Abstract
In this work, the aim is to find the optimal operating conditions of olefin production process. To achieve this, the methanol to olefin (MTO) process was investigated and optimized through Aspen-Hysys coupled with statistical approach. The MTO process is comprised of three sections, including reaction, conditioning, and separation section. The separation section consisted of three distillation columns. Central composite design was used to design of experimental layout and optimizing the performance of separation section. The statistical analysis was performed to screen the important operative factors. The effects of operative factors including column stages (A), reflux ratio (B), and pressure (C) on the distillation columns were investigated. Accordingly a statistical model was developed and was used for optimization the performance of separation section. Optimization was performed for each three units of separation section. Based on optimization procedure, the optimum condition of distillation columns were suggested. This may be used to propose the values of effective factors in operating the separation section.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light olefins are widely used in different industries. Ethylene, propylene, butylene, and butadiene are termed as light olefins. Steam cracking of ethane, propane, and naphtha is the conventional process of ethylene production. Generally, thermal cracking of hydrocarbon is of interest due to conversion of low value raw materials to high value productions. This process has some disadvantage such as huge energy consumption and dependence on oil supply (Keyvanloo et al. 2012). Methanol to olefin (MTO) is an innovative method for ethylene and propylene production. This new alternative route provides more flexible ethylene to propylene ratio (Taheri Najafabadi et al. 2012). Further, lower operating temperature as well as higher light to heavy olefin selectivity motivate this method. The feedstock of this route is methanol that could be produced from different carbon sources. It is important to note that ethylene and propylene have widespread applications in different industries (Bahri Laleh et al. 2016). Generally, the outlet of MTO reactor includes lighter hydrocarbon fractions in comparison with the outlet of conventional thermal cracking process. Further, low price of natural gas and coal as raw materials for methanol production is a critical point. Huge amount of natural gas resources has motivated the methanol production. Ethylene also could be produced from ethane. In recent years, using new technology in shale gas extraction has provided huge amount of ethane (Gaffney et al. 2021). Other types of olefins such as poly (α-olefins) (PAO) has widely used in production of synthetic lubricants (Hanifpour et al. 2022).
In MTO process, selection of appropriate catalyst is a crucial point. Small pore silico alumino phosphate molecular sieve (SAPO-34) is widely used in catalytic dehydrogenation of methanol due to its porous structure with small pores (Taheri Najafabadi et al. 2012). High conversion rate of methanol and selectivity of light olefin are the advantages of this catalyst (Sedighi et al. 2014). Dubois et al. investigated the performance of SAPO-34 modified by transient metals. They reported that MnSAPO-34 has the longer lifetime in comparison with conventional SAPO-34 (Dubois et al. 2003). It is important to note that there are several side reactions that lead to formation and deposition of carbonaceous material on catalyst particles. This causes catalyst deactivation and subsequent lower performance. The catalyst has a porous structure. So, the compound with a large size could not go through. This leads to coke formation. Coke formation influences the catalyst performance in terms of activity and selectivity. Soundararajan et al. simulated MTO process in a circulating fluidized bed (CFB). The operative temperature and pressure of reactor was 450 °C and 1 atm, respectively. The results of their modeling showed that the coke deposition on catalyst leads to an increase in selectivity toward ethylene. They reported that this observation could be due to cage effect. Further, methanol conversion decreased with increase in coke deposition. The optimum coke deposit on catalyst was 5 wt% (Soundararajan et al. 2001). Chen et al. investigated the coke formation on SAPO-34 as a function of temperature, methanol partial pressure, and space velocity during the MTO process. They reported that as the temperature increased, the capacity of the catalyst increased while the partial pressure and velocity of the methanol space had no effect (Chen et al. 2000).
To develop a robust model, identifying the reaction kinetics is necessary. But describing the kinetics of MTO is controversial. There are several studies that have developed kinetic models to describe the MTO process (Alwahabi and Froment 2004). These models aim to advance the production performance through finding optimum condition. Park and Forment developed kinetic models to describe olefin production in MTO over HZSM-5 catalyst with Si/AL ratio of 200 (Park and Froment 2001). Sedighi et al. developed a kinetic model to describe MTO process on SAPO-34. They reported that high space time increased the production rate of light olefins. Further, by increasing the operative temperature, the ethylene yield increased (Sedighi et al. 2014). Zhaung et al. investigated olefin production through MTO process in a fixed bed plug flow reactor (PFR). They used computational fluid dynamics (CFD) code to investigate the flow behaviour in reactor. The effects of feed temperature, space velocity, and feed composition of reaction were investigated. They reported a close relation between methanol conversion and deactivation of catalyst due to coke deposition (Zhuang et al. 2012). Taheri et al. developed a kinetic model based on data obtained from a micro catalytic reactor. They optimized the parameters using genetic algorithm optimization method. A Comparison between experimental and predicted data was done that showed a good model fit (Taheri Najafabadi et al. 2012). Qian et al. investigated the poly-generation systems. They reported that poly-generation system of conversion of natural gas to electricity and light olefins is more efficient than single purpose (Qian et al. 2009). In order to simulate and model the ethylene production process by oxidative dehydrogenation of ethane Gaffney et al. utilized HYSYS simulator (Gaffney et al. 2021).
In the MTO process, the performance of different units depends on several factors and interactions with different complexities. Accordingly, selection of robust statistical approach to identify the importance of each factor is of interest. One-factor-at-a-time (OFAT) approach is not able to cover the interaction between parameters. Further, the number of experiments in this approach is huge. So, in this study design of experiment (DoE) technique was used. Note, less experiments number and considering the interactions between parameters make the DoE approach a shining alternative option. This methodology was used in different fields of engineering for design of experimental layout (Gholamzadehdevin and Pakzad 2019; Mahmoudian et al. 2022; Kazemzadeh et al. 2016; Al-Dahhan et al. 2017; Aghasadeghi et al. 2017; Moghaddam et al. 2016; Zeynali et al. 2016). DoE including a broad range of practical statistical techniques. In current work, response surface methodology (RSM) is used to make the design layout.
The novelty of this work is to use the DoE method in a way that the simulation of the process was done using Aspen-HYSYS and a model based on statistical approach is developed. Further, finding the optimum condition was done using desirability function. First, the separation process in MTO is described and simulated using Aspen-HYSYS V9 (35.0.0.270). The separation section of MTO process is comprised of three distillation columns. Each of these units are assessed using RSM to find the important factors and develop a robust statistical model. The developed models are used for optimization of each unit. DoE and subsequent optimization were performed by Design Expert V7. The main goal of this optimization is to develop a tool to improve the yield of olefin separation in MTO process as an economic and green route. By developing the polynomial model, the performance of distillation columns will be predicted. According to author’s knowledge, researches on modelling and simulation of olefins production from methanol are scares. The conceptual design of this work is based on the work of Yu and Chien (Yu and Chien 2016).
Methodology & procedure
Process mechanism
The mechanism of MTO is not completely determined. Methanol is converted to dimethyl ether (DME) through dehydration as an intermediate substance. In the next step, DME is converted to olefin and water. It is important to note that the involved reactions are more complex. Indeed, there is a network of reactions in this process (Abdulwahab 2018). Taheri et al. reported 10 elementary reactions to describe MTO process as follows (Taheri Najafabadi et al. 2012):
The order of reactions 1 and 2 is assumed to be 1 and the other reactions are elementary. The conversion value of this process is assumed to be 95% (Abdulwahab 2018). The MTO reaction is exothermic and operates at 350–550 °C and 2–3 bar in vapour phase. This is reported that higher operative temperature and pressure lead to higher propylene/ethylene ratio (Yu and Chien 2016).
Process description
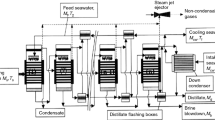
The component in this process are methanol, ethylene, dimethyl ether, and water. The MTO process is comprised of three sections, including reaction, conditioning, and separation section. Since the MTO reaction is exothermic, the effluent gases from MTO reactor have high temperature and could be used for generating of heat and power. In Fig. 1, the block flow diagram of MTO process has been presented. Methanol feed after passing through heat exchangers enters into the MTO reactor. This reactor is a fluidized bed type. This type of reactor is efficient in removal of reaction heat (Dimian and Bildea 2018). The outlet from MTO reactor is cooled and enters into two quenching columns. In these columns raw olefin is separated from unreacted methanol, catalyst particles, carboxylic acids, and heavy hydrocarbons that are soluble in produced water. After conditioning step, the olefin stream enters into two separation sections. In first separation section, this stream is divided into three parts. The first part (including methane, hydrogen, carbon monoxide etc.) could be used as fuel gas. The second part includes C4 and C5 (heavy products). And the third part (including ethylene, ethane, propylene, and propane) enters into second separation section. The second separation section comprises of three columns termed C2 stripper, dethanizer, and C3 stripper. The goal of this section is to produce pure ethylene and pure propylene.
Design of experiment
Design of experiment (DoE) is important to perform a well-planned investigation of a complicated system. As mentioned earlier, without DoE, the analysis of collected data is not statistically correct. Indeed DoE helps researchers to investigate the effects of important operative parameters on the responses of a process to establish a mathematical relation between input–output parameters. RSM provides better tuning to find optimum condition in comparison with other DoE techniques such as factorial and fractional factorial design. So, RSM requires more data than two level factorial techniques. There are three type of points termed as full or fractional factorial, centre, and star or axial points. Centre points and star points provide a robust estimate of pure error and quadratic effects, respectively. The number of experiments designed through RSM is determined as follows:
where n and C are the numbers of factors and centre points, respectively. It is clear that by increasing the number of factors, the number of experiments rises exponentially. So, the cost and time of running the study would increase. To face this issue, two-level factorial designs approach could be used to explore which parameters are important. And RSM would be used to design experimental layout only for important factors.
Result and disscussion
In this section, the second separation section of MTO process is investigated and simulated by Aspen Hysys coupled with DoE method. The properties of stream entered into this section is obtained from the work of Yu and Chien (Yu and Chien 2016). The separation section included three units: C2/C3 separation column, deethanizer, and propylene/propane separation column. In Fig. 2, the process flow (PFD) diagram of this separation section has been shown.
In C2/C3 distillation column, the sum of absolute values of condenser and reboiler power as well as the mole fraction of C2 (ethylene and ethane) in top stream were selected as the index of separation performance. The distillation column was simulated using Aspen Hysys. To assess the effects of operative variables on separation yield, RSM was selected for designing the experimental layout. The ranges of the operational variables are presented in Table 1.
The analysis of variance (ANOVA) is a method to investigate the effect of model terms. The modified ANOVA for the sum of absolute values of condenser and reboiler power is shown in Table 2. Due to p-value less than 0.05, it is concluded that the model is significant. According to ANOVA table, A, B, C, A2, B2, and BC are significant model terms. R2 value is 0.9998. Further, the values of adjusted and predicted R2 are 0.9996 and 0.9991, respectively. Similar assessment was done for the mole fraction of C2 in top stream. For this response, R2 value is 0.9259. But adjusted and predicted R2 values are less than the R2 values related to the other responses.
The 3D plot of the power consumption of C2/C3 distillation column in terms of reflux ratio and pressure while the number of stages was held constant at 29 is presented in Fig. 3. It is found that the effect of reflux ratio on the power consumption in condenser and reboiler is severe than column pressure. The effect of column pressure on power consumption increases by increasing the reflux ratio. By increasing the reflux ratio, the power consumption increases, either at low or high pressure.
In optimization approach of C2/C3 distillation column, two different goals have been considered simultaneously:
-
Ι: Maximizing the C2 fraction in top stream
-
ΙΙ: Minimizing the sum of absolute values of condenser and reboiler power
Due to technical limitation, the lower temperature of top stream was set at − 20 °C. Desirability function method was used to find the optimum condition. Some conditions have been presented in Table 3.
The first row of Table 3 was selected as optimum condition and the C2/C3 separation was designed according to this condition. The composition of top and bottom stream of C2/C3 separation column operated in optimum condition is presented in Table 4.
Deethanizer column
The top stream of C2/C3 separation column was entered into the deethanizer column. Similar to C2/C3 separation column, RSM was used to design of experiment, modelling, and optimization of deethanizer performance. It should be noted that the pressure of deethanizer needs to be equal to or less than the C2/C3 separation column. The range of reflux ratio and column pressure are similar to C2/C3 separation column. But, the range of separation stages is 20–70.
The ANOVA table for the ethylene composition in top stream was modified using backward elimination. It is found that A, B, C, AB, BC, A2 and B2 are significant model terms. R2 value is 0.9956. Further, the values of adjusted and predicted R2 are 0.9911 and 0.9776, respectively. In Fig. 4, the ethylene fraction in top stream of deethanizer is shown in terms of reflux ratio and number of stages. As shown, the number of separation stages could not change the ethylene fraction value in top stream, effectively. But, the amount of ethylene would increase with increase in reflux ratio.
Optimization strategy was selected similar to C2/C3 separation column one. Accordingly, the optimum condition was determined. The optimum No. stages, reflux ratio, and column pressure are 55, 1.48, and 2516 kpa, respectively. So, the deethanizer was designed according to this condition. The properties of top and bottom stream of deethanizer column in optimum design mode is presented in Table 5.
Propane/propylene separation column
The bottom stream of C2 stripper was entered into the propane/propylene separation column. The fraction of propylene in top stream, the required power for condenser and reboiler, and temperature of top distillate were selected as responses. The range of reflux ratio and column pressure are similar to C2/C3 separation column. But, the range of separation stages is 50–199.
In Fig. 5, the changes of distillate temperature are depicted in terms of column pressure and number of separation stages. As shown, the response would not change by increasing or decreasing the number of separation stages. Adversely, an increase in column pressure results in increasing the temperature. For optimization, the aim is to have maximum purity of propylene as well as minimum power consumption. In contrast with previous units, in propylene/propane separation column, there is no restriction for temperature. Accordingly the optimum condition is presented in Table 6.
Conclusion
In this work, methanol to olefin (MTO) process has been studied. The second separation section of the process has been simulated using Aspen-Hysys coupled with design of experiment (DoE) approach. The effect of column stage, reflux ratio, and column pressure on the performance of different units of second separation section were assessed. The sum of absolute values of condenser and reboiler power, component composition in top stream of columns, and temperature of top stream were selected as responses. R2 value of statistical model for the sum of absolute values of condenser and reboiler power in C2/C3 separation column is 0.9998. The model developed for the prediction of ethylene composition in top stream of deethanizer column has the R2 value of 0.9956. The R2 value of model developed for the temperature of top stream of C3 stripper column was 0.9996.
References
Abdulwahab G (2018) Design of a methanol-to-olefin process using aspen HYSYS: material and energy balances. ARPN J Eng Appl Sci 13:3325–3333
Aghasadeghi K, Csordas M, Peldszus S, Latulippe DR (2017) Combining LC-OCD analysis with design-of-experiments methods to optimize an advanced oxidation process for the treatment of industrial wastewater. Can J Chem Eng 95:1943–1952
Al-Dahhan M, Aradhya S, Taofeeq H (2017) Prediction of spout diameter in gas-solid spouted beds using factorial design of experiments approach with the aid of advanced optical fibre probe. Can J Chem Eng 95:1463–1470
Alwahabi SM, Froment GF (2004) Single event kinetic modeling of the methanol-to-olefins process on SAPO-34. Ind Eng Chem Res 43:5098–5111
Bahri Laleh N, Nekoomanesh-Haghighi M, Sadjadi S, Pajouhan A (2016) Polyolefin and olefin production in Iran: current and future capacities. Polyolefins J 3:11–22
Chen D, Rebo H, Grønvold A, Moljord K, Holmen A (2000) Methanol conversion to light olefins over SAPO-34: kinetic modeling of coke formation. Microporous Mesoporous Mater 35:121–135
Dimian AC, Bildea CS (2018) Energy efficient methanol-to-olefins process. Chem Eng Res Des 131:41–54
Dubois DR, Obrzut DL, Liu J, Thundimadathil J, Adekkanattu PM, Guin JA, Punnoose A, Seehra MS (2003) Conversion of methanol to olefins over cobalt-, manganese- and nickel-incorporated SAPO-34 molecular sieves. Fuel Process Technol 83:203–218
Gaffney AM, Sims JW, Martin VJ, Duprez NV, Louthan KJ, Roberts KL (2021) Evaluation and analysis of ethylene production using oxidative dehydrogenation. Catal Today 369:203–209
Gholamzadehdevin M, Pakzad L (2019) Hydrodynamic characteristics of an activated sludge bubble column through computational fluid dynamics (CFD) and response surface methodology (RSM). Can J Chem Eng 97:967–982
Hanifpour A, Bahri-Laleh N, Mohebbi A, Nekoomanesh-Haghighi M (2022) Oligomerization of higher α-olefins to poly(α-olefins). Iran Polym J 31:107–126
Kazemzadeh A, Ein-Mozaffari F, Lohi A, Pakzad L (2016) Effect of the rheological properties on the mixing of Herschel-Bulkley fluids with coaxial mixers: applications of tomography, CFD, and response surface methodology. Can J Chem Eng 94:2394–2406
Keyvanloo K, Sedighi M, Towfighi J (2012) Genetic algorithm model development for prediction of main products in thermal cracking of naphtha: comparison with kinetic modeling. Chem Eng J 209:255–262
Mahmoudian F, Moghaddam AH, Davachi SM (2022) Genetic-based multi-objective optimization of alkylation process by a hybrid model of statistical and artificial intelligence approaches. Can J Chem Eng 100:90–102
Moghaddam AH, Shayegan J, Sargolzaei J (2016) Investigating and modeling the cleaning-in-place process for retrieving the membrane permeate flux: Case study of hydrophilic polyethersulfone (PES). J Taiwan Inst Chem Eng 62:150–157
Park T-Y, Froment GF (2001) Kinetic modeling of the methanol to olefins process. 1. Model formulation. Ind Eng Chem Res 40:4172–4186
Qian Y, Liu J, Huang Z, Kraslawski A, Cui J, Huang Y (2009) Conceptual design and system analysis of a poly-generation system for power and olefin production from natural gas. Appl Energy 86:2088–2095
Sedighi M, Bahrami H, Towfighi J (2014) Kinetic modeling formulation of the methanol to olefin process: Parameter estimation. J Ind Eng Chem 20:3108–3114
Soundararajan S, Dalai AK, Berruti F (2001) Modeling of methanol to olefins (MTO) process in a circulating fluidized bed reactor. Fuel 80:1187–1197
Taheri Najafabadi A, Fatemi S, Sohrabi M, Salmasi M (2012) Kinetic modeling and optimization of the operating condition of MTO process on SAPO-34 catalyst. J Ind Eng Chem 18:29–37
Yu BY, Chien IL (2016) Design and optimization of the methanol-to-olefin process part i: steady-state design and optimization. Chem Eng Technol 39:2293–2303
Zeynali V, Sargolzaei J, Hedayati Moghaddam A (2016) Optimization of several hydrodynamic and non-hydrodynamic operating parameters in treatment of synthetic wastewater containing wheat starch in a sequencing batch reactor (SBR) using response surface methodology. Desalin Water Treat 57:24240–24256
Zhuang Y-Q, Gao X, Zhu Y-P, Luo Z-H (2012) CFD modeling of methanol to olefins process in a fixed-bed reactor. Powder Technol 221:419–430
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hedayati Moghaddam, A. Simulation and optimization of separation section in methanol to olefin (MTO) process based on statistical approach. Chem. Pap. 76, 4787–4794 (2022). https://doi.org/10.1007/s11696-022-02190-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02190-4