Abstract
Imipramine hydrochloride (IMIP) is a tricyclic antidepressant utilized in the treatment of depression and chronic pain in some certain cases together with pain medication. The side effects of anxiety, insomnia, crying attacks, personality change and tachycardia are seen in imipramine overdose; therefore, determination of imipramine is an important issue. In this study, a novel potentiometric PVC membrane ion-selective sensor (ISE) was developed for monitoring of IMIP. MIL-53(Al) metal–organic framework was utilized for the first time as an electroactive material in the construction of imipramine-selective PVC membrane sensor. The sensor membrane consisting of 3.0% MIL-53(Al), 64.0% dibutylphthalate (DBP), 32.0% polyvinylchloride (PVC) and 1.0% potassium tetrakis(4-chlorophenyl)borate (KTPClPB) exhibited the most satisfied potentiometric performance characteristics. The sensor displayed a linear response for imipramine hydrochloride in the concentration range of 1.0 × 10−7 M-1.0 × 10−1 M with a slope of 57.7 mV/decade and detection limit of 5.0 × 10−8 M. The operational pH range of the sensor was determined as 3.7–8.5. The sensor showed highly reproducible and stable potentiometric responses with the response time of less than 5 s. The IMIP content of a pharmaceutical used in the treatment of depression was successfully determined with the proposed imipramine-selective sensor. Additionally, the analytical applicability of the sensor in real biological samples was demonstrated by performing imipramine determinations in spiked human blood serum and urine samples.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
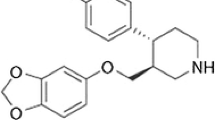
Imipramine (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N,N-dimethylpropan-1-amine) hydrochloride (IMIP) (Scheme 1) is a tricyclic antidepressant. It is a drug active ingredient used in the treatment of depression, attention deficit hyperactivity disorder, and chronic pain in combination with pain medication in some cases (Dargan et al. 2005; Breaud et al. 2010). Common side effects of imipramine use are blurred vision, xerostomia, dizziness, loss of weight and appetite. Moreover, anxiety, insomnia, crying attacks, personality change and tachycardia are among the potential side effects of imipramine overdose (Fayez and Gupta 2021). Therefore, dose setting of imipramine throughout the treatments is quite vital. The therapeutic doses of IMIP vary considerably; however, the maximum proposed daily dose is between 100 and 300 ng/mL. It was reported that the daily intake more than 500 ng/mL eventuated in overdose and more than 1 μg/mL daily intake could be even fatal (Bailey et al. 1978; Brunton et al. 2012). The monitoring of IMIP levels in blood and pharmaceutical preparations has crucial significance for its safe and effective use because its therapeutic and harmful dose levels are close to each other (Amitai and Frischer 2004).
Imipramine hydrochloride can be determined by several analytical techniques such as spectrophotometry (Mansour et al. 2017; Azmi et al. 2022; Patel and Patel 2010; Soni et al. 2011; Susmitha et al. 2013; Deepakumari et al. 2013), spectrofluorimetry (Sobhani et al. 2019; Azmi et al. 2017), atomic absorption spectrophotometry (EI-Ansary et al. 2001), flow injection methods (Acedo-Valenzuela et al. 2005; El-Gendy et al. 2006), gas chromatography (Monney et al. 2019), capillary electrophoresis (Quirino and Breadmore 2012; Wu et al. 2014; Sanagi et al. 2014), high-performance liquid chromatography (Zhao et al. 2016; Zilfidou et al. 2019) and high-performance thin-layer chromatography (Patel and Patel 2010; Srivastava et al. 2016). However, most of these techniques have some limitations such as the needs of time-consuming, tedious and complex pre-treatment processes, sophisticated instruments, skilled technicians, large volume of organic solvents and expensive consumables. In this aspect, it is important to develop a fast, simple, delicate and inexpensive alternative methods for the determination of imipramine hydrochloride.
Studies on the developments and applications of ion-selective electrodes (ISE) started at the end of the 1960s and are still continuing to attract attentions day by day (Pretsch 2007). In recent years, potentiometric ion-selective electrodes with improved performance characteristics (Ramanavicius and Ramanavicius 2021; Ramanavicius et al. 2021) have been used in numerous areas for the determinations of various species as alternative to above-mentioned expensive detection methods because of their advantages, such as high selectivity, wide operating range, low detection limits, high accuracy and precision, short analysis time, simple design, low cost, no damage to the measured material, no pre-treatment steps, determination even in colored and turbid solutions (Çoldur et al. 2016).
The literature survey indicates that all-solid-state PVC membrane-type potentiometric sensors constitute a major class of potentiometric sensors. In this type of sensors, the most important component responsible for the selective and sensitive potentiometric response of the electrode toward target species is electroactive substance, called as ionophore. Up to now, various materials have been used as ionophore substances for a large number of different chemical species. In most of the drug selective electrodes reported in the literature, the ion-pair complexes of the target drug active substances were the most preferred ionophore type among the others. However, the drug selective electrodes based on ion-pair type ionophores have usually some limitations such as high detection limit, short life-time and low selectivity.
A new group of functional material called metal–organic frameworks (MOFs) was firstly reported by Yaghi et al. (1995). MOFs are porous crystalline hybrid materials including metal centers and electron-donating multifunctional organic ligands (Zhou et al. 2012). MOFs are largely utilized in the applications of gas storage (Xiao and Yuan 2009), drug delivery (Liao et al. 2018), sensors (Kukkar et al. 2018; Li et al. 2018), catalysis (Joharian et al. 2018; Joharian and Morsali 2019) and adsorption and separation (Chen et al. 2013; Wang et al. 2016; Ma et al. 2016, 2018) owing to their ultra-high level of porosity, large specific surface area, adjustable pore size and volume, stable framework structure, tailorable structure and functionality, wide range of thermal and chemical stability, non-toxic nature, chemical functionality, perfect biocompatibility and unsaturated metal sites (ZareKarizi et al. 2018).
The MIL-53(Al) MOF structure was comprised from the chains of trans-corner-sharing [AlO4(OH)2] octahedral which are connected to each other by 1,4 benzenedicarboxylate (BDC) ligands, and thus, three-dimensional framework in which one-dimensional channels run parallel to the inorganic backbone of the structure is constructed. Several studies have been conducted on the use of aluminum-based metal–organic framework in adsorption (Trung et al. 2008; Patil et al. 2011; Zhou et al. 2013; Xie et al. 2014), gas adsorption and isolation processes (Himsl et al. 2009; Hu and Zhang 2010; Maes et al. 2010).
In recent years, in parallel to technological advances in nanoscience and nanotechnology, significant advances have been made for the use of nanostructured MOFs in electrochemical sensor applications (Zhao et al. 2019). Hence, MOFs as highly sensitive platforms in sensor studies have recently attracted increasing interest of electrochemical scientists. The redox and catalytically active sites formed by the active metals and/or ligands bring electrochemical sensing capabilities to MOFs (Lei et al. 2014; Wang et al. 2015; Kumar et al. 2017; Liu et al. 2018). Although MOFs have been broadly used in electrochemical sensor field recently, the use of MOFs in potentiometric sensor applications remained limited. There are a few studies related to use of MOFs in potentiometric sensors. In one of them, a synthesized two-dimensional conductive MOF was utilized as an ion to electron transducer on a glassy carbon electrode to enhance the potentiometric performance characteristics of the sensitive membrane covered on MOF layer (Mendecki and Mirica 2018). In a very recent study, a new cadmium-based metal–organic framework (Cd-MOF) was synthesized and incorporated as an ionophore into a Cu(II)-selective carbon paste electrode (Deghadi et al. 2021). In another recent study, an Al(III)-selective carbon paste electrode has been developed based on a novelly prepared Cu-MOF as ionophore (Mahmoud et al. 2021). In addition, a novel nano-composite based on boron-doped graphene oxide-aluminum fumarate metal–organic framework has been also reported for the fabrication of a bromide-selective electrode (Kaur et al. 2020).
This study aims to develop imipramine-selective electrode by using Mil-53 (Al) electrodes from metal–organic frameworks as ionophore material. The developed PVC membrane electrode was successfully used for determining the imipramine hydrochloride content of some curatives applied in depression treatment.
Experimental
Chemicals and apparatus
Tetrahydrofuran (THF), imipramine hydrochloride (IMIP-HCl), Mil-53 (Al), high-molecular-weight-poly (vinyl chloride) (PVC), o-nitrophenyloctylether (NPOE), and dibutylphthalate (DBF) were obtained from Sigma-Aldrich (Germany). The epoxy (TP3100) and hardener (Desmodur RFE) used in solid-contact preparation were obtained from Denlaks (Turkey)) and Bayer (Germany) companies, respectively. All chemicals used for the preparation of the solutions throughout this study were obtained from Sigma-Aldrich (Germany). Analyzed pharmaceutical (Tofranil®, 25 mg) was purchased from a local pharmacy. The human blood serum and urine samples employed in the potentiometric applications were obtained from Van Yuzuncu Yil Research Hospital. Deionized water (18.3 MΩ) used in the study was obtained from the Human Corporation Zeener Power II (Korea) water purification system. All potentiometric measurements were performed by using a laboratory-made computer-controlled potentiometric measurement system. Gamry (USA) brand saturated Ag/AgCl electrode was used as reference electrode in all potential measurements.
Fabrication of the electrodes and measurement procedure
Fabrication of the electrodes generally consisted of two steps. The first step was the preparation of the solid contacts to obtain conductive interfaces compatible for membrane covering. For the preparation of solid contacts, a homogenized mixture composed of 50% (w/w) graphite (250 mg), 35% (w/w) epoxy (175 mg) and 15% (w/w) hardener (75 mg) in 2.5 mL was formed in THF. The mixture was mixed for 5–8 min to let to reach the appropriate consistency. In follow, one open end of copper wires was dipped into this mixture several times and let to dry at room temperature for one night. At the second step, membrane cocktails consisting of the studied compositions (the masses of the membrane ingredients were 100 mg in total) were prepared by dissolving in 2 mL THF. The surfaces of the previously fabricated solid contacts were coated with the studied membrane cocktails by dipping into the membrane cocktails several times. The electrode membranes attained on the solid contact surfaces were dried at room temperature at least 12 h (Fig. 1). Finally, the fabricated electrodes were conditioned in 10−2 M 20 mL imipramine hydrochloride solution for 12 h. The potentiometric measurement cell used in the current study can be represented as in follow.
Preparation of the samples
For the preparation of the pharmaceutical sample, 10 tablets of the pharmaceutical were taken, crushed in a mortar and homogenized. Afterward, a mass corresponding to the amount of one tablet on average is weighed and dissolved in 100 mL of deionized water. The attained pharmaceutical solution was analyzed by using direct calibration method.
For the preparations of urine samples, a volume of the supplied imipramine-free urine enough for the preparation of the studied samples was centrifuged at 4500 rpm for 5 min. The obtained supernatant was employed directly for the preparation of imipramine-spiked urine samples which contain imipramine hydrochloride at the studied concentration levels. The resultant urine samples were analyzed by using standard addition method without any pre-treatment step. The procedure similar to preparation of spiked urine sample was followed for the preparation of imipramine-spiked serum samples.
Results and discussion
Investigation of optimum membrane composition
Potentiometric characteristics of a PVC membrane ion-selective electrode largely depend on the membrane composition. The ratios of ionophore, plasticizer, ionic additive and PVC as far as plasticizer and ionic additive type are substantial factors that affect the potentiometric performance characteristics of an electrode. Therefore, these membrane parameters were investigated by taking into account 22 different membrane compositions. The potentiometric performance characteristics of the prepared membrane compositions were compared to each other in terms of slope, detection limit, linear working range, and R2 value to determine the membrane composition performed the best potentiometric performance features. The studied membrane compositions and their potentiometric performance characteristics are given in Table S1 and Table S2, respectively. The data in Table S2 show that Electrode 6 exhibits the best potentiometric performance characteristic as the optimum membrane when compared to the other studied membrane compositions in terms of sensitivity, detection limit and linear operating range. Figure 2 illustrates the potentiometric responses of the imipramine-selective electrode toward the standard imipramine hydrochloride solutions within the concentration range of 1.0 × 10−1–1.0 × 10−8 M.
Determination of linear working range, detection limit and slope
The measurements taken in the series of imipramine hydrochloride standard solutions (1.0 × 10−1–1.0 × 10−8 M) were used to construct the calibration plot, and the obtained calibration plot was employed for the calculations of the slope, linear concentration range and detection limit of the imipramine-selective electrode according to IUPAC recommendations (Buck and Lindner 1994). For this, potential measurements were performed at 25 ± 1 °C from low to higher concentrations of imipramine hydrochloride. The recorded potential values were plotted versus the logarithm of the corresponding imipramine hydrochloride concentrations (log [IMIP]). The resulting calibration plot is illustrated in Fig. 3. Electrode exhibited linear response within the concentration range of 1.0 × 10−7–1.0 × 10−1 M with a slope of 57.7 mV/decade. The detection limit of the proposed electrode was also calculated as 5.0 × 10−8 M.
Determination of response time
For the investigation of its response time, the electrode was dipped into each calibration solution from low to high concentration and vice-versa, and the potential changes were monitored depending on time. While the measurement solutions were stirred at a constant speed, the required time period for the potential stabilization after each solution change was documented (t95). The average time period in which the potentials stabilized was expressed as response time of the electrode (Buck and Lindner 1994). The recorded real-time continuous potentiometric responses of the proposed electrode in 10−3 M and 10−4 M imipramine hydrochloride solutions are illustrated in Fig. 4. As seen, the response of the electrode to imipramine is quite rapid. This fact proves the presence of a fast equilibrium between the aqueous and membrane phases. The average response time of the electrode was calculated as about 3 s.
Determination of repeatability
To demonstrate the repeatability of the IMIP-Selective electrode consecutive measurements were also taken in 10−2 M, 10−3 M and 10−4 M imipramine hydrochloride solutions. The obtained potential measurements are shown in Fig. 5. The mean and standard deviation values for 10−2 M, 10−3 M and 10−4 M IMIP solutions were calculated as 2916.6 ± 1.3 mV, 2862.2 ± 2.1 mV and 2802.5 ± 0.7 mV, respectively.
Determination of pH working range
In order to examine the pH working range of the electrode 25 mL 10−3 M solutions of imipramine hydrochloride were prepared. The solution was stirred by a magnetic stirrer at 300 rpm. The reference electrode and IMIP-selective electrode were dipped into this solution. Moreover, the combined pH meter was also simultaneously immersed into the same solution. The pH of the solution was altered by gradual addition of concentrated HCl and NaOH solutions. The pH of the solution with pH electrode and the potential values from the potentiometric cell were recorded after each addition. The measured mV values were plotted versus the corresponding pH values. The obtained graph is displayed in Fig. 6. The graph indicates that the potential values obtained from the ion-selective electrode system in the pH range of 3.7–8.4 did not changed significantly. This finding proves that the potentiometric response of the electrode in the pH range 3.7–8.4 is not influenced by the pH of the measurement media. Below the pH 3.7, the increase in the measured potential can be attributed to the interference effect of hydronium ions which present in the measurement media at high level. On the other hand, it was observed that the electrode potentials begin to decrease rapidly above the pH = 8.4. The reason for this decrease may be explained by the gradual decrease in the amount of protonated imipramine at higher pHs, as expected.
Determination of selectivity
The general potentiometric response curves of the present electrode for imipramine hydrochloride and some studied interferents are shown in Fig. 7. Potentiometric selectivity coefficient defines the ability of an ISE to distinguish between the primary and interfering ions in the same solution. In the current study, separate solution method (EA = EB) (Umezawa et al. 2000) was preferred in the calculation of selectivity coefficients for certain organic molecules such as cysteamine, sunset yellow, fructose, glucose, maltose, lactose and sucrose, some common alkali metals, alkaline earth metals and heavy metals. For the calculation of the selectivity coefficients, the potential values in 1.0 × 10−2 M solutions of the interfering ions were obtained and the obtained potential values were used for the calculations of corresponding imipramine hydrochloride concentrations from the linear equation of the calibration line. The determined imipramine hydrochloride concentration values and 1.0 × 10−2 M concentration value of interferents were replaced by the equation employed for the calculation of selectivity coefficients, and thus, the selectivity coefficients of the electrode were determined for each interferent. The calculated selectivity coefficients are presented in Table S3. The obtained selectivity coefficients revealed that the electrode is very selective against the studied chemical species.
MIL-53(Al) is an infinite octahedral chain formed by coordination of Al(III) with terephthalate and OH- groups. The terephthalate ligands point in four directions, making 1D lozenge-shaped pores. The reason of this very high selectivity of the sensor toward IMIP is the synergistic effect emerged from some structural properties of MIL-53(Al) (high porosity, suitable pore size, internal pore accessibility through tunnel structure, flexibility, and high adsorption capability) and membrane ingredients. The main mechanism for selective interaction between IMIP and MIL-53(Al) is the electrostatic interactions between the protonated IMIP and negatively charged MIL-53(Al) centers (OH- groups) in addition to π–π interaction/stacking between the aromatic parts of IMIP and aromatic part of terephthalate on MOF. The presence of metallic centers, OH- groups, and shape-sensitive channels in MOF structure may be the causation of selective interactions between MIL-53(Al) and IMIP. Even so, we should express that the mechanism of this selective behavior of MIL-53(Al) for IMIP has been not clarified yet and more research is required on this fact.
Determination of life-time
For the evaluation of the sensor’s life-time, the changes in the slopes of the sensor were monitored depending on time. For this, potentiometric measurements were taken on sequential days by using the electrode in imipramine hydrochloride standard solutions which have imipramine hydrochloride in the concentration range of 1.0 × 10−1–1.0 × 10−7 M. The obtained data were utilized for the construction of the regarding calibration plots, and the slopes of the calibration plots were calculated. Before each daily calibration study, IMIP-selective electrode was conditioned in 1.0 × 10−2 M imipramine hydrochloride solution. The electrode was kept in room temperature in a closed and dark environment when not in use. The plot indicating the slopes of the proposed electrode (for three repetitions) depending on time is given with their error bars in Fig. 8. Figure 8 shows that excluding the slight decrease in the first a few days, there is no significant change in the slope of the electrode within a period of 55-days. After the slight decrease in the first 4 days, the electrode slope stayed nearly unchanged and quite stable until 55th day. After this day, slope and stability of the electrode started to decrease significantly. We can express that the proposed electrode can be used without any sensitivity problem for about two months.
Analytical application of IMIP-selective electrode
Following the evaluation of potentiometric performance characteristics of the produced electrode, its analytical application was carried out by performing the determinations of imipramine contents of an antidepressant drug, spiked human serum and urine samples.
The imipramine content of the prepared pharmaceutical solution was determined 5 times by the constructed calibration plot with the use of direct calibration method. Potentiometric responses for the calibration standards and pharmaceutical sample are illustrated in Fig. 9. The average imipramine content of the corresponding drug tablet (containing 25 mg Imipramine) was calculated as 25.08 ± 0.09 for N = 5 replicates with a recovery value of 100.32% (Relative Error % = 0.32).
Before the imipramine determinations in serum and urine samples, we examined whether the electrode behavior in serum and urine samples differed significantly compared to the aqueous solution matrix due to the matrix effect. The concentration range studied was chosen considering the therapeutic concentration range of imipramine. For this, we compared the slope values of the electrode response in these matrices in the concentration range of 2.0 × 10−6–1.0 × 10−7 M. The potentiometric responses of the electrode and related calibration plots are illustrated in Fig. 10. As can be seen in Fig. 10, the slope values for serum and urine matrix are 26.5 and 28.8 mV/decade, respectively, and these are lower compared to that obtained in aqueous solutions (39.0 mV/decade). These results indicate that the calibration plots constructed with the aqueous external standards cannot be employed for the accurate imipramine determinations in serum and urine matrixes. Therefore, standard addition method was applied for the determination of imipramine contents of spiked serum and urine samples. The spiked imipramine amounts and the imipramine amounts attained by standard addition method are summarized with the recovery values in Table 1. As can be seen in Table 1, the recovery% values for serum samples and urine samples are changed between 82.6–116.3 and 84.2–110.3, respectively.
Conclusions
We have proposed a MOF (MIL-53 (Al))-based potentiometric PVC membrane sensor for the selective determination of imipramine hydrochloride drug active substance. The membrane consisting of 3% Mil-53, 64% DBF, 32% PVC and 1% KTpClPB was determined as the optimum membrane composition in terms of potentiometric performance characteristics. The sensor allows highly accurate determination of imipramine in complex matrices due to that it exhibits high selectivity for imipramine in the presence of commonly found potential interferents in the measurement media. The sensor has a broad linear working range of 1.0 × 10−7 M-1.0 × 10−1 M with a sensitivity and detection limit of 57.7 mV/decade and 5.0 × 10−8 M, respectively. The analytical performance characteristics of the proposed sensor and various imipramine detection methods available in the literature are comparatively summarized in Table 2. As can be seen in Table 2, the proposed sensor has potential use as an alternative to sophisticated measurement techniques that require more expensive, time-consuming processes due to its ease of preparation, low cost, short response time, sensitivity and selectivity, wide linear operating range and low detection limit. Particularly, the short response time of the sensor makes it a convenient candidate for the employment as a detector in automated systems such as flow injection analysis. The ability of the sensor to detect imipramine in a very wide linear concentration range is also its another important feature when compared to the other existing methods in the literature. The developed sensor was successfully applied for the determination of the imipramine in the pharmaceutical, spiked human blood serum and spiked human urine samples. The most prominent aspect of the current work is that we manufactured a drug selective potentiometric sensor by using MOFs as ionophore for the first time. The satisfactory results show the potential usability of MOFs as ionophore in potentiometric sensor development and encourage the attempts to develop potentiometric sensors with lower detection limit and wider working range. In this context, the current study can be considered as a promising and pioneering work in the potentiometric sensor field for the future studies.
References
Acedo-Valenzuela MI, Galeano-Diaz T, Mora-Diez N, Silva-Rodriguez A (2005) Response surface methodology for the optimisation of flow-injection analysis with in situ solvent extraction and fluorimetric assay of tricyclic antideprassants. Talanta 66(4):952–960. https://doi.org/10.1016/j.talanta.2004.12.044
Amitai Y, Frischer H (2004) Excess fatality from desipramine and dosage recommendations. Ther Drug Monit 26(5):468–473. https://doi.org/10.1097/00007691-200410000-00002
Azmi SNH, Al-Hattali MK, Al-Hinai RK, Al-Ajmi IM (2017) A New spectrofluorimetric approach for the quantitation of imipramine HCl in commercial dosage forms. JAPST 1(3):29–47. https://doi.org/10.14302/issn.2328-0182.japst-17-1714
Azmi SNH, Al-Masrouri ZN, Al-Lamki IR, Al-Jabri AK, Rahman N, Nasir M, Abdelrahman K, Fnais MS, Alam M (2022) Development and validation of spectrophotometric method for determination of imipramine hydrochloride in tablets (solid materials). J King Saud Univ Sci 34(2):101823. https://doi.org/10.1016/j.jksus.2022.101823
Bailey DN, Langou RA, Jatlow PI (1978) Tricyclic antidepressants: plasma levels and clinical findings in overdose. Am J Psychiatry 135(11):1325–1328. https://doi.org/10.1176/ajp.135.11.1325
Breaud AR, Harlan R, Di Bussolo JM, McMillin GA, Clarke W (2010) A rapid and fully-automated method for the quantitation of tricyclic antidepressants in serum using turbulent-flow liquid chromatography-tandem mass spectrometry. Clin Chim Acta 411(11–12):825–832. https://doi.org/10.1016/j.cca.2010.02.067
Berm EJJ, Paardekooper J, Brummel-Mulder E, Hak E, Wilffert B, Maring JG (2013) Determination of the tricyclic antidepressants amitriptyline, nortriptyline, imipramine, desipramine, clomipramine and desmethylclomipramine in dried blood spots using LC-MS/MS. Ther Drug Monit 35(5):683
Brunton LL, Chabner BA, Knollmann BC (2012) As Bases Farmacológicas da Terapêutica de Goodman & Gilman, 12th edn. McGraw Hill, Porto Alegre
Buck RP, Lindner E (1994) Recomendations for nomenclature of ion-selective electrodes. Pure Appl Chem 66(12):2527–2536. https://doi.org/10.1351/pac199466122527
Chen XF, Ding N, Zang H, Yeung H, Zhao RS, Cheng CG, Liu JH, Chan TWD (2013) Fe3O4@MOF core–shell magnetic microspheres for magnetic solid-phase extraction of polychlorinated biphenyls from environmental water samples. J Chromatogr A 1304:241–245. https://doi.org/10.1016/j.chroma.2013.06.053
Çoldur F, Boz H, Onder A (2016) Bütünüyle katı hal PVC membran izoniazid-seçici potansiyometrik sensör. EÜ Fen Bil Enst Dergisi 9(1):29–39. https://doi.org/10.18185/eufbed.94522
Dargan PI, Colbridge MG, Jones AL (2005) The management of tricyclic antidepressant poisoning: the role of gut decontamination, extracorporeal procedures and fab antibody fragments. Toxicol Rev 24:187–194. https://doi.org/10.2165/00139709-200524030-00011
Deepakumari HN, Prashanth MK, Revanasiddappa HD (2013) Validated and sensitive spectrophotometric method for the determination of amitriptyline hydrochloride. Chem Sci J 4(2):1–5
Deghadi RG, Eliwa AS, Ali AE, Hosny WM, Mohamed GG (2021) Preparation, characterization of novel cadmium-based metal-organic framework for using as a highly selective and sensitive modified carbon paste electrode in determination of Cu(II) ion. Comments Inorg Chem 41(4):189–212. https://doi.org/10.1080/02603594.2020.1870963
EI-Ansary AL, El-Hawary WF, Issa YM, Ahmed AF (2001) Preparation characterization and thermodynamic studies of promazine chlorpromazine promethazine imipramine and cıprofloxacin ion-associates with some metal complexs ions. Synth React Inorg Met-Org Chem 31(3):441–456. https://doi.org/10.1081/SIM-100002231
El-Gendy AE, El-Bardicyy MG, Loutfy HM, El-Tarras MF (2006) Flow injection analysis of pharmaceutical compounds VI. Determination of some central nervous system acting drugs by UV-spectrophotometric detection. Spectrosc Lett 26(9):1649–1660. https://doi.org/10.1080/00387019308010764
Fayez R, Gupta V (2021) Imipramine. In: StatPearls. StatPearls publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK557656/. Accessed 9 Sep 2021
Himsl D, Wallacher D, Hartmann M (2009) Improving the hydrogen-adsorption properties of a hydroxy-modified MIL-53(Al) structural analogue by lithium doping. Angew Chem Int Ed 48(25):4639–4642. https://doi.org/10.1002/anie.200806203
Hu YH, Zhang L (2010) Hydrogen storage in metal-organic frameworks. Adv Mater 22(20):E117–E130. https://doi.org/10.1002/adma.200902096
Joharian M, Morsali A (2019) Ultrasound-assisted synthesis of two new fluorinated metal-organic frameworks (F-MOFs) with the high surface area to improve the catalytic activity. J Solid State Chem 270:135–146. https://doi.org/10.1016/j.jssc.2018.10.046
Joharian M, Morsali A, Tehrani AA, Carlucci L, Proserpio DM (2018) Water-stable fluorinated metal-organic frameworks (F-MOFs) with hydrophobic properties as efficient and highly active heterogeneous catalysts in aqueous solution. Green Chem 20:5336–5345. https://doi.org/10.1039/C8GC02367K
Kaur N, Kaur J, Badru R, Kaushal S, Singh PP (2020) BGO/AlFu MOF core shell nano-composite based bromide ion-selective electrode. J Environ Chem Eng 8(5):104375. https://doi.org/10.1016/j.jece.2020.104375
Khodari M, Mansour H, Salah El-Din H (1997) Preconcentration and determination of the tricyclic antidepressant drug - imipramine at modified carbon paste electrode. Anal Lett 30(10):1909–1921. https://doi.org/10.1080/00032719708001707
Kukkar D, Vellingiri K, Kim KH, Deep A (2018) Recent progress in biological and chemical sensing by luminescent metal-organic frameworks. Sens Actuators B-Chem 273:1346–1370. https://doi.org/10.1016/j.snb.2018.06.128
Kumar P, Vellingiri K, Kim KH, Brown RJC, Manos MJ (2017) Modern progress in metal-organic frameworks and their composites for diverse applications. Micropor Mesopor Mater 253:251–265. https://doi.org/10.1016/j.micromeso.2017.07.003
Lei J, Qian R, Ling P, Cui L, Ju H (2014) Design and sensing applications of metal–organic framework composites. TrAC Trends Anal Chem 58:71–78. https://doi.org/10.1016/j.trac.2014.02.012
Li Y, Xiao AS, Zou B, Zhang HX, Yan KL, Lin Y (2018) Advances of metal–organic frameworks for gas sensing. Polyhedron 154:83–97. https://doi.org/10.1016/j.poly.2018.07.028
Liao ZL, Zhang J, Yu EY, Cui YJ (2018) Recent progress in metal–organic frameworks for precaution and diagnosis of Alzheimer’s disease. Polyhedron 151:554–567. https://doi.org/10.1016/j.poly.2018.06.013
Liu L, Zhou Y, Liu S, Xu M (2018) The applications of metal−organic frameworks in electrochemical sensors. Chem Electro Chem 5(1):6–19. https://doi.org/10.1002/celc.201700931
Ma JP, Yao ZD, Hou LW, Lu WH, Yang QP, Li JH, Chen LX (2016) Metal organic frameworks (MOFs) for magnetic solid-phase extraction of pyrazole/pyrrole pesticides in environmental water samples followed by HPLC-DAD determination. Talanta 161:686–692. https://doi.org/10.1016/j.talanta.2016.09.035
Ma JP, Wu GG, Li S, Tan WQ, Wang XY, Li JH, Chen LX (2018) Magnetic solid phase extraction of heterocyclic pesticides in environmental water samples using metal-organic frameworks coupled to high performance liquid chromatography determination. J Chromatogr A 1553:57–66. https://doi.org/10.1016/j.chroma.2018.04.034
Maes M, Vermoortele F, Alaerts L, Couck S, Kirschhock CEA, Denayer JFM, De Vos DE (2010) Separation of styrene and ethylbenzene on metal−organic frameworks: analogous structures with different adsorption mechanisms. J Am Chem Soc 132(43):15277–15285. https://doi.org/10.1021/ja106142x
Mahmoud NF, Fouad OA, Ali AE, Mohamed GG (2021) Potentiometric determination of the Al(III) ion in polluted water and pharmaceutical samples by a novel mesoporous copper metal-organic framework-modified carbon paste electrode. Ind Eng Chem Res 60(6):2374–2387. https://doi.org/10.1021/acs.iecr.0c06288
Mansour MF, ElKady EF, El-Guindi NM, El-Moghazy SM, Schepdael AV, Adams E (2017) Simultaneous spectrophotometric determination of imipramine hydrochloride with chlordiazepoxide and nortriptyline hydrochloride with fluphenazine hydrochloride. Anal Lett 50(11):1778–1802. https://doi.org/10.1080/00032719.2016.1251448
Mendecki L, Mirica KA (2018) Conductive metal-organic frameworks as ion-to-electron transducers in potentiometric sensors. ACS Appl Mater Interf 10(22):19248–19257. https://doi.org/10.1021/acsami.8b03956
Monney A, Adjei JK, Tetteh S, Zugle R (2019) Quantification of imipramine, amitriptyline and their major metabolites in urine samples of depressed patients by gas chromatography-mass spectrometry. Front Chem Res 1(1):31–34. https://doi.org/10.22034/fcr.2019.37149
Patel SK, Patel NJ (2010) Simultaneous determination of imipramine hydrochloride and chlordiazepoxide in pharmaceutical preparations by spectrophotometric, RP-HPLC, and HPTLC methods. J AOAC Int 93:904–910. https://doi.org/10.1093/jaoac/93.3.904
Patil DV, Rallapalli PBS, Dangi GP, Tayade RJ, Somani RS, Bajaj HC (2011) MIL-53(Al): an efficient adsorbent for the removal of nitrobenzene from aqueous solutions. Ind Eng Chem Res 50(18):10516–10524. https://doi.org/10.1021/ie200429f
Paz JLL, Townshend A (1996) Flow injection chemiluminescence determination of imipramine and chlorpromazine. Anal Commun 33:31–33. https://doi.org/10.1039/AC9963300031
Pretsch E (2007) The new wave of ion-selective electrodes. Trac-Trend Anal Chem 26(1):46–51. https://doi.org/10.1016/j.trac.2006.10.006
Quirino JP, Breadmore MC (2012) Acid-induced transient isotachophoretic stacking of basic drugs in co-electroosmotic flow capillary zone electrophoresis. J Sep Sci 35(1):60–65. https://doi.org/10.1002/jssc.201100788
Ramanavicius S, Ramanavicius A (2021) Conducting polymers in the design of biosensors and biofuel cells. Polymers 13(1):49. https://doi.org/10.3390/polym13010049
Ramanavicius S, Jagminas A, Ramanavicius A (2021) Advances in molecularly imprinted polymers based affinity sensors. Polymers 13(6):974. https://doi.org/10.3390/polym13060974
Sanagi MM, Hanapi NSM, Ismail AK, Ibrahim WAW, Saim N, Yahaya N (2014) Two phase electrodriven membrane extraction combined with liquid chromatography for the determination of tricyclic antidepressants in aqueous matrices. Anal Methods 6:8802–8809. https://doi.org/10.1039/C4AY01700E
Sobhani R, Rezaei B, Shahshahanipour M, Ensafi AA, Mohammadnezhad G (2019) Simple and green synthesis of carbon dots (CDs) from valerian root and application of modified mesoporous boehmite (AlOOH) with CDs as a fluorescence probe for determination of imipramine. Anal Bioanal Chem 411:3115–3124. https://doi.org/10.1007/s00216-019-01779-1
Soni P, Sar SK, Kamavisdar A, Patel R (2011) Simple and sensitive spectrophotometric method for determination of tricyclic antidepressant imipramine. J Anal Chem 66:596–599. https://doi.org/10.1134/S1061934811060153
Srikantha D, Raju RR (2015) RP-HPLC estimation of imipramine hydrochloride and diazepam in tablets. Indian J Pharm Sci 77(3):343–347. https://doi.org/10.4103/0250-474x.159672
Srivastava V, Kumar P, Jat RK (2016) Validated stability indicating HPTLC method for diazepam and imipramine in bulk and combined dosage form. Int J Drug Res Tech 6(1):7–21
Susmitha K, Thirumalachary M, Vinod Kumar T, Venkateshwarlu G (2013) Spectrophotometric determination of imipramine HCl in pure and pharmaceutical forms. Der Pharma Chem 5:271–279
Toledo RAD, Santos MC, Shim H, Mazo LH (2015) Electroanalytical determination of imipramine in reconstituted serum with a graphite-polyurethane composite electrode. Int J Electrochem Sci 10:6975–6985
Trung TK, Trens P, Tanchoux N, Bourrelly S, Llewellyn PL, Loera-Serna S, Serre C, Loiseau T, Fajula F, Ferey G (2008) Hydrocarbon adsorption in the flexible metal organic frameworks MIL-53(Al, Cr). J Am Chem Soc 130(50):16926–16932. https://doi.org/10.1021/ja8039579
Umezawa Y, Buhlmann P, Umezawa K, Tohda K, Amemiya S (2000) Potentiometric selectivity coefficients of ion-selective electrodes Part 1 Inorganic cation. Pure Appl Chem 72(10):1851–2082. https://doi.org/10.1351/pac200072101851
Wang Z, Liu H, Wang S, Rao Z, Yang Y (2015) A luminescent Terbium-Succinate MOF thin film fabricated by electrodeposition for sensing of Cu2+ in aqueous environment. Sens Actuators B Chem 220:779–787. https://doi.org/10.1016/j.snb.2015.05.129
Wang T, Zhao P, Lu N, Chen HC, Zhang CL, Hou XH (2016) Facile fabrication of Fe3O4/MIL-101(Cr) for effective removal of acid red 1 and orange G from aqueous solution. Chem Eng J 295:403–413. https://doi.org/10.1016/j.cej.2016.03.016
Wu H-F, Kailasa SK, Yan J-Y, Chin C-C, Ku H-Y (2014) Comparison of single-drop microextraction with microvolume pipette extraction directly coupled with capillary electrophoresis for extraction and separation of tricyclic antidepressant drugs. J Ind Eng Chem 20(4):2071–2076. https://doi.org/10.1016/j.jiec.2013.09.034
Xiao B, Yuan QC (2009) Nanoporous metal organic framework materials for hydrogen storage. Particuology 7(2):129–140. https://doi.org/10.1016/j.partic.2009.01.006
Xie L, Liu D, Huang H, Yang Q, Zhong C (2014) Efficient capture of nitrobenzene from waste water using metal–organic frameworks. Chem Eng J 246:142–149. https://doi.org/10.1016/j.cej.2014.02.070
Yaghi OM, Li HM, Li HL (1995) Selective binding and removal of guests in a microporous metal-organic framework. Nature 378:703–706. https://doi.org/10.1038/378703a0
ZareKarizi F, Joharian M, Morsali A (2018) Pillar-layered MOFs: functionality, interpenetration, flexibility and applications. J Mater Chem 6:19288–19329. https://doi.org/10.1039/C8TA03306D
Zhao J, Shin Y, Chun K-H, Yoon H-R, Lee J (2016) A simple, rapid and reliable method to determine imipramine and desipramine in mouse serum using ultra-high-performance liquid chromatography–quadrupole-time-of-flight mass spectrometry. J Chromatogr Sci 54(4):561–568. https://doi.org/10.1093/chromsci/bmv187
Zhao F, Sun T, Geng F, Chen P, Gao Y (2019) Metal-organic frameworks-based electrochemical sensors and biosensors. Int J Electrochem Sci 14:5287–5304. https://doi.org/10.20964/2019.06.63
Zhou HC, Long JR, Yaghi OM (2012) Introduction to metal-organic frameworks. Chem Rev 112(2):673–674. https://doi.org/10.1021/cr300014x
Zhou M, Wu YN, Qiao J, Zhang J, McDonald A, Li G, Li F (2013) The removal of bisphenol A from aqueous solutions by MIL-53(Al) and mesostructured MIL-53(Al). J Colloid Interface Sci 405:157–163. https://doi.org/10.1016/j.jcis.2013.05.024
Zilfidou E, Kabir A, Furton KG, Samanidou V (2019) An improved fabric phase sorptive extraction method for the determination of five selected antidepressant drug residues in human blood serum prior to high performance liquid chromatography with diode array detection. J Chromatogr B Biomed Appl 125:121720. https://doi.org/10.1016/j.jchromb.2019.121720
Acknowledgements
We thank to Van Yuzuncu Yil University Research Foundation for their financial support on the current work by the grant of FYL-2020-9337.
Funding
Van Yuzuncı Yil University Research Foundation, FYL-2020-9337, Gulsah Saydan Kanberoglu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Subasi, Y., Kanberoglu, G.S., Coldur, F. et al. Development of MOF-based PVC membrane potentiometric sensor for determination of imipramine hydrochloride. Chem. Pap. 76, 5105–5117 (2022). https://doi.org/10.1007/s11696-022-02210-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02210-3