder neck contractures. Urol Clin N Am 40(3):371–380
Sokoloff MH, Michel K, Smith RB (2010) Complications of transurethral resection of the prostate. In: Taneja SS (ed) Complications of urologic surgery—prevention and management, 4th edn. Saunders Elsevier, Philadelphia, pp 279–294
Barry MJ, Fowler FJ Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT (1992) The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 148(5):1549–1557
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Rassweiler J, Pini G, Gözen AS, Klein J, Teber D (2010) Role of laparoscopy in reconstructive surgery. Curr Opin Urol 20(6):471–482
Musch M, Hohenhorst L, Pailliart A, Loewen H, Davoudi Y, Kroepfl D (2013) Robot-assisted reconstructive surgery of the distal ureter: single institution experience in 16 patients. BJU Int 111(5):773–783
MacAlister CL (1958) The treatment of bladder-neck sclerosis by retropubic reconstruction. Br J Urol 30(1):31–33
Colabawalla BN (1969) Adult bladder neck contracture—100 Y-V plasties. Br J Urol 41(5):601
Acknowledgements
We would like to thank Ms. Hilary Coleman (medical translator) for revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Michael Musch, Jan Lukas Hohenhorst, Anne Vogel, Heinrich Loewen, Susanne Krege and Darko Kroepfl declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Musch, M., Hohenhorst, J.L., Vogel, A. et al. Robot-assisted laparoscopic Y-V plasty in 12 patients with refractory bladder neck contracture. J Robotic Surg 12, 139–145 (2018). https://doi.org/10.1007/s11701-017-0708-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-017-0708-y