Abstract
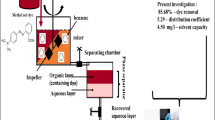
Liquid–liquid extraction has gained huge attention in chemical industries for the removal of valuable products. The aim of this research to optimize the percentage dye removal, distribution coefficient and solvent capacity for liquid–liquid extraction of methyl red dye from its aqueous solution with benzene as an extractant. In this work, effect of parameters such as dye concentration in feed (20–100 ppm), extraction time (10–30 min), and dye solution to solvent ratio (1–3 mL/mL) was examined at constant pH, and temperature of 3, and 27 ± 2 °C. Response surface methodology (RSM)-based Box–Behnken design was used for optimization by considering independent and dependent factors. The optimization results reveal that the percentage dye removal, distribution coefficient and solvent capacity of 81.28%, 4.33 and 21.7 mg/L were achieved at dye concentration in feed, extraction time, and dye solution to solvent ratio of 45 ppm, 27 min, and 1.5 mL/mL, respectively. Thus, benzene could be the potential solvent for liquid–liquid extraction of methyl red dye from its aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The textile industry is one of the important industries generating a large amount of industrial wastewater (Khan and Malik 2014). The main attraction of any fabric is its colour from synthetic dyes (Husain 2006). Synthetic dyes offer a wide range of bright colours. However, their toxicity has caused great concern to environmental activists (Frick 2003). The use of synthetic dyes has a negative effect on all types of life. Sulphur, naphthalene, vat dyes, nitrates, acetic acid, soaps, enzymes, chromium compounds, copper, arsenic, lead, cadmium, mercury, nickel, cobalt and some by-products make textile waste highly toxic. These organic substances react with many disinfectants, especially chlorine, to generate carcinogenic substances (Kuppusamy et al. 2016). If this wastewater enters the fields, it will clog the soil pores and reduce the productivity of the soil (Abou-Hadid 2003).
According to the United States Geological Survey (USGS), if wastewater is not treated properly, the environment and human health will be adversely affected (Missimer and Maliva 2018). Vulnerabilities include fish and wildlife populations, lack of oxygen, beach closures and other restrictions on recreational water use (Topare et al. 2011). There are two main stages in wastewater treatment: primary and secondary treatment (Udayakumar et al. 2021). In the primary condition, the solids are allowed to settle, and the waste is removed from the water. They use biological processes to further purify secondary wastewater (Asthana et al. 2017). Sometimes these conditions are combined, and in some cases, additional treatment such as tertiary treatment and advanced sewage treatment is used (Nascimben Santos et al. 2020). The primary treatment involves screening and sedimentation. Screening removes large floating objects such as rags and sticks that can clog pipes or damage equipment. After the sewage is filtered, it goes to a tank where solids can be removed by sedimentation (Nithya et al. 2021).
Wastewater is treated by conventional methods such as oxidation, adsorption, and membrane processes (Kapoor and Sivamani 2021). In addition, liquid–liquid extraction is one of the methods that are not employed in broad wastewater research due to its high operational cost (Fredij et al. 2015). Liquid–liquid extraction, also known as solvent extraction and separation, is a method of separating compounds or metal complexes into two different unmixed liquids, usually water and an organic solvent. From a liquid, the net transfer of one or more organisms from one liquid state to another, usually happens from water to organic matter (Huddleston et al. 1999). The exchange is driven by chemical energy, i.e., the overall composition of the chemical components that make up the solvents and solvents during the transfer is in a stable structure low free energy (Rosenthal et al. 1996). However, liquid–liquid extraction methods are required when factors such as low volatility, heat-sensitive materials, high operating costs/investments, and complex process sequences are involved (De Castro and Garcıa-Ayuso 1998).
Next, it is necessary to consider the solvent recovery features because they will also affect the overall process economics (Degryse et al. 2009). In most cases, the bulk of the capital expenditure is paid in the extraction column. Materials that cannot withstand boiling compounds (azeotropes) or filtration temperatures can often be separated even under vacuum by liquid–liquid extraction (Bidari et al. 2013). In dilute solutions in equilibrium, the ratio of the concentrations of a solution in two phases is called the distribution coefficient or distribution constant. Thus, the distribution coefficient based on the weight fraction of the solution can be given in two stages. The concentration of the solution and the ratio of the solvent in the solution are called the selective or separation factor. Higher values of the distribution coefficient are generally desirable because a given extraction duty will require less solvent. The solvent must be insoluble in the original liquid, and it must be highly soluble to dissolve (Sprakel and Schuur 2019).
From the analysis of literature, methylene blue dye is removed using 0.072 M salicylic acid (Soniya and Muthuraman 2015), and mixture of edible paraffin oil and heptane in the volume ratio of 4:1 (Zereshki et al. 2018), and reported 93 and 95% removal, respectively, through liquid membrane technologies. Similarly, astacryl golden yellow, Rhodamine B, and methyl violet dyes are removed using palm oil (Muthuraman and Palanivelu 2006), sunflower oil (Muthuraman and Teng 2009a, b), and waste vegetable oil (Shokri et al. 2020), respectively, and reported > 90% removal through liquid membranes (Chang 2020). Hence, in the present work, solvent extraction is attempted for the removal of methyl red dye from its aqueous solution using benzene as a solvent, and optimized using three-factor-three-level Box–Behnken design.
Materials and methods
Chemicals
Methyl red, benzene, and separating funnel were used from the laboratories of University of Technology and Applied Sciences Salalah, Oman. Double distilled water was used unless otherwise specified.
Batch liquid–liquid extraction studies
A stock solution (1000 ppm) of methyl red dye was prepared by dissolving 1 g of dye in 1 L of water. Standard solutions were prepared at the concentration of 20, 60 and 100 ppm. The dye solution of 100 mL was mixed with benzene solvent at a specified time. The mixture was allowed to equilibrium in a separating funnel. After equilibrium, extract (top) and bottom (raffinate) layers form. The dye concentration in raffinate was determined using UV–visible spectrophotometer to calculate percentage dye removal, distribution coefficient, and solvent capacity, as given in Equations (1) to (4) below.
Statistical optimization of liquid–liquid extraction by Box–Behnken design
Response surface methodology develops second-order polynomial equation with minimum trials and error. Also, it is used to study the interaction between independent factors (Sivamani et al. 2020). It has two designs: Box–Wilson or central composite design and Box–Behnken design. According to their principle, number of experiments performed for Box–Wilson design is 2f + 2*f + m and 2*f*(f − 1) + m for Box–Behnken design, where f is number of independent factors and m is number of centre points (Sivamani and Baskar 2018; Joaquin et al. 2021). Normally, Box–Behnken design provides more accurate results with a lesser number of experiments.
An original version of Design-Expert 13, from Stat-Ease, Inc. USA, was used to perform the response surface design and analysis. Box–Behnken design is used in this research to maximize percentage dye removal, distribution coefficient and solvent capacity at optimum values of dye concentration in feed (20–100 ppm), extraction time (10–30 min), and dye solution to solvent ratio (1–3 mL/mL). In total, 15 experiments were performed for three independent factors with three centre points. All the experiments were performed as per the Box–Behnken design matrix, and the experimental data were tested to fit linear, 2FI (two-factor interactive), quadratic and cubic models for their significance. Then, the data were fitted to the significant quadratic equation to investigate the effect of independent factors on the response as represented in Eq. (4) and model coefficients evaluated using Eq. (5). After evaluation, the coefficients were substituted in Eq. (4) for checking the deviation between experimental and predicted values. The significance of the model was tested using analysis of variance (ANOVA) based on high F-value and low p-value with 95% confidence level (Sivamani et al. 2020). Also, regression coefficient R2, difference between adjusted and predicted R2, and predicted residual error sum of squares (PRESS) values were also assessed to find the goodness-of-fit between experimental and predicted values (Vijayanand et al. 2021).
where βo is an intercept; βi, βii, βij are linear, squared and interaction coefficients, respectively, Y is the response; Xi and Xj are independent factors; and ε is a random error.
Results and discussion
Statistical optimization of liquid–liquid extraction by Box–Behnken design
Table 1 shows the independent factors and levels selected for liquid–liquid extraction of methyl red dye from its aqueous solution with benzene as an extractant. The number of experiments performed in Box–Behnken design is 2*f*(f−1) + m = 2 × 3 × 2 + 3 = 15 according to the principle.
Table 2 shows the Box–Behnken matrix of 15 experiments for liquid–liquid extraction of methyl red dye from its aqueous solution with benzene as an extractant. The various models, viz., linear, 2FI, quadratic and cubic models were tested for liquid–liquid extraction of methyl red dye from its aqueous solution with benzene as an extractant.
The values of F, p, R2, difference between adjusted and predicted R2 and PRESS were used to check the significance of model. A sufficient F-value, low p-value of < 0.05, R2 > 0.8, difference between adjusted and predicted R2 < 0.2 and a least value of PRESS for quadratic model implied that it is significant and suitable for the liquid–liquid extraction of methyl red dye from its aqueous solution with benzene as an extractant.
The experimental data were fitted to the quadratic model, as represented in Eqs. (6), (7) and (8) for percentage dye removal, distribution coefficient and solvent capacity, respectively. All the intercept, linear, interactive and quadratic coefficients were alternative at negative and positive signs, respectively. The responses, percentage dye removal, distribution coefficient and solvent capacity tend to increase with increase in independent factors, dye concentration in feed, extraction time, and dye solution to solvent ratio in the studied range. According to the principle of maxima and minima, negative coefficients in linear terms and positive coefficients in quadratic terms tend to decrease the response and vice versa. Similarly, a decline in positive coefficient terms tends to diminish the dependent factor and vice versa (Chandrasekaran and Sivamani 2018). In Eqs. (6)–(7), positive values of coefficients in linear terms and negative values of coefficients in quadratic terms tend to maximize the responses. Also, all the data points lie within ± 10% of predicted and experimental values, which is in agreement with the statistical inference (Table 3).
Table 4 shows the analysis of variance (ANOVA) for quadratic model developed for liquid–liquid extraction of methyl red dye using benzene. From the ANOVA table, it is evident that the models were significant with high F-values of 5.83, 5.80 and 191.6 for percentage dye removal, distribution coefficient, and solvent capacity, respectively, and low p-values of < 0.05. ANOVA shows difference in significance of model terms for solvent capacity than percentage dye removal and distribution coefficient. For percentage removal and distribution coefficient, interactive terms and quadratic terms involving dye concentration in feed and dye solution to solvent ratio were not significant with p-values > 0.05. But, for solvent capacity, an interactive term involving dye concentration in feed and extraction time was not significant with p-value > 0.05. All the remaining terms are significant with p-value < 0.05. This means that linear terms are contributing more to the maximizing the responses. Also, it is evident that standard deviation of response values is 0.5029 which is less than ± 5%. Coefficient of variance (C.V.) of 6.25% implied that the experimental values are in accordance with the predicted values.
Table 4 shows the statistical significance for response factors for liquid–liquid extraction of methyl red dye using benzene. The deviation between experimental and predicted values were studied through standard deviation, coefficient of variation, determination coefficient (R2), adjusted and predicted R2, PRESS, AICc and BIC.
Standard deviation, coefficient of variation, determination coefficient (R2), difference between adjusted and predicted R2 are used as model fitting parameters for response factors. They should be < 10%, between 5 and 10, > 0.8 and < 0.2, respectively. PRESS, BIC and AICc are used as model comparison parameters for dependent variables. They should be minimum, maximum and minimum, respectively, for statistical significance. Table 4 shows that all the criteria were satisfied by the models.
Effect of process parameters on percentage dye removal
Figure 1a shows the 3D interactive effect between dye concentration in feed and extraction time on percentage dye removal at constant dye solution to solvent ratio. The interactive effect was studied by varying dye concentration in feed from 20 to 100 ppm and extraction time from 10 to 30 min at dye solution to solvent ratio of 2 mL/mL. The dye removal of 46.28% was obtained at the lowest dye concentration in feed and extraction time of 20 ppm and 10 min, respectively. From this point, when the dye concentration in feed increased to 100 ppm, the dye removal enhanced to 48%.
Similarly, the dye removal increased to 80.57% at the dye concentration in feed and extraction time of 20 ppm and 30 min, respectively. The dye removal decreased to 57.42% when the dye concentration in feed further increased to 100 ppm at the extraction time and dye solution and solvent ratio of 30 min and 2 mL/mL. Also, the dye removal of 67.71% was obtained at extraction time of 20 min at the dye concentration in feed of 60 ppm. From the interactive effect between dye concentration in feed and extraction time at constant dye solution to solvent ratio, the optimal conditions were found to be dye concentration in feed and extraction time of 20 ppm and 30 min, respectively, to obtain dye removal of 80.57%.
Figure 1b shows the 3D interactive effect between dye concentration in feed and dye solution to solvent ratio on percentage dye removal at constant extraction time. The interactive effect was studied by varying dye concentration in feed from 20 to 100 ppm and dye solution to solvent ratio from 1 to 3 mL/mL at extraction time of 20 min. The dye removal of 69.42% was obtained at the lowest dye concentration in feed and dye solution to solvent ratio of 20 ppm and 1 mL/mL, respectively. From this point, when the dye concentration in feed increased to 100 ppm, the dye removal decreased to 47.14%.
Similarly, the dye removal increased to 81.42% at the dye concentration in feed and dye solution to solvent ratio of 20 ppm and 3 mL/mL, respectively. The dye removal decreased to 55.71% when the dye concentration in feed further increased to 100 ppm at the dye solution to solvent ratio and extraction time of 3 mL/mL and 20 min. Also, the dye removal of 67.71% was obtained at dye solution to solvent ratio of 2 mL/mL at the dye concentration in feed of 60 ppm. From the interactive effect between dye concentration in feed and dye solution to solvent ratio at constant extraction time, the optimal conditions were found to be dye concentration in feed and dye solution to solvent ratio of 20 ppm and 3 mL/mL, respectively, to obtain dye removal of 81.42%.
Figure 1c shows the 3D interactive effect between extraction time and dye solution to solvent ratio on percentage dye removal at constant dye concentration in feed. The interactive effect was studied by varying extraction time from 10 to 30 min and dye solution to solvent ratio from 1 to 3 mL/mL at dye concentration in feed of 60 ppm. The dye removal of 45.42% was obtained at the lowest extraction time and dye solution to solvent ratio of 10 min and 1 mL/mL, respectively. From this point, when the extraction time increased to 30 min, the dye removal decreased to 56.57%.
Similarly, the dye removal increased to 58.28% at the extraction time and dye solution to solvent ratio of 10 min and 3 mL/mL, respectively. The dye removal increased to 66% when the extraction time further increased to 30 min at the dye solution to solvent ratio and dye concentration in feed of 3 mL/mL and 60 ppm. Also, the dye removal of 67.71% was obtained at dye solution to solvent ratio of 2 mL/mL at the extraction time of 20 min. From the interactive effect between extraction time and dye solution to solvent ratio at constant dye concentration in feed, the optimal conditions were found to be extraction time and dye solution to solvent ratio of 20 min and 2 mL/mL, respectively, to obtain dye removal of 67.71%.
Effect of process parameters on distribution coefficient
Figure 2a shows the 3D interactive effect between dye concentration in feed and extraction time on distribution coefficient at constant dye solution to solvent ratio. The interactive effect was studied by varying dye concentration in feed from 20 to 100 ppm and extraction time from 10 to 30 min at dye solution to solvent ratio of 2 mL/mL. The distribution coefficient of 2.85 was obtained at the lowest dye concentration in feed and extraction time of 20 ppm and 10 min, respectively. From this point, when the dye concentration in feed increased to 100 ppm, the distribution coefficient enhanced to 2.96.
Similarly, the distribution coefficient increased to 4.97 at the dye concentration in feed and extraction time of 20 ppm and 30 min, respectively. The distribution coefficient decreased to 3.54 when the dye concentration in feed further increased to 100 ppm at the extraction time and dye solution and solvent ratio of 30 min and 2 mL/mL. Also, the distribution coefficient of 4.17 was obtained at extraction time of 20 min at the dye concentration in feed of 60 ppm. From the interactive effect between dye concentration in feed and extraction time at constant dye solution to solvent ratio, the optimal conditions were found to be dye concentration in feed and extraction time of 20 ppm and 30 min, respectively, to obtain distribution coefficient of 4.97.
Figure 2b shows the 3D interactive effect between dye concentration in feed and dye solution to solvent ratio on distribution coefficient at constant extraction time. The interactive effect was studied by varying dye concentration in feed from 20 to 100 ppm and dye solution to solvent ratio from 1 to 3 mL/mL at extraction time of 20 min. The distribution coefficient of 4.28 was obtained at the lowest dye concentration in feed and dye solution to solvent ratio of 20 ppm and 1 mL/mL, respectively. From this point, when the dye concentration in feed increased to 100 ppm, the distribution coefficient decreased to 2.90.
Similarly, the distribution coefficient increased to 5.02 at the dye concentration in feed and dye solution to solvent ratio of 20 ppm and 3 mL/mL, respectively. The distribution coefficient decreased to 3.44 when the dye concentration in feed further increased to 100 ppm at the dye solution to solvent ratio and extraction time of 3 mL/mL and 20 min. Also, the distribution coefficient of 4.18 was obtained at dye solution to solvent ratio of 2 mL/mL at the dye concentration in feed of 60 ppm. From the interactive effect between dye concentration in feed and dye solution to solvent ratio at constant extraction time, the optimal conditions were found to be dye concentration in feed and dye solution to solvent ratio of 20 ppm and 3 mL/mL, respectively, to obtain distribution coefficient of 5.02.
Figure 2c shows the 3D interactive effect between extraction time and dye solution to solvent ratio on distribution coefficient at constant dye concentration in feed. The interactive effect was studied by varying extraction time from 10 to 30 min and dye solution to solvent ratio from 1 to 3 mL/mL at dye concentration in feed of 60 ppm. The distribution coefficient of 2.8 was obtained at the lowest extraction time and dye solution to solvent ratio of 10 min and 1 mL/mL, respectively. From this point, when the extraction time increased to 30 min, the distribution coefficient decreased to 3.49.
Similarly, the distribution coefficient increased to 3.59 at the extraction time and dye solution to solvent ratio of 10 min and 3 mL/mL, respectively. The distribution coefficient increased to 4.07 when the extraction time further increased to 30 min at the dye solution to solvent ratio and dye concentration in feed of 3 mL/mL and 60 ppm. Also, the distribution coefficient of 4.17 was obtained at dye solution to solvent ratio of 2 mL/mL at the extraction time of 20 min. From the interactive effect between extraction time and dye solution to solvent ratio at constant dye concentration in feed, the optimal conditions were found to be extraction time and dye solution to solvent ratio of 20 min and 2 mL/mL, respectively to obtain distribution coefficient of 4.17.
Effect of process parameters on solvent capacity
Figure 3a shows the 3D interactive effect between dye concentration in feed and extraction time on solvent capacity at constant dye solution to solvent ratio. The interactive effect was studied by varying dye concentration in feed from 20 to 100 ppm and extraction time from 10 to 30 min at dye solution to solvent ratio of 2 mL/mL. The solvent capacity of 4.62 mg/L was obtained at the lowest dye concentration in feed and extraction time of 20 ppm and 10 min, respectively. From this point, when the dye concentration in feed increased to 100 ppm, the solvent capacity enhanced to 24 mg/L.
Similarly, the solvent capacity decreased to 8.05 mg/L at the dye concentration in feed and extraction time of 20 ppm and 30 min, respectively. The solvent capacity decreased to 2.87 mg/L when the dye concentration in feed further increased to 100 ppm at the extraction time and dye solution and solvent ratio of 30 min and 2 mL/mL. Also, the solvent capacity of 20.31 mg/L was obtained at extraction time of 20 min at the dye concentration in feed of 60 ppm. From the interactive effect between dye concentration in feed and extraction time at constant dye solution to solvent ratio, the optimal conditions were found to be dye concentration in feed and extraction time of 20 ppm and 30 min, respectively, to obtain solvent capacity of 24 mg/L.
Figure 3b shows the 3D interactive effect between dye concentration in feed and dye solution to solvent ratio on solvent capacity at constant extraction time. The interactive effect was studied by varying dye concentration in feed from 20 to 100 ppm and dye solution to solvent ratio from 1 to 3 mL/mL at extraction time of 20 min. The solvent capacity of 13.88 mg/L was obtained at the lowest dye concentration in feed and dye solution to solvent ratio of 20 ppm and 1 mL/mL, respectively. From this point, when the dye concentration in feed increased to 100 ppm, the solvent capacity decreased to 47.14 mg/L.
Similarly, the solvent capacity decreased to 5.42 mg/L at the dye concentration in feed and dye solution to solvent ratio of 20 ppm and 3 mL/mL, respectively. The solvent capacity increased to 18.57 mg/L when the dye concentration in feed further increased to 100 ppm at the dye solution to solvent ratio and extraction time of 3 mL/mL and 20 min. Also, the solvent capacity of 20.31 mg/L was obtained at dye solution to solvent ratio of 2 mL/mL at the dye concentration in feed of 60 ppm. From the interactive effect between dye concentration in feed and dye solution to solvent ratio at constant extraction time, the optimal conditions were found to be dye concentration in feed and dye solution to solvent ratio of 20 ppm and 3 mL/mL, respectively, to obtain solvent capacity of 20.31 mg/L.
Figure 3c shows the 3D interactive effect between extraction time and dye solution to solvent ratio on solvent capacity at constant dye concentration in feed. The interactive effect was studied by varying extraction time from 10 to 30 min and dye solution to solvent ratio from 1 to 3 mL/mL at dye concentration in feed of 60 ppm. The solvent capacity of 27.25 mg/L was obtained at the lowest extraction time and dye solution to solvent ratio of 10 min and 1 mL/mL, respectively. From this point, when the extraction time increased to 30 min, the solvent capacity decreased to 33.94 mg/L.
Similarly, the solvent capacity decreased to 11.65 mg/L at the extraction time and dye solution to solvent ratio of 10 min and 3 mL/mL, respectively. The solvent capacity increased to 13.2 mg/L when the extraction time further increased to 30 min at the dye solution to solvent ratio and dye concentration in feed of 3 mL/mL and 60 ppm. Also, the solvent capacity of 20.31 mg/L was obtained at dye solution to solvent ratio of 2 mL/mL at the extraction time of 20 min. From the interactive effect between extraction time and dye solution to solvent ratio at constant dye concentration in feed, the optimal conditions were found to be extraction time and dye solution to solvent ratio of 20 min and 2 mL/mL, respectively, to obtain solvent capacity of 20.31 mg/L. Table 5 compares outcomes of liquid–liquid extraction of various dyes on solvents.
Conclusion
The presented work aimed to maximize the percentage dye removal, distribution coefficient and solvent capacity by varying dye concentration in feed (20–100 ppm), extraction time (10–30 min), and dye solution to solvent ratio (1–3 mL/mL) was examined at constant pH and temperature of 3 and 27 ± 2 °C. The optimal values show that the percentage dye removal, distribution coefficient, and solvent capacity of 81.28%, 4.33, and 21.7 mg/L were achieved at dye concentration in feed, extraction time, and dye solution to solvent ratio of 45 ppm, 27 min, and 1.5 mL/mL, respectively. The results showed the best goodness-of-fit between experimental and predicted values by Box–Behnken design. Thus, benzene could be the potential solvent for liquid–liquid extraction of methyl red dye from its aqueous solution.
Abbreviations
- β o :
-
Intercept
- β i :
-
Linear coefficient
- β ii :
-
Squared coefficient
- β ij :
-
Interaction coefficient
- Y :
-
Response matrix
- X :
-
Input matrix
- ε :
-
Random error
- A :
-
Concentration of dye in feed
- B :
-
Extraction time
- C :
-
Dye solution to solvent ratio
- Y 1 :
-
Dye removal
- Y 2 :
-
Distribution coefficient
- Y 3 :
-
Solvent capacity
References
Abou-Hadid AF (2003) The use of saline water in agriculture in the Near East and North Africa Region: present and future. J Crop Prod 7(1–2):299–323
Asthana M, Kumar A, Sharma BS (2017) Wastewater treatment. In: Principles and applications of environmental biotechnology for a sustainable future. Springer, Singapore, pp 173–232
Bidari E, Irannejad M, Gharabaghi M (2013) Solvent extraction recovery and separation of cadmium and copper from sulphate solution. J Environ Chem Eng 1(4):1269–1274
Chandrasekaran AP, Sivamani S (2018) Statistical modeling and optimization of pretreatment for fermentable sugars production from cotton gin waste. Energy Sources Part a: Recov Util Environ Effects 40(4):400–405
Chang SH (2020) Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery—a review. Environ Sci Pollut Res 27(26):32371–32388
De Castro ML, Garcıa-Ayuso LE (1998) Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal Chim Acta 369(1–2):1–10
Degryse F, Smolders E, Parker DR (2009) Partitioning of metals (Cd Co, Cu, Ni, Pb, Zn) in soils: concepts, methodologies, prediction and applications—a review. Eur J Soil Sci 60(4):590–612
Fredj SB, Nobbs J, Tizaoui C, Monser L (2015) Removal of estrone (E1), 17β-estradiol (E2), and 17α-ethinylestradiol (EE2) from wastewater by liquid–liquid extraction. Chem Eng J 262:417–426
Frick D (2003) The coloration of food. Rev Prog Color Relat Top 33(1):15–32
Huddleston JG, Willauer HD, Griffin ST, Rogers RD (1999) Aqueous polymeric solutions as environmentally benign liquid/liquid extraction media. Ind Eng Chem Res 38(7):2523–2539
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26(4):201–221
Joaquin AA, Nirmala G, Kanakasabai P (2021) Response surface analysis for sewage wastewater treatment using natural coagulants. Polish J Environ Stud 30(2):1
Kapoor RT, Sivamani S (2021) Exploring the potential of Eucalyptus citriodora biochar against direct red 31 dye and its phytotoxicity assessment. Biomass Convers Biorefinery 1:1–12
Khan S, Malik A (2014) Environmental and health effects of textile industry wastewater. In: Environmental deterioration and human health. Springer, Dordrecht, pp 55–71
Kuppusamy S, Palanisami T, Megharaj M, Venkateswarlu K, Naidu R (2016) In-situ remediation approaches for the management of contaminated sites: a comprehensive overview. Rev Environ Contam Toxicol 236:1–115
Missimer TM, Maliva RG (2018) Environmental issues in seawater reverse osmosis desalination: Intakes and outfalls. Desalination 434:198–215
Muthuraman G (2011) Extractive removal of astacryl blue BG and astacryl golden yellow dyes from aqueous solutions by liquid–liquid extraction. Desalination 277(1–3):308–312
Muthuraman G, Palanivelu K (2006) Transport of textile dye in vegetable oils based supported liquid membrane. Dyes Pigm 70(2):99–104
Muthuraman G, Teng TT (2009a) Extraction of methyl red from industrial wastewater using xylene as an extractant. Prog Nat Sci 19(10):1215–1220
Muthuraman G, Teng TT (2009b) Use of vegetable oil in supported liquid membrane for the transport of Rhodamine B. Desalination 249(3):1062–1066
Muthuraman G, Teng TT (2010) Solvent extraction of methyl violet with salicylic acid from aqueous acidic solutions. Desalination 263(1–3):113–117
Muthuraman G, Teng TT, Tan SH (2012) Liquid–liquid extraction of Cibacron Red FN-R by TBAB as an extractant. Desalination 284:135–141
Muthuraman G, Teng TT, Leh CP, Norli I (2009) Extraction and recovery of methylene blue from industrial wastewater using benzoic acid as an extractant. J Hazard Mater 163(1):363–369
Nascimben Santos E, László Z, Hodúr C, Arthanareeswaran G, Veréb G (2020) Photocatalytic membrane filtration and its advantages over conventional approaches in the treatment of oily wastewater: A review. Asia-Pac J Chem Eng 15(5):e2533
Nithya K, Sathish A, Sivamani S (2021) In situ synthesis of mesostructured iron oxide nanoparticles embedded in L. camara: adsorption insights and modeling studies. Biomass Convers Biorefinery 1:1–12
Rosenthal A, Pyle DL, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enzyme Microb Technol 19(6):402–420
Shokri A, Daraei P, Zereshki S (2020) Water decolorization using waste cooking oil: an optimized green emulsion liquid membrane by RSM. J Water Process Eng 33:101021
Sivamani S, Baskar R (2018) Process design and optimization of bioethanol production from cassava bagasse using statistical design and genetic algorithm. Prep Biochem Biotechnol 48(9):834–841
Sivamani S, Baskar R, Chandrasekaran AP (2020) Response surface optimization of acid pretreatment of cassava stem for bioethanol production. Environ Prog Sustainable Energy 39(2):e13335
Soniya M, Muthuraman G (2015) Comparative study between liquid–liquid extraction and bulk liquid membrane for the removal and recovery of methylene blue from wastewater. J Ind Eng Chem 30:266–273
Sprakel LMJ, Schuur B (2019) Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep Purif Technol 211:935–957
Topare NS, Attar SJ, Manfe MM (2011) Sewage/wastewater treatment technologies: a review. Sci Revs Chem Commun 1(1):18–24
Udayakumar GP, Muthusamy S, Selvaganesh B, Sivarajasekar N, Rambabu K, Sivamani S, Hosseini-Bandegharaei A (2021) Ecofriendly biopolymers and composites: preparation and their applications in water-treatment. Biotechnol Adv 11:07815
Vijayanand M, Varahamoorthi R, Kumaradhas P, Sivamani S (2021) Modelling and optimisation of hardness in citrate stabilised electroless nickel boron (ENi-B) coatings using back propagation neural network–Box Behnken design and simulated annealing–genetic algorithm. Trans IMF 1:1–12
Zereshki S, Daraei P, Shokri A (2018) Application of edible paraffin oil for cationic dye removal from water using emulsion liquid membrane. J Hazard Mater 356:1–8
Acknowledgements
We would acknowledge our heartfelt thanks to the President, University of Technology and Applied Sciences, Muscat and the Management of University of Technology and Applied Sciences (Salalah College of Technology), Sultanate of Oman, for the wonderful opportunity, continuing support and encouragement by providing necessary facilities for executing the research work.
Funding
The authors received no financial support for the research, authorship, and publication of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by PK and SS; methodology was done by PK and SS; software was done by SS; validation was done by PK; formal analysis was done by PK and SS; investigation was done by PK; data curation was done by PK; writing—original draft were done by PK and SS; writing—review and editing were done by PK, SS, and KT; supervision was done by SS; project administration was done by PK and SS.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanakasabai, P., Sivamani, S. & Thirumavalavan, K. Box–Behnken design and analysis for liquid–liquid extraction of methyl red dye from its aqueous solution with benzene. Chem. Pap. 77, 7225–7235 (2023). https://doi.org/10.1007/s11696-023-03013-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03013-w