Abstract
Solution combustion method is a low-cost, simple, fast and productive technique for the synthesis of nanosized particles, and therefore, it has been used for the production of a variety of fine complex oxide powders for several advanced applications, including catalysts, fuel cells and energy storage. In this study, porous cobalt oxide nanospheres have been successfully synthesized by combustion method using cobalt nitrate hexahydrate (Co (NO3)2.6H2O) and urea (NH2CONH2) as precursors at low temperature. The microstructural, dielectric, magnetic and electrochemical properties of the prepared cobalt oxide nanospheres are studied. The XRD spectra exhibited (220), (311), (222), (400), (422), (511), (440) orientations which corresponds to cubic structure of Co3O4 with Fd \(\bar 3\) m (227) space group. The crystallite size is estimated using Scherrer’s formula and is found to be 8 nm. The SEM analysis reveals the appearance of spherical grains with an average grain size of 30 nm and variable pores of 7 nm in size. The presence of cobalt–oxygen bonding and the microstructure of the synthesized sample are confirmed from the Raman and FTIR studies. The dielectric studies show that the dielectric constant and dielectric loss of the sample decrease with frequency. The magnetic modules show the ferromagnetic nature of Co3O4 nanospheres. The supercapacitive behavior of Co3O4 nanospheres in 1 M KOH aqueous electrolyte shows a high specific capacitance of 182 Fg−1 at a current density of 0.5 Ag−1 and good electrochemical stability even after 2000 cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energy conversion and storage technique has to be greatly explored in the field of research in order to meet the demands of energy in the present world and next generation. In the present world, the most important energy storage devices are batteries, capacitors and electrochemical capacitors (ECs). Among all these energy storage devices, the electrochemical capacitors are using to fill the power and energy gap between batteries and conventional capacitor owing to its long life span, high power density and good charging/discharging characteristics. As one of the key electrochemical energy storage devices, electrochemical capacitors also known as supercapacitors have, especially, shown great potential in recent years to meet the short-term power needs and energy demands over the timescale of 0.1–100 s (P. J. Hall et al. 2010). Their excellent power handling characteristics when combined with various primary energy sources allow engineers to utilize them as an attractive power solution for an increasing number of applications in order to match the specification of the primary energy sources closer to that of the average power demand in each particular case. However, to fully exploit the potential of ECs in the field of energy storage, advanced materials/electrolytes are required (M. Mirzaeian et al. 2017). One of the advances is exploring the nature of electrode material with their suitable electrolyte. The nature of electrode material governs the storing and releasing the energy of supercapacitors by accumulating charges at the interface of electrolyte and electrode or by speedy reversible oxidation–reduction reaction or by both. Nanosized metal oxide particles with porous nature are extensively used in electrochemical chemical capacitors due to high specific surface area-to-volume ratio. Transition metal oxides like MnO2, IrO2, RuO2,V2O5, NiO, Mn3O4 and TiO2 are a group of very promising supercapacitor electrode materials. Among transition metal oxides, ruthenium oxides with multiple redox states and good electrical conductivity are commonly used as a pseudocapacitor electrode (DPMD Shaik et al. 2018). However, due to expensive and toxic nature of RuO2 (Wang et al. 2014), use of RuO2 is limited; hence, alternate electrode materials for supercapacitors have to be explored. Henceforth, developing the electroactive materials with high reversible oxidation–reduction reactions and high specific surface area is an investigative area of electrochemists. Moreover, these materials must be economical. Researchers suggest the low energy density and high self-discharge of supercapacitors can be resolved by improving the capacitance of the supercapacitors and which can be further improved with the selection of suitable electrode material (Naresh et al. 2019). Works like synthesis of three-dimensional porous nanostructures with high specific surface area essential to enhance the capacitance of supercapacitors demonstrated better electrochemical performance due to its delightful structural morphology (Naresh et al. 2021).
Research shows that the spinel porous nanostructured Co3O4 is found to be one of the potential electrode materials because of its being economical, less toxic nature and possessing intrinsically high capacity (Zhang et al. 2013). Among the library of various transition metal oxides, cobalt oxide (Co3O4) emerged as a shining star on the horizon of metal oxides. It has high surface-to-volume ratio, simple preparation method, outstanding chemical durability, promising ratio of surface atoms and diverse morphology, which makes it more prominent in the family of transition metal oxides (Numan et al. 2016). Added to this, Co3O4 has greater stability and it finds many applications in various areas (Naresh et al. 2019; DPMD Shaik et al. 2021; Yildirim and Kilis 2019; Salunkhe et al. 2015). It is worthy to be noted that earlier work has been done with cobalt oxide nanoparticles as tubular structures with the integration of Co3O4 hollow nanoparticle by Zhang et al. (2017). Cobalt oxide materials can be considered as superior materials for supercapacitor application, due to fact that they have high theoretical capacitance values (~ 3,600 Fg−1), low cost, environmental friendliness, high electrochemical stability, etc.(Salunkhe et al. 2015; Liu et al. 2016; Farhadi et al. 2013). The other points which reflect that Co3O4 electrode is efficient are its resistivity to corrosion and retention of performance for long time. There are fewer reports available on the electrochemical properties of Co3O4 nanospheres in different electrolytes along with dielectric and magnetic properties. Therefore, in the present investigation, solution combustion method (Gu et al. 2007; Wen et al. 2012) has been used to synthesize porous Co3O4 with nanodimensions at low temperatures and a detailed study has been executed to explore the electrochemical properties of prepared porous Co3O4 nanospheres in different aqueous electrolytes for their effective use in supercapacitors along with their dielectric and magnetic properties.
Experimental procedure
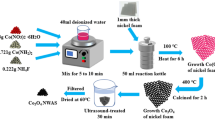
In the typical preparation, the porous Co3O4 nanospheres are synthesized by solution combustion technique. The procedure starts with the collection of two precursors, cobalt nitrate hexahydrate (Co(NO3)2.6H2O) and urea (NH2CONH2) in equimolar ratio in 100 ml of deionized water and kept under continuous stirring for half an hour. Then, the solution is kept in a pre-heated muffle furnace at 150 °C for 1 h. During the synthesis, exothermic reactions are taking place with release of gases. Then, the furnace is turned off and left for 8 h to bring it to room temperature. Finally, the Co3O4 black powder is collected and the microstructural, morphological, dielectric, magnetic and electrochemical properties are studied systematically by means of techniques compiled in Table 1.

This solution combustion method was executed due to availability of resources. The work of Byoung et al. highlights the importance of choosing a better method of synthesis in order to have a better electrochemical performance of synthesized material (Lee et al. 2016).

Material characterization
The information on studies on structural properties and chemical analysis of as prepared nanospheres is given below.
Preparation of electrode
The working electrode is prepared using synthesized black Co3O4 nanopowder at 80%, to improve electrical conductivity carbon black taken at 10% and the excellent binder which combines both carbon black and Co3O4, namely polyvinylidene fluoride taken at 10%. The above mixture was grinded for an hour. Then, it was homogenized with excellent polar solvent named N-methyl-2-pyrrolidone. This was followed by adhering the slurry on an activated and chemically cleaned 3D nickel foam substrate. Finally, the solvent was evaporated from the prepared electrode by heating in hot air oven for 2 h at 100 °C.
Preparation of 3 electrode cell
To explore the essential electrochemical behavior of Co3O4 nanospheres, a three-electrode glass cell was set up. Details of the three-electrode system are provided in Tables 2, 3 and 4.
Results and discussion
XRD analysis
The typical X-ray powder diffraction spectrum of as-synthesized porous Co3O4 nanospheres recorded in the diffraction angle 20°–80° is displayed in Fig. 1a. The XRD pattern revealed the (311) predominant orientation peak at 2θ = 37.19° with other different characterization peaks (220), (222), (400), (422), (511), (440) at Bragg angles 31.48°, 38.3°, 44.57°, 56.24°, 60° and 65.66° respectively, indicating the presence of crystalline Co3O4 (Packiaraj et al. 2019). All reflection peaks are indexed to the cubic structure of Co3O4 with Fd \({\bar{3}}\) m (227) space group with lattice constant, a = 8.072 Ǻ. The average crystalline size of the synthesized Co3O4 nanospheres is calculated using the following Debye–Scherrer Eq. (1) (Packiaraj et al. 2019) from the major diffraction peak (311) and is found to be 8 nm
Raman spectroscopy
As shown in Fig. 1b, Raman spectra of Co3O4 synthesized nanospheres were recorded in the range 350–750 cm−1 to determine the vibrational modes present in the sample. The Raman spectrum shows distinct peaks at 472, 510, 609 and 677 cm−1, which proved the Co3O4 structure, support the XRD findings. The work of as-referred Diallo et al. highlights that the Co3O4 with Co2+ (3d7) and Co3+ (3d6) located at tetrahedral and octahedral sites, respectively, crystallizes in a spinel configuration and further added that all observed modes are in good agreement with the presence of pure cobalt oxide (Diallo et al. 2015). As shown in Fig. 1b, the main peak located at 677 cm−1 corresponding to Co3+ in octahedral coordination and Co–O vibrations due to stretching (double-degenerate Eg symmetrical mode). The peak at 472 cm−1 (Eg symmetry mode) is due to Co–O out-of-plane bending vibration and 510 cm−1 due to bridge oxygen (Co–O–Co) which has asymmetric stretching. All of the peaks in the spectrum are indicating typical Raman spectrum of cubic structured Co3O4 and its purity.
FTIR analysis
Chemical bonds in the sample prepared are shown by vibrational studies on Co3O4 prepared by FTIR with 500–4000 cm−1 being the range of wavenumber in Fig. 1c. The absorption broad band at 3735.45 cm−1 is attributed to O–H. The absorption bands around 1038.25 cm−1 and 1516.55 cm−1 may be attributed to O–H bending vibrations combined with Co atoms. The strong absorption band observed at 659.51 cm−1 is attributed to stretching vibration mode of Co+2–O bonds which are tetrahedral sites. The second band at 551.39 cm−1 is due to stretching vibrational mode of Co+3–O at octahedral sites. The FTIR characteristic peaks further proved the spinel structure of cobalt oxide. The above vibrational studies are elucidated by the important work on cobalt oxide characterization which concludes the spinel Co3O4 (Simonot et al. 1997; Toniolo et al. 2010).
SEM and EDS analysis
The SEM analysis has been executed to examine the morphology of porous Co3O4 nanospheres which are prepared. It is observed that the morphology consists of agglomerated spheres with small pores. 30 nm being the average size as depicted in Fig. 2a, b and c, which vividly shows the symmetrical nature of the spheres. This small-size and nanoporous morphology of the synthesized nanospheres enhances the electrochemical performance of supercapacitors and hence makes it good electrode material in supercapacitors. The above statement is supported by earlier research reports of SEM micrographs where presence of more active ions in porous electrode enhances specific capacitance by pseudocapacitance effect (Eslam et al. 2018) and also that NiCo2O4 nanoparticles with good spherical morphology possess superior specific capacitance which will be considered as good candidate for supercapacitor electrode applications (Priyadharshini and Ganesh. 2017).
EDS was performed to investigate the chemical composition of the newly formed porous Co3O4 nanospheres. The EDS spectrum shows presence of only elements cobalt and oxygen, indicating complete chemical reduction of the hexahydrated Co(NO3)2·6H2O precursor (Diallo et al. 2015). EDS measurement is shown in Fig. 2d which shows corresponding binding energy peaks of Co and O present in the sample. Added to this, the synthesized samples purity is also reflected in EDS studies with 78.57% being the estimated amount of Co and 21.53%.The presence of only cobalt and oxygen revealed by EDS also is an indication of complete combustion as reflected by the work of Toniolo et al. which is due to the characteristics of chemical reagents such as the fuels consumed and the oxidant nature of nitrate (Toniolo et al. 2010).
XPS studies
XPS is a surface-sensitive process which provides information about atomic composition and oxidation states of the samples. Figure 3a shows the XPS survey spectrum of Co3O4 nanospheres. Figure 3b shows the Co 2P spectrum of prepared sample where the two peaks clearly indicate two oxidation states. It displays Co 2p 3/2 and Co 2p ½ with peaks at binding energies observed at 780.74 and 796 eV phases or species intrinsic to the surface of spinel. This indicates the type of surface termination for the spinel orientation which shows presence of Co+2 and Co+3 cations. According to the findings (Yildirim and Kilis 2019), a spinel orientation contains two Co+2 cations in the tetrahedral sites, two Co+3 cations in the octahedral sites and four O−2 anions which is close to the present study including the separation of the peaks 15.26 eV. Figure 3c represents O1s spectra at 530.51 eV binding energy.
Dielectric properties
Dielectric constant
Figure 4a depicts the variation of Co3O4’s dielectric constant with frequency. It is observed that the dielectric constant drops as the frequency rises. At lower frequencies, the value of dielectric constant is significantly higher. As frequency rises, it gets smaller and drops to extremely low at high frequencies where it is frequency independent. Similar types of behavior have also seen by other studies (Numan et al. 2016; DPMD Shaik et al. 2021). Space charge polarization may provide an explanation for the fluctuation in the dielectric constant. It is made up of several well-conducting grains that are spaced apart by thin, in conductive grain borders. The local movement of electrons in the direction of the electric field, which controls the polarization in Co3O4, is what causes the electronic exchange between Co2+ and Co3+. With increasing frequency, polarization falls off until it reaches a constant value. This is due to the fact that the electron exchange Co2+ and Co3+ cannot follow the alternating field above a specific amount of external field frequency (Zhang et al. 2017; Salunkhe et al. 2015).
Dielectric loss
Figure 4b depicts the variation of dielectric loss with frequency. It is observed that with an increase in frequency, dielectric loss decreases. The reason for this is because as frequency rises, polarization decreases. It eventually settles on a fixed value (Liu et al. 2016). At lower frequencies, dielectric loss has a significant value. The prevalence of species including Co2+ ions, oxygen vacancies, grain boundary defects, interfacial dislocation pileups, voids, etc., is the reason (Salunkhe et al. 2015; Wen et al. 2012). Due to any species contributing to polarizability being discovered to lag behind the applied electric field at higher frequencies, the decreasing trend in with an increase in frequency is natural (Zhang et al. 2013).
Magnetic properties
The magnetic properties of Co3O4 nanospheres at 5 and 300 K temperatures are shown in Fig. 5. As shown in Fig. 5, the hysteresis curve of Co3O4 nanospheres prepared at 473 K displays a ferromagnetic behavior at both the temperatures (5 and 300 K) with a saturation magnetization of 0.113 emu g−1 at the maximum field of 8.9 kOe applied. The ferromagnetic behavior of the sample could be attributed to the uncompensated surface spins and finite size effects (Reddy et al. 2021; Sylla et al. 2019; Yang et al. 2007).
Electrochemical properties
The electrochemical properties of synthesized Co3O4 nanospheres with porous framework and increased surface area are evaluated by cyclic voltammetry (CV), chronopotentiometry (CP) and electrochemical impedance spectroscopy (EIS) using a three-electrode aqueous method. Here, Co3O4 is used as a working electrode, Ag/AgCl as a counter, and platinum foil as a reference electrode and four electrolytic solutions of KOH, NaOH, Na2SO4 and K2SO4 are used. Figure 6a shows the cyclic voltammetry (CV) curves of the porous Co3O4 nanospheres at scan rate 5 mVs−1 having −0.2–+ 0.8 V as potential window in the above aqueous electrolytes. The CV curves display the capacitive-like characteristics from the quick reversal in the currents during the change in the potential direction (Naresh et al. 2019). In addition, CV curves show quasi-rectangular shape and more area in 1 M KOH electrolyte when compared to aqueous solutions of NaOH, Na2SO4 and K2SO4 which shows better electrochemical performance. The values of specific capacitance of the prepared sample were calculated using Eq. (2):
a CV curves of porous Co3O4 nanospheres in different electrolytes at 5 mVs−1, b CV curves of the sample in 1 M KOH at different scan rates, c charge–discharge curves in different electrolytes, d charge–discharge curves at different current densities in 1 M KOH, e specific capacitance with cycle number, f Nyquist plot before and after 2000 cycles
The specific capacitance values are 175 Fg−1 (KOH), 97 Fg−1 (NaOH), 64 Fg−1 (K2SO4) and 29 Fg−1 (Na2SO4) at 5 mVs−1 scan rate in various electrolyte solutions. The better electrochemical performance of porous Co3O4 nanospheres in aqueous KOH solution, when compared to other electrolytes like NaOH, Na2SO4 and K2SO4, is due to the greater size of hydrated Na+ ion than that of hydrated K+ ion. Added to this, other parameter is ionic conductivity which depends on ionic size that will be lower for Na+ ion hydrated compared to K+ ion. The hydrated ion size and their conductivities are compiled in Table 5.
Therefore, SO4− has lower ionic conductivity than OH− due to larger size. The ionic conductivity variation in water and other solvents exhibits an anomalous dependence on the ionic radius. Molecular dynamics simulations suggest that this has origin in the levitation effect which suggests that the diffusivity is maximum for the ion with diameter comparable to the neck diameter of the void network within the solvent. The origin of such an anomalous dependence lies in the mutual cancellation of forces (Rahimi-Nasrabadi et al. 2017; Kumar and Yashonath 2019). The CV curves of Co3O4 in 1 M KOH at different scan rates are shown in Fig. 6b. The specific capacitance values are calculated using Eq. (2) and are found to be 175, 148, 104 and 77 Fg−1 at 5, 10, 20 and 50 mVs−1, respectively. The study shows that at lower scan rates, K+ electrolytic cations penetrate into the pores of the material which leads to higher capacitance, whereas at greater scan rates they are on outer surface of the material which results in lower capacitance.
The galvanostatic charge–discharge measurements of the Co3O4 nanospheres in four electrolytes are carried out at 0.5 Ag.−1 current density. The almost symmetric nature of the charge–discharge curves of Co3O4 in KOH electrolyte shows good electrochemical reversibility of the electrode. At the same time, Co3O4 shows greater discharge time in KOH electrolyte (Fig. 6c) which leads to greater discharge capacitance. The discharge specific capacitance values are calculated using Eq. (3) (Table 6)
The Co3O4 nanospheres discharge specific capacitance in various electrolyte solutions are 182 Fg−1 (KOH), 106 Fg−1 (NaOH), 73 Fg−1 (K2SO4) and 37 Fg−1 (Na2SO4). It is to be noted that the higher specific capacitance of 182 Fg−1 was exhibited by Co3O4 which is in support with CV results. Figure 6d represents the Co3O4 charge–discharge curves at 0.5 Ag−1 current density in 1 M KOH electrolyte solution. The galvanostatic charge–discharge measurements of the Co3O4 nanospheres in 1 M KOH are carried out at different current densities ranging from 0.5 to 3 Ag−1 and are presented in Fig. 6d. The symmetric nature of the charge–discharge curves of Co3O4 in KOH electrolyte shows good electrochemical reversibility of the electrode. The specific capacitance value is calculated using Eq. (3) and is found to be 182, 154, 118 and 81 Fg−1 at 0.5, 2, 2.5 and 3 Ag−1, respectively.
Added to specific capacitance values, long-term cyclic stability is a good electrochemical characterization study to assess the fabricated porous Co3O4 nanospheres as electrode in 1 M KOH electrolyte. Figure 6e shows that after 2000 continuous cycles at 5 mVs−1 scan rate the prepared sample had 71% capacitive retention than initial value (Yang et al. 2007; Kumar and Yashonath 2019). There is a decrease in specific capacitance value with cycle number as active material is used up in the electrolyte during the early charging/discharging cycles (Wang et al. 2013) as shown in Fig. 6e. The high SCs achieved for Co3O4 nanospheres may be owing to the improved electric double-layer capacitance behavior of the sample. In particular, this porous nanostructure of Co3O4 can act as an “ion buffer reservoir” (Wang et al. 2013; Priyadharsini et al. 2020; Huang et al. 2019; Dezfuli et al. 2015). These findings have shown that with better cyclic stability of porous Co3O4 electrode it may be a long-lasting candidate for electrode material for next-generation electrochemical energy storage applications.
The electrochemical impedance spectroscopy is a core technique for studying the kinetic behavior of the ions in an electrolyte, and Fig. 6f represents EIS analysis of synthesized Co3O4 in 1 M KOH aqueous solution over a frequency range of 1 Hz–1 MHz. In Nyquist plots, at high-frequency region, the solution resistance (Rs) is taken by the intersection made on horizontal axis. Increase in solution resistance from 4–5Ω after 2000 cycles was the key observation. Charge transfer resistance (Rct) is the semicircle in high to medium-frequency region in the Nyquist plot. It was observed that after 2000 cycles the Rct of the porous sample was increased to 8–6Ω. The increase in Rs and Rct values after 2000 cycles suggests the decrease in specific capacitance with increase in cycle number. The characteristic behavior of supercapacitor is reflected in the synthesized sample by diffusive resistance in low-frequency region with a straight line behavior. Table 7 shows the comparison of the present work with previous literature.
Conclusion
Porous Co3O4 nanospheres are successfully prepared by a solution combustion technique at low temperatures for supercapacitor applications. XRD and Raman data were confirmed cubic structure of Co3O4 with an estimated crystallite size of 8 nm. SEM studies revealed a spherical shape of grains with an average grain size of 30 nm. The dielectric studies proved the frequency dependence of dielectric constant and dielectric loss. The magnetic properties of porous Co3O4 showed ferromagnetic nature of the sample. The electrochemical studies of Co3O4 nanospheres exhibited a specific capacitance of 182 Fg−1 at 0.5 Ag−1 current density in 1 M KOH aqueous electrolyte. The porous Co3O4 nanosphere electrode with good capacitive retention during cycling makes it a future potential candidate for supercapacitors. To further enhance the capacitive performance of Co3O4, the study can be extended at various fuel-to-oxidation ratios and optimizing the synthesis and annealed temperature.
References
Deori K, Ujjain SK, Sharma RK, Deka S (2013) Morphology controlled synthesis of nanoporous Co3O4 nanostructures and their charge storage characteristics in supercapacitors. ACS Appl Mater Interface 5(21):10665–10672. https://doi.org/10.1021/am4027482
Dezfuli AS, Ganjali MR, Naderi HR, Norouzi P (2015) A high performance supercapacitor based on a ceria/graphene nanocomposite synthesized by a facile sonochemical method. RSC Adv 5(57):46050–46058. https://doi.org/10.1039/c5ra02957k
Diallo A, Beye AC, Doyle TB, Park E, Maaza M (2015) Green synthesis of Co3O4 nanoparticles via aspalathus linearis: physical properties. Green Chem Lett Rev 8(3–4):30–36. https://doi.org/10.1080/17518253.2015.1082646
Eslam KFC, Aboelazm AA, Gomaa-Ali AM (2018) Cobalt oxide supercapacitor electrode recovered from spent lithium-ion battery. Chem Adv Mater 3(4):67–74
Farhadi S, Pourzare K, Sadeghinejad S (2013) Simple preparation of ferromagnetic Co3O4 nanoparticles by thermal dissociation of the [CoII(NH3)6](NO3)2 complex at low temperature. J Nanostruct Chem 3(1):4–10. https://doi.org/10.1186/2193-8865-3-16
Gu F, Li C, Hu Y, Zhang L (2007) Synthesis and optical characterization of Co3O4 nanocrystals. J Cryst Growth 304(2):369–373. https://doi.org/10.1016/j.jcrysgro.2007.03.040
Hall PJ et al (2010) Energy storage in electrochemical capacitors: designing functional materials to improve performance. Energy Environ Sci 3(9):1238–1251. https://doi.org/10.1039/c0ee00004c
Huang D, Liu H, Li T, Niu Q (2019) Template-free synthesis of NiO skeleton crystal octahedron and effect of surface depression on electrochemical performance. J Sol-Gel Sci Technol 89(2):511–520. https://doi.org/10.1007/s10971-018-4908-3
Kumar P, Yashonath S (2019) Ionic conductivity in aqueous electrolyte solutions: insights from computer simulations. J Mol Liq 277:506–515. https://doi.org/10.1016/j.molliq.2018.12.090
Lee BS et al (2016) Silicon/carbon nanotube/BaTiO3 nanocomposite anode: evidence for enhanced lithium-ion mobility induced by the local piezoelectric potential. ACS Nano 10(2):2617–2627. https://doi.org/10.1021/acsnano.5b07674
Liu XY, Gao YQ, Yang GW (2016) A flexible, transparent and super-long-life supercapacitor based on ultrafine Co3O4 nanocrystal electrodes. Nanoscale 8(7):4227–4235. https://doi.org/10.1039/c5nr09145d
Mirzaeian M et al (2017) Electrode and electrolyte materials for electrochemical capacitors. Int J Hydrogen Energy 42(40):25565–25587. https://doi.org/10.1016/j.ijhydene.2017.04.241
Naresh-Kumar-Reddy P, Shaik DP, Ganesh V, Nagamalleswari D, Thyagarajan K, Vishnu Prasanth P (2019) Structural, optical and electrochemical properties of TiO2 nanoparticles synthesized using medicinal plant leaf extract. Ceram Int 45(13):16251–16260. https://doi.org/10.1016/j.ceramint.2019.05.147
Naresh Kumar Reddy P, Shaik DPMD, Ganesh V, Nagamalleswari D, Thyagarajan K, Vishnu Prasanth P (2021) High electrochemical activity of 3D flower like nanostructured TiO2 obtained by green synthesis. Appl Surf Sci 561(May):150092. https://doi.org/10.1016/j.apsusc.2021.150092
Numan A, Duraisamy N, Saiha Omar F, Mahipal YK, Ramesh K, Ramesh S (2016) Enhanced electrochemical performance of cobalt oxide nanocube intercalated reduced graphene oxide for supercapacitor application. RSC Adv 6(41):34894–34902. https://doi.org/10.1039/c6ra00160b
Packiaraj R, Devendran P, Venkatesh KS, Asath-Bahadur S, Manikandan A, Nallamuthu N (2019) Electrochemical investigations of magnetic Co3O4 nanoparticles as an active electrode for supercapacitor applications. J Supercond Nov Magn 32(8):2427–2436. https://doi.org/10.1007/s10948-018-4963-6
Priyadharshini BST, Ganesh GRV (2017) Hydrothermal synthesis of spherical NiCO2O4 nanoparticles as a positive electrode for pseudocapacitor applications. J Sol-Gel Sci Technol. https://doi.org/10.1007/s10971-017-4504-y
Priyadharsini CI et al (2020) Sol–Gel synthesis of Co3O4 nanoparticles as an electrode material for supercapacitor applications. J Sol-Gel Sci Technol 96(2):416–422. https://doi.org/10.1007/s10971-020-05393-x
Rahimi-Nasrabadi M, Naderi HR, Karimi MS, Ahmadi F, Pourmortazavi SM (2017) Cobalt carbonate and cobalt oxide nanoparticles synthesis, characterization and supercapacitive evaluation. J Mater Sci Mater Electron 28(2):1877–1888. https://doi.org/10.1007/s10854-016-5739-z
Reddy PNK, Shaik DPMD, Nagamalleswari D, Thyagarajan K, Prasanth PV (2021) Electrochemical activity of TiO2 nanoparticles in NaOH electrolyte via green synthesis using calotropis gigantea plant leaf extract. Indian J Sci Technol 14(34):2766–2772. https://doi.org/10.17485/ijst/v14i34.1424
Salunkhe RR, Tang J, Kamachi Y, Nakato T, Kim JH, Yamauchi Y (2015) Asymmetric supercapacitors using 3D nanoporous carbon and cobalt oxide electrodes synthesized from a single metal-organic framework. ACS Nano 9(6):6288–6296. https://doi.org/10.1021/acsnano.5b01790
Shaik DPMD, Rosaiah P, Ganesh KS, Qiu Y, Hussain OM (2018) Improved electrochemical performance of Mn3O4 thin film electrodes for supercapacitors. Mater Sci Semicond Process 84:83–90. https://doi.org/10.1016/j.mssp.2018.03.013
Shaik DPMD, Kumar MVS, Reddy PNK, Hussain OM (2021) High electrochemical performance of spinel Mn3O4 over Co3O4 nanocrystals. J Mol Struct 1241:130619. https://doi.org/10.1016/j.molstruc.2021.130619
Simonot L, Garin F, Maire G (1997) A comparative study of LaCoO3, Co3O4 and LaCoO3–Co3O4: I. Preparation, characterisation and catalytic properties for the oxidation of CO. Appl Catal B Environ 11(2):167–179. https://doi.org/10.1016/S0926-3373(96)00046-X
Sylla NF et al (2019) Effect of porosity enhancing agents on the electrochemical performance of high-energy ultracapacitor electrodes derived from peanut shell waste. Sci Rep 9(1):1–15. https://doi.org/10.1038/s41598-019-50189-x
Toniolo JC, Takimi AS, Bergmann CP (2010) Nanostructured cobalt oxides (Co3O4 and CoO) and metallic Co powders synthesized by the solution combustion method. Mater Res Bull 45(6):672–676. https://doi.org/10.1016/j.materresbull.2010.03.001
Tummala R, Guduru RK, Mohanty PS (2012) Nanostructured Co3O4 electrodes for supercapacitor applications from plasma spray technique. J Power Source 209:44–51. https://doi.org/10.1016/j.jpowsour.2012.02.071
Wang J, Du N, Wu H, Zhang H, Yu J, Yang D (2013) Order-aligned Mn3O4 nanostructures as super high-rate electrodes for rechargeable lithium-ion batteries. J Power Source 222:32–37. https://doi.org/10.1016/j.jpowsour.2012.08.056
Wang X, Yan C, Sumboja A, Lee PS (2014) High performance porous nickel cobalt oxide nanowires for asymmetric supercapacitor. Nano Energy 3:119–126. https://doi.org/10.1016/j.nanoen.2013.11.001
Wen W, Wu JM, Tu JP (2012) A novel solution combustion synthesis of cobalt oxide nanoparticles as negative-electrode materials for lithium ion batteries. J Alloy Compd 513:592–596. https://doi.org/10.1016/j.jallcom.2011.11.019
Yang CM et al (2007) Nanowindow-regulated specific capacitance of supercapacitor electrodes of single-wall carbon nanohorns. J Am Chem Soc 129(1):20–21. https://doi.org/10.1021/ja065501k
Yildirim Z, Kilis S (2019) Posting patterns of students’ social presence, cognitive presence, and teaching presence in online learning. Online Learn J. https://doi.org/10.24059/olj.v23i2.1460
Zallouz S, Réty B, Vidal L, Le-Meins JM, Matei-Ghimbeu C (2021) Co3O4 Nanoparticles embedded in mesoporous carbon for supercapacitor applications. ACS Appl Nano Mater 4(5):5022–5037. https://doi.org/10.1021/acsanm.1c00522
Zhang X, Yu P, Zhang D, Zhang H, Sun X, Ma Y (2013) Room temperature synthesis of Mn3O4 nanoparticles: characterization, electrochemical properties and hydrothermal transformation to γ-MnO2 nanorods. Mater Lett 92:401–404. https://doi.org/10.1016/j.matlet.2012.11.022
Zhang H, Nai J, Yu L, David-Lou XW (2017) Metal-organic-framework-based materials as platforms for renewable energy and environmental applications. Joule 1(1):77–107. https://doi.org/10.1016/j.joule.2017.08.008
Acknowledgements
The authors acknowledge Dr. Anoop Mukharjee, Assistant Professor, and Mrs. Babita Mukharjee, Lecturer, from Prince Sultan University, Riyadh, Saudi Arabia, for their support in checking the plagiarism time to time.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afrooze, A., Shaik, D.P.M.D. Porous Co3O4 nanospheres synthesized via solution combustion method for supercapacitors. Chem. Pap. 77, 1201–1211 (2023). https://doi.org/10.1007/s11696-022-02535-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02535-z