Abstract
Biliopancreatic diversion with duodenal switch (BPD/DS) is considered the most effective surgical option for morbidly obese patients. Several techniques have been described: open, laparoscopic, and the combination of open and laparoscopic. Only a few centers in the world perform robotically-assisted laparoscopic BPD/DS and the published literature is limited. We describe our experience using this technique as a safe alternative for treatment of morbid obesity. A review of a prospectively maintained database from 2008 to 2011 was conducted. A total of 107 consecutive patients (F:M = 83:24) were included in this series. Average age was 44.76 years (range 20–67), body mass index 49.97 kg/m2 (range 37–70), and the number of preoperative comorbidities was 6.24 (range 3–11). The mean operative time for a typical BPD/DS with or without an appendectomy was 264 min (range 192–413), which increased to 298 min (range 210–463) when lysis of adhesion or additional procedures were performed. All study cases were completed using a minimally invasive approach. There were no intraoperative or 30-day major postoperative complications. Two patients returned to the operating room: one for endoscopic release of an inadvertently-sutured nasogastric tube during creation of the duodeno-ileal anastomosis and another patient for a port-site infection. Minor postoperative complications included carpal tunnel syndrome exacerbation (n = 1), which did not require surgical intervention. The median length of stay was 3.0 days (range 2–13). Two patients were readmitted within 30 day due to fluid retention and incarcerated umbilical hernia. The percentages of excess body weight loss (EBWL) at 1, 3, 6, 9, 12, and 18 months were 18.9, 36.4, 54.5, 67.4, 73.9, and 82.42 %, respectively. No mortality occurred in this study. Robotically-assisted laparoscopic technique for BPD/DS is a feasible, safe, and effective alternative for weight loss surgery with excellent outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliopancreatic diversion with duodenal switch (BPD/DS) is often referred to as the duodenal switch operation, and is a modification of the original biliopancreatic diversion, as described by Scopinaro in 1979 [1], and the duodenal switch operation described by Demeester in 1987 [2]. In BPD/DS, a vertical sleeve gastrectomy is performed, thus preserving the pylorus, whereas a distal gastrectomy sacrifices the pylorus in the original Scopinaro operation. Both operations have three major components in common: the stomach pouch that has a capacity of 120–250 mL, an alimentary limb of 150–250 cm in length that results in malabsorption from distal Roux-en-Y reconstruction of the intestine, and a common channel of 50–100 cm [3]. The original Scopinaro operation resulted in excellent long-term weight loss; however, it carries a significant risk of postgastrectomy syndromes that are related to the distal gastrectomy, including dumping, diarrhea, and ulceration in the area of anastomosis [4].
Currently, laparoscopic BPD/DS is performed using several methods which differ primarily in the technique for duodenoileostomy. These methods include the hand-assisted technique using a linear stapler [5], totally intracorporeal technique using a circular [6] or linear stapler [7], conventional laparoscopic hand-sewn technique [8], and the robotically-assisted hand-sewn technique [9]. BPD/DS through a midline laparotomy had been the only option until 2000 when Ren et al. [6] reported the results of the first human laparoscopic BPD/DS series. In the same year, the first robotically-assisted BPD/DS procedure with a totally intracorporeal approach was reported by Sudan et al. [10]. In the current study, we describe our experience using this approach as a safe alternative for treatment of morbid obesity, especially in high-body mass index (BMI) obese patients.
Materials and methods
A prospectively maintained database of all consecutive patients who underwent a robotically-assisted laparoscopic BPD/DS between December 2008 and July 2011 in a teaching hospital was reviewed. Patient characteristics, intraoperative details, perioperative complications (anastomotic leak, hemorrhage, intra-abdominal organ injury, intra-abdominal abscess, thromboembolic events, and superficial skin infection) weight loss outcomes, and potential benefits of this technique were analyzed.
Patient selection
The standard criteria for bariatric surgery selection were BMI > 40 kg/m2 without comorbidities, or BMI > 35 kg/m2 with at least one obesity-related comorbidity. All patients underwent a comprehensive preoperative medical evaluation, a detailed psychological assessment, relevant laboratory and radiologic testing, and esophagogastroduodenoscopy. A sleep apnea test was performed in most patients based on clinical suspicion of obstructive sleep apnea. All patients were counseled about other surgical options, including the standard laparoscopic Roux-en-Y gastric bypass (RYGB), laparoscopic vertical sleeve gastrectomy (VSG), and laparoscopic adjustable gastric banding (AGB). Patients with a high BMI (>50 kg/m2) and/or comorbidities, such as diabetes mellitus, were recommended to consider BPD/DS.
Surgical technique
The patient is positioned supine with the left arm tucked to the body. All pressure points are carefully padded and protected to avoid soft tissue and nerve injuries. Pneumoperitoneum is established using a Verees needle (Auto-Suture, Norwalk, CT, USA). A 5-mm port (Ethicon Endosurgery, Cincinnati, OH, USA) is used to enter the abdominal cavity in the left upper quadrant. Five additional ports are carefully inserted, as shown in Fig. 1. The 15-mm supraumbilical and the left lateral 11-mm ports are used as robotic arm ports, while the 11-mm right upper quadrant port is used as camera port. A Flex liver retractor (Snowden-Pencer/Cardinal Health, Dublin, OH, USA) is placed through a 5-mm right flank port under direct visualization. Dissection along the greater curvature is started 6 cm from the pylorus to the angle of His using a Harmonic™ ultrasonic dissector (Ethicon Endosurgery). A green load 60-mm Echelon linear stapler (Ethicon Endosurgery) is applied from the dissection point toward the incisura angularis, followed by sequential applications of blue load 60-mm staplers superiorly alongside the lesser curvature. A 42-French bougie is used to guide the vertical sleeve gastrectomy. The staple line is routinely imbricated using an Endo Stitch™ device (Covidien, Norwalk, CT, USA) and 2-0 Surgitek® sutures (Medical Engineering Corporation, Racine, WI, USA). Duodenal dissection is started approximately 3 cm distal to the pylorus. A Penrose drain is placed to elevate the duodenum anteriorly following adequate retroduodenal plane dissection. Duodenal transection is performed using a blue load Echelon 60™ linear stapler.
A mark is made 100 cm proximal to the terminal ileum (common channel), and a subsequent 150 cm of small bowel (alimentary limb) is measured from this point proximally. Appendectomy is routinely performed. The alimentary limb is then brought up towards the duodenal stump in an antecolic fashion. Endo Stitch™ is used to place the posterior layer of the duodeno-ileal (DI) anastomosis. The biliopancreatic limb is then divided from the alimentary limb, and an ileo-ileal (II) anastomosis is then created. The mesenteric defect is closed using Endo Stitch™ and 2-0 Surgitek® suture.
Following the passage of a 16-French nasogastric tube through an opening in the duodenal stump, the da Vinci® robotic system (Intuitive Surgical, Sunnyvale, CA, USA) is brought into the field. Two robotic needle holders are inserted via existing ports. A 3-0 Vicryl on an SH needle is used to complete a two-layered robotically-assisted DI anastomosis (Fig. 2).
Sixty mL of methylene blue is injected via the nasogastric tube after occlusion of the alimentary limb using a laparoscopic bowel clamp. An abdominal drain is routinely placed in proximity to the DI anastomosis and the gastric sleeve staple line. The stomach remnant is removed through the supraumbilical port.
Results
A total of 107 patients were analyzed in the study. The average age was 44.7 years, with female predominance, and mean excess body weight of 82 kg. Patient demographics are displayed in Table 1. The procedure was suggested for patients with a BMI of approximately 50 kg/m2 or higher, as well as those with diabetes mellitus. This suggestion was based on data published by Prachand et al. [11, 12] showing that patients with a BMI greater than 50 kg/m2 lose more weight and experienced faster resolution of diabetes and hypertension with a BPD/DS than after a Roux-en-Y gastric bypass. The mean BMI in our study patients was 49.9 kg/m2, with more than 45 % of patients suffering from diabetes mellitus.
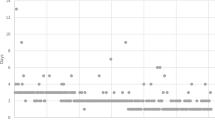
All study cases were completed using a minimally invasive, single-stage approach. Our mean ± standard deviation operative time was 264 ± 54.2 min in standard cases, with or without an appendectomy. The time increased to almost 300 min when extensive lysis of adhesion from previous open operations was warranted or other add-on procedures such as cholecystectomy, ventral, or umbilical hernia repair had to be performed (Fig. 3). Our overall operative time improved steadily from an average of 350 min in the first 15 cases to approximately 200 min in the last 15 cases. Mean blood loss was described as minimal (<50 mL) in all cases.
There were no intraoperative or 30-day major postoperative complications (Table 2). Two patients were returned to the operating room: one on postoperative day one for an endoscopic release of a nasogastric tube that had been inadvertently sutured during robotically-assisted creation of the DI anastomosis [13] and the other returned on postoperative day two because of a port-site infection. Minor postoperative complications included carpal tunnel syndrome exacerbation in one patient, which did not require a surgical intervention. The median length of stay was 3.0 days (range 2–13). Two patients stayed for 9 and 13 days due to carpal tunnel syndrome exacerbation and port-site infection, respectively. The 30-day readmission rate was 1.87 %. One patient was readmitted for fluid retention, and the other for an incarcerated umbilical hernia.
The effectiveness of BPD/DS was measured by the percentages of excess body weight loss (EBWL) at 1, 3, 6, 9, 12, and 18 months postoperatively (Fig. 4). Follow-up data was available in 86 % of the patients. No 30-day BPD/DS mortality occurred in this study.
Discussion
Marceau et al. were the first to describe a reduction in postgastrectomy syndrome associated with standard Scopinaro BPD. In this procedure, a tube along the lesser curvature of the stomach is made, preserving the pylorus. The duodenum was stapled (but not divided) proximal to the ampulla of Vater, and the first part of the duodenum was anastomosed to the alimentary limb, which enabled the gastric content to bypass the pancreatic and biliary secretions. This operation was successful in reducing postgastrectomy syndrome; however, the duodenal staple line reopened spontaneously in several patients, re-establishing the stomach–duodenum continuity [14].
In 1998, the technique was modified further by Hess and Hess, by completely dividing the duodenal staple line [15]. In the same year, Marceau et al. [14] published their results of BPD/DS with a divided duodenum and demonstrated that weight loss was equivalent, but the postgastrectomy symptoms were significantly reduced. Many authors have described single- and two-stage approaches in BPD/DS [16].
Compared with RYGB, open BPD/DS was found to have improved short-term weight loss outcomes, better long-term weight loss maintenance, lack of dumping syndrome, and improved quality of life [11, 12]. As opposed to its counterpart, RYGB, laparoscopic or robotically-assisted BPD/DS has been much slower in gaining popularity, much slower in being published in the literature with new and improved data [17], rather sluggish in transitioning from the open technique, and has somewhat higher complication rates compared to laparoscopic RYGB [8]. This may be explained by the higher technical complexity of the laparoscopic or robotically-assisted BPD/DS. Only a few centers in the world are preparing trainees/fellows to perform these rather complex operations. Hospitals also lack highly trained laparoscopic or dedicated robotic teams to achieve excellent results.
In our series, no deaths occurred postoperatively. The operative morbidity rate is also minimal compared to those from the older series of BPD/DS. The operative mortality in a large open BPD/DS series from 1993 to 2007 was approximately 1 %, with a range of 0.57–1.9 % [14, 15, 18]. These series also reported a leak rate of 2.7–3.75 %. The operative mortality rate in the first reported laparoscopic BPD/DS was 2.5 % [15], and for patients with BMI > 60 kg/m2, the rate was high as 6.5 % [19].
Although our surgeons are proficient in laparoscopic intracorporeal suturing following a formal minimally invasive fellowship training, or an extensive clinical experience, we feel that the da Vinci robotic system still offers significant advantages, especially during creation of the DI anastomosis. Improved ease of operation, visualization, precision, and range of motion, especially during critical parts of the operation, are the main benefits. The robotic system enables a bariatric surgeon to create the anastomosis in a fashion similar to the open technique.
We use a conventional laparoscopic approach to perform the vertical sleeve gastrectomy and the II anastomosis. We have achieved excellent results in our laparoscopic vertical sleeve gastrectomy and Roux-en-Y gastric bypass experience. The incidence of gastric staple lines or anastomotic leaks in our center is <1 %. Therefore, we do not feel the necessity to convert from laparoscopic stapling to robotic hand sewing. Our hope is that we will be able to perform full BPD/DS robotically in the near future with the da Vinci’s new robotic stapling devices.
The DI anastomosis is a unique anastomosis in many regards. Primarily, in this anastomosis, tissue preservation is of the utmost importance. Only a limited length of tissue is present from the pylorus to the common bile duct (±4 cm), where important nutrients and minerals are absorbed in this area. The use of the stapling technique uniformly results in tissue loss caused by the stapler width. Therefore, we prefer the robotically-assisted handsewn anastomosis technique for tissue preservation. Besides the tissue preservation, the angulation and technical access to the anastomosis via traditionally placed ports and conventional laparoscopic needle holders are often very challenging, especially in patients with BMI > 50 kg/m2. Excessive torque on the instruments caused by the thick abdominal wall and uncomfortable access result in a technically suboptimal DI anastomosis. The time needed to complete the anastomosis is also less predictable based on the patient’s abdominal morphology.
When the robotic technology is utilized, the torque and angulation problems are eliminated. The three-dimensional view produces a high degree of precision for anastomotic suturing. Additionally, the six degrees of freedom with wrist motion overcomes any angulation problems. In our experience, the DI anastomosis is consistently completed within 30 min without sacrificing perfection and precision of stitching, regardless of the patient’s weight. The importance of creating a perfect DI anastomosis is explained by several factors. Firstly, preservation of the duodenal tissue potentially improves the absorption of nutrients and minerals. Secondly, a well performed anastomosis decreases the incidence of leak and stricture. Low incidence of stricture at the DI anastomosis subsequently decreases potential leaks at the vertical sleeve suture line caused by distal obstruction.
All cases in this study were performed with the same technique—the robotically-assisted laparoscopic technique. Comparison could be made only to a historical cohort. Leak and stricture rates of 1 and 0.5 %, respectively, were reported in a modern study of duodenal switch by Buchwald et al. [20]. In our series, we have not encountered anastomotic leak or stricture in more than 150 patients to date.
Despite the advantages, lack of tactile feedback contributed to an inadvertent suturing of the nasogastric tube in one patient in our series. With the current generation of the robotic system, the operating surgeon depends only on visual inspection to avoid excessive application of force to the tissues. We hope that the future generations of the robotic system will be equipped with haptic feedback. In a majority of large hospitals such as ours, the robotic system is utilized by the department of urology (mainly for prostatectomy, and complex urinary tract reconstructions), as well as gynecology (mainly for hysterectomy and myomectomy). With already available tools and trained staff, robotically-assisted BPD/DS allows the bariatric surgeon to increase the utilization of the robotic system. This helps minimize the costs for investment and maintenance of the da Vinci system. Well-trained operating room staff decrease the average time to set up the robotic system to approximately 9–10 min. This emphasizes the importance of a dedicated team and trained personnel.
Conclusions
The robotic surgical system is a useful advanced tool in laparoscopic BPD/DS. It allows a complex procedure to be performed through a minimally invasive approach, rather than requiring conversion to an open operation. The main advantages of the robotic system includes precise suturing related to improved visualization, range of motion, and elimination of tremor. It also allows the surgeon to overcome significant torque from a thick abdominal wall in bariatric patients. The absence of tactile feedback is a disadvantage of this technique. Advances in robotic technology in the future will likely eliminate these disadvantages and improve applicability of the system. Robotically-assisted laparoscopic BPD/DS is safe and effective, and should be considered for high-BMI bariatric patients when an appropriately trained operating room team is available.
References
Scopinaro N, Gianetta E, Civalleri D et al (1979) Bilio-pancreatic bypass for obesity: initial experience in man. Br J Surg 66(9):618–620
Demeester TR, Fuchs KH, Ball CS, Albertucci M, Smyrk TC, Marcus JN (1987) Experimental and clinical results with proximal end-to-end duodenojejunostomy for pathologic duodenogastric reflux. Ann Surg 206(4):414–426
Scopinaro N, Gianetta E, Civallery D et al (1979) Bilio-pancreatic bypass for obesity: 1. An experimental study in dogs. Br J Surg 66(9):613–617
Scopinaro N, Adami GF, Marinari GM et al (1998) Biliopancreatic diversion. World J Surg 22(9):936–946
Rabkin RA, Rabkin JM, Metcalf B et al (2003) Laparoscopic technique for performing duodenal switch with gastric reduction. Obes Surg 13(2):263–268
Ren CJ, Patterson E, Gagner M (2000) Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg 10(6):514–523
Ramos AC, Galvao Neto M, Santana Galvao M et al (2007) Simplified laparoscopic duodenal switch. Surg Obes Relat Dis 3(5):565–568
Sudan R, Puri V, Sudan D (2007) Robotically assisted biliary pancreatic diversion with a duodenal switch: a new technique. Surg Endosc 21(5):729–733
Baltasar A (2007) Hand-sewn laparoscopic duodenal switch. Surg Obes Relat Dis 3(1):94–96
Sudan R, Sudan D (2002) Development of a totally intracorporeal robotic assisted biliary pancreatic diversion with duodenal switch. Obes Surg 12:205
Prachand VN, Davee RT, Alverdy JC (2006) Duodenal switch provides superior weight loss in the super-obese (BMI ≥ 50 kg/m2) compared with gastric bypass. Ann Surg 244(4):611–619
Prachand VN, Ward M, Alverdy JC (2010) Duodenal switch provides superior resolution of metabolic comobidities independent of weight loss in the super-obese (BMI ≥ 50 kg/m2) compared with gastric bypass. J Gastrointest Surg 14(2):211–220
Sucandy I, Antanavicius G (2011) A novel use of endoscopic cutter: endoscopic retrieval of a retained nasogastric tube following a robotically assisted laparoscopic biliopancreatic diversion with duodenal switch. N Am J Med Sci 3:486–488
Marceau P, Biron S, Bourque RA et al (1993) Biliopancreatic diversion with a new type of gastrectomy. Obes Surg 3(1):29–35
Hess DS, Hess DW (1998) Biliopancreatic diversion with a duodenal switch. Obes Surg 8(3):267–282
Milone L, Strong V, Gagner M (2005) Laparoscopic sleeve gastrectomy is superior to endoscopic intragastric balloon as a first-stage procedure for superobese patients (BMI ≥ 50 kg/m2). Obes Surg 15:612–617
Sudan R, Jacobs DO (2011) Biliopancreatic diversion with duodenal switch. Surg Clin N Am 91:1281–1293
Marceau P, Biron S, Hould FS et al (2007) Duodenal switch: long term results. Obes Surg 17(11):1421–1430
Kim WW, Gagner M, Kini S et al (2003) Laparoscopic vs open biliopancreatic diversion with duodenal switch: a comparative study. J Gastrointest Surg 7(4):552–557
Buchwald H, Kellogg TA, Leslie DB et al (2008) Duodenal switch operative mortality and morbidity are not impacted by body mass index. Ann Surg 248(4):541–548
Acknowledgments
We thank Thomas Leibrandt for editorial assistance during the writing of the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Podium presentation at the Society of American Gastrointestinal and Endoscopic Surgeons Annual Meeting 2012, San Diego, California, USA.
Rights and permissions
About this article
Cite this article
Antanavicius, G., Sucandy, I. Robotically-assisted laparoscopic biliopancreatic diversion with duodenal switch: the utility of the robotic system in bariatric surgery. J Robotic Surg 7, 261–266 (2013). https://doi.org/10.1007/s11701-012-0372-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-012-0372-1