Abstract
Myrtus communis L. is a common medicinal plant with a wide range of biological properties. In this study, an attempt was made to improve its essential oil’s antioxidant, anticancer, and antibacterial activities by preparing a nanogel dosage form. Alpha-pinene (29.7%), 1,8-cineole (25.8%), linalool (9.1%), linalool acetate (5.9%), and geranyl acetate (3.4%) were identified as five major compounds in the essential oil using GC–MS analysis. Optimum nanoemulsion with a droplet size of 179 ± 7 nm and a narrow droplet size distribution (SPAN 0.96) was gelified by the addition of carboxymethyl cellulose (3 w/v %). The rheometry analysis at shear rates of 0.1–100 1/s showed the viscosity was fully fitted with the Carreau-Yasuda model. The nanogel with IC50 132.6 µg/mL was 4 folds more potent than the bulk essential oil (IC50: 580.8 µg/mL) against A-375 melanoma cells. Besides, after treatment of Escherichia coli and Staphylococcus aureus with 1000 µg/mL of the nanogel and the bulk essential oil, their growths were observed at 37.5 and 59.1% as well as 21.4 and 40.6%. Besides, antioxidant activity was investigated using DPPH assay; the nanogel was significantly more potent (P < 0.001) than that of bulk essential oil at all examined concentrations (62.6–1000 µg/mL). Furthermore, polyethylene oxide-gelatin electrospun nanofibers (diameter of 359 ± 36 nm) with no effects on the cancer cell and the bacterial growth were proposed as lesion dressing after-treatment with the nanogel. Therefore, the stained nanofiber with the nanogel could be considered a natural potent anticancer and antibacterial agent in vivo study.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancers were responsible for about 10 million deaths in 2020 (WHO 2021). Among them, skin cancers are by far on the list of prevalent cancers; they are categorized in non-melanoma and melanoma (Hardy et al. 2010). Melanoma is the most aggressive and deadliest type of skin cancer (Domingues et al. 2018). There are 42 melanoma cell lines on the surface of transcriptomes (Vincent and Postovit 2017). Studies indicate that the A-375 cell line is more aggressive with low sensitivity to chemotherapy treatment (Maksimović-Ivanić et al. 2019; Massi et al. 2014).
Staphylococcus aureus is a gram-positive bacteria that cause various diseases in humans such as wound, skin, urinary and respiratory tract infections, inflammations (i.e., endocarditis, osteomyelitis), toxic shock and scalded skin syndromes, and necrosis (i.e., necrotizing fasciitis and necrotizing pneumonia) (Francis et al. 2005; Miller et al. 2005). Escherichia coli is a gram-negative bacterium that is one of the most common causes of urinary tract infections, neonatal meningitis, acute enteritis, traveler’s diarrhea, dysentery-like disease, and hemorrhagic colitis (Percival and Williams 2014; Wanke and Sears 2008).
Drug resistance in cancers or microorganisms has become a health challenge in two past decades (Housman et al. 2014; Longley and Johnston 2005). The development of natural drugs has thus been received more attention. For instance, Myrtus communis L. (myrtle) in the Myrtaceae family is traditionally used in cough, gastrointestinal disorders (i.e., peptic ulcers, diarrhea, and hemorrhoids), urinary diseases (i.e., urethritis), and skin ailments (i.e., reddened skin), inactivation microorganisms and for the wound healing (Alipour et al. 2014; Messaoud et al. 2005). Furthermore, anticancer, antibacterial, antiviral, antifungal, anti-parasitic, and anti-inflammatory properties of M. communis essential oil (EO) are due to flavonoids and polyphenols compounds (Aleksic and Knezevic 2014; Alipour et al. 2014).
Nanotechnology is defined as targeted materials in the nanoscale (1–200 nm) to obtain size-dependent properties; preparation of nanostructures containing EOs is a promising approach for improving their efficacy (Esmaili et al. 2021; Osanloo et al. 2020b; Zhou et al. 2021). Hydrogels are 3D structures of swelled polymers in an aqueous media; biodegradability, biocompatibility, and high drug loading capacity are some of their advantages (Guo et al. 2020; Zhang and Khademhosseini 2017). Furthermore, if a nanoemulsion is gelified (nanoemulsion based gel), advantages of nanosystems (improving efficacy, better cellular penetration, loading of hydrophobic materials) are also achievable (Hussain et al. 2016; Qasemi et al. 2021).
In this study, chemical composition of M. communis EO was first investigated. An attempt was then made to improve its biological activities (antioxidant, anticancer, and antibacterial) by preparing nanoemulsion-based gel. Moreover, a polyethylene oxide-gelatin (PEO-Gel) electrospun nanofibers mat was prepared as a dressing after treatment.
Materials and methods
Materials
Melanoma cell line A-375 (ATCC CRL-1619), S. aureus (ATCC 25923), and E. coli (ATCC 25922), provided by the Pasteur Institute of Iran. M. communis EO (99.99%) was purchased from Tabib Daru Co., Iran. Merck Co., Germany, provided PEO, Gel, and tween 20 and 80. Penicillin–streptomycin, Dimethyl Sulfoxide (DMSO), and RPMI cell culture medium were supplied by Shellmax (China).
Method
GS-MS analysis
Myrtus communis EO was analyzed as described in our previous study using a gas chromatography device (Agilent 6890, HP-5MS column, USA) connected to a mass spectrometer (Agilent 5973, USA) (Ghanbariasad et al. 2021). Briefly, the initial column temperature was set at 40 °C and fixed for 1 min. The final temperature was set at 250 °C, and the injection port and detector temperature were fixed at 250 and 230 °C. Helium (He) gas 99.999% was applied as the carrier gas. Mass spectra were taken at 70 eV ionization energy, and full scan mode (50–350 m/z) was performed. Myrtus communis EO’ compounds were identified by combining the temperature programmed retention indices and mass spectra of ADAMS and NIST 17 (Adams 2007, Standards & Technology 2008).
Preparation and characterization of fifteen nanoemulsions
The spontaneous approach (without external energy such as ultra-sonication and homogenizer) was used to prepare nanoemulsion to prevent evaporation of EO components (Abedinpour et al. 2021). A defined amount of the EO (0.2% w/v) with different amounts of emulsifiers (tween 20 or tween 80; n = 15) was first stirred at 2000 rpm for 5 min. Then, phosphate-buffered saline (PBS) or normal saline as aqueous phase was added dropwise and stirred for 40 min. The mean droplet size of the samples and droplet size distribution (SPAN) were measured by dynamic light scattering (DLS) using a Scatteroscope (K-one Ltd. Korea). The mean size of droplets has been considered as D50 and SPAN calculated by Eq. (1). Where D stands for diameter, and 10, 50, and 90 are percentages of particles with smaller sizes than mentioned ones.
Preparation and characterization of a nanoemulsion-based gel
Amongst the prepared nanoemulsions, a sample with a mean droplet size < 200 nm and SPAN value < 1 was selected as the optimum nanoemulsion. Then, the nanoemulsion was gelified by adding carboxymethylcellulose (3% w/v); the mixture was stirred overnight at 2000 rpm. Moreover, the blank gel was also prepared similarly, only without the EO. Finally, the viscosity of the nanogel was investigated in different shear rates of 0.1 to 100 S−1 utilizing a rheometer machine (MCR-302, Anton Paar-Austria).
Preparation and characterization of an electrospun nanofiber
The electrospinning technique was used to prepare nanofibers; PEO-Gel (10–8% w/v) were first dissolved in distilled water (2000 rpm, overnight, room temperature). The prepared sample was then filled in a 10 mL syringe that connected to a blunted needle (gauge 22) and situated in the syringe pump in an electrospinning device (Fanavaran Nano-Meghyas (FNM), Iran). Instrumental parameters were set: injection rate 0.3 mL/h, the distance between needle and collector 100 mm, collector speed 100 rpm, and voltage 15 kV.
A scanning electronic microscopy (SEM) instrument (Vega 3, TESCAN Co., Czech Republic) was used to investigate the size and morphology of nanofibers 0.5 cm2, which was sputter-coated by gold vapor (Quorum Technologies, Q150R-ES). The contact angle measurement equipment (CA-500 Å model, Sharif Solar Co., Iran) was used to investigate nanofibers’ surface; 7 µL deionized water was injected, and its contact angle (θ) with the surface was recorded immediately. Fourier Transform Infrared (FTIR) spectroscopy was used to assess the functional groups of the PEO, GEL, and PEO-GEL nanofibers (Tensor II model—Bruker–Germany) in a wavenumber of 400–4000 cm−1. Noteworthy, nanofibers 0.5 cm2 were used as an independent sample for investigating its antioxidant, antibacterial, and anticancer activities, as described in the next sections.
Investigation of antioxidant activity
DPPH assay was used to investigate the antioxidant activities of the EO and nanogel in a concentration range of 62.5–1000 µg/mL. DPPH powder (394.32 g/mole), the EO, and nanogel were first dissolved in absolute ethanol containing 0.5% DMSO to reach 0.3 mM and the mentioned serial dilutions. After that, DPPH solution (150 µL/well) and serial dilutions (50 µL/well) were added to a 96 well plate and incubated 30 min away from light for completing the reaction. Six well/plate was considered a control group (150 µL DPPH solution and 50 µL ethanol), i.e., was not treated with samples. Eventually, each well’s optical density (OD) was read at 517 nm using a plate reader device (Synergy HTX Multi-Mode Reader, USA). The antioxidant activities were calculated by Eq. (2). In addition, the antioxidant activities of blank gel, nanofiber, and ascorbic acid (1000 µg/mL, as positive control) were also investigated.
Investigation of the cytotoxicity activity
The EO and nanogel were dissolved in normal saline containing 0.5% DMSO. The anticancer effect of the EO and nanogel (in a concentration range of 62.5–1000 µg/mL) was investigated against A-375 cells using MTT assay as described in our previous research (Valizadeh et al. 2021). Cultured cell in RPMI (containing 10% FBS and 1% antibiotics) was added to 96-well plate (100 µL/well) and incubated at 37 °C, and CO2 (5%) to attach the cells to the bottom reached 80% confluence. After that, the liquids of wells were replaced with 50 µL of fresh culture medium, and serial dilutions were added, 50 µL/well. In each plate, 6 was considered the control group filled with 50 µL fresh culture medium and normal saline containing 0.5% DMSO. Treated plates were incubated for 24 h, and the liquid content was replaced with 50 µL mtt solution (0.5 mg/mL) and then incubated for 4 h. To dissolve the created formazan crystals, 50 µL/well DMSO was added, and OD of wells was read at 570 nm. The cell viability at each concentration was determined using Eq. (3). In addition, the cytotoxic activities of blank gel and nanofiber were also investigated.
Investigation of antibacterial activity
Antibacterial activity of the EO and nanogel in a concentration range of (62.5–1000 µg/mL) against E. coli and S. aureus was investigated using the 96-well plate microdilution method (Osanloo et al. 2020a). The EO and nanogel were dissolved in Muller Hinton broth containing 0.5% w/v DMSO. Bacterial colonies were also dispersed in the Muller Hinton broth medium to reach 0.5 McFarland turbidity (1.5 × 108 CFU/mL); 20 µL/well of the bacterial suspension was added to a 96-wells plate. After that, 30 and 50 µL/well Muller Hinton broth and as-prepared serial dilutions were added, and the plates were incubated for 24 h at 37 °C. In each plate, control wells (n = 6) were filled with 20 and 80 µL of the bacteria suspension and the Muller Hinton Broth culture medium containing 0.5% w/v DMSO. The OD of each well was read at 630 nm using the plate reader, and bacteria growth was measured using Eq. (4). In addition, the antibacterial activities of blank gel and nanofiber were also investigated.
Statistical analysis
All experiments were carried out in triplicates, and data were expressed as Mean ± standard deviation. An independent sample T-test was used to determine statistically significant differences between groups. All analyses were conducted using computer-based statistical software (STATA v. 16); a P value < 0.05 was accepted as statistically significant. CalcuSyn software (Free version, BIOSOFT, UK) was used to calculate half-maximal inhibitory concentration (IC50); no overlap between the upper and lower limit of IC50 was considered significant.
Results
Identified compounds in the EO
Identified compounds with a higher portion than 1% in the EO are listed in Table 1. Five major compounds are α-pinene (29.7%), 1,8-cineole (25.8%), linalool (9.1%), linalool acetate (5.9%), and geranyl acetate (3.4%).
Prepared nanoemulsions and viscosity of the nanogel
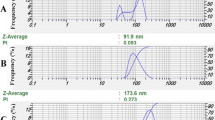
Ingredients and size analyses of the prepared nanoemulsion are listed in Table 2. One sample (My11) only possesses proper size characteristics (droplet size 179 ± 7 nm and SPAN 0.96); its DLS profile is depicted in Fig. 1. The viscosity profile of the nanogel containing M. communis followed the Carreau-Yasuda regression model (Fig. 2). It is a well-known empirical equation that has been used for non-Newtonian fluids such as biopolymer and polymeric solutions, emulsions, and protein solutions (Avazmohammadi and Castañeda 2015; Bird et al. 1987). The viscosity of non-Newtonian decreases with increasing the shear rates, while, Newtonian substances have constant viscosity at all shear rates (Kwack and Masud 2014; Zare et al. 2019).
Physicochemical properties of the nanofibers
The SEM image of smooth, randomly oriented, and beadles PEO-GEL electrospun nanofibers are depicted in Fig. 3. Besides, the contact angle (θ) of water with the nanofibers was assessed to determine the degree of hydrophilicity of the surface. In the current study, θ was < 90º (i.e., 19° ± 2), the hydrophilic surface is confirmed (data not shown).
Furthermore, FTIR analysis was carried out to receive the exact information about the functional groups and molecular interactions in electrospun PEO-Gel nanofibers (Kuppan et al. 2013). In Fig. 4, the spectra of the pure components (PEO and Gel powder) are compared with the prepared nanofibers. In the FTIR spectrum of pure PEO, a strong peak at 2878 cm−1 is associated with the asymmetric stretching of CH2 in PEO. Notably, a characteristic triplet (1144, 1094, and 1059 cm−1) was observed with a maximum at 1094 cm−1, which is assigned to C–O–C vibration in the structure of PEO. The presence of this triplet in the spectrum of PEO greatly reveals the high crystallinity of PEO. The peaks observed at 960 and 841 cm−1 can be ascribed to the –CH2–CH2 rocking and CH2 rocking bending, respectively. Moreover, the absorption bands of PEO appeared at 1466, and 1359 cm−1 are attributed to CH2 groups (Anilkumar et al. 2017; Zhou et al. 2014). In the spectrum of pure Gel, a broad peak at 3271 cm−1 and a weak band at 2945 cm−1 are attributed to the hydroxyl (O–H) and CH2 groups, respectively. The main characteristic peaks of Gel were located at 1628 cm−1 (amide I), 1523 cm−1 (amide II), and 1238 cm−1 (amide III), respectively (Cai et al. 2019; Merk et al. 2021). In the FTIR spectrum of electrospun PEO-GEL, peaks for PEO and Gel are evidence for a perfect combination of these two polymers. Although, the position and intensity of some peaks were slightly changed due to molecular interactions between Gel and PEO. For instance, the peaks at 1628, 1523, and 1238 cm−1 for Gel shifted to 1632, 1556, and 1240 cm−1 in the PEO/Gel nanofibers spectrum, respectively.
Antioxidant activities of M. communis EO and nanogel
Antioxidant activities of the EO and the nanogel are shown in Fig. 5. The nanogel was significantly more potent than the EO at all examined concentrations. P value for all concentrations is < 0.001. Ascorbic acid 1000 µg/mL (positive control) showed 92% antioxidant effect. Besides, blank gel and nanofibers did not show antioxidant effects.
Cytotoxic activities of M. communis EO and nanogel
The viability of A-375 cells after treatment with different concentrations of the EO and nanogel is shown in Fig. 6. The efficacy of the nanogel was significantly more than the EO at concentrations of 62.5, 250, 500, and 1000 µg/mL (P value 0.0421, 0.0000, 0.0000, and 0.0003). The IC50 of the nanogel and the EO were obtained as 132.6 (74–237) µg/mL and 580.8 (328–1026) µg/mL (Table 3). Besides, the viability of cells after treatment with blank gel was only reduced by less than 10%. Moreover, the nanofibers did not significantly affect the viability of cells.
Antibacterial activities of M. communis EO and nanogel
The antibacterial activities of the EO and nanogel on E. coli are illustrated in Fig. 7. At the highest concentration (1000 µg/mL) efficacy of the nanogel was significantly more potent than the EO (P value 0.0020). However, the potency of the EO at concentrations of 62.5 and 125 µg/mL was significantly more potent than the nanogel (P value 0.0208 and 0.0031). Besides, IC50 values of the nanogel and the EO was observed as 583 (437–777) µg/mL and 4547 (2574–8034) µg/mL (Table 3).
The antibacterial activities of the EO and nanogel on S. aureus are shown in Fig. 8. The potency of nanogel at 1000 µg/mL was significantly more potent than the EO (P value 0.0001). However, the potency of the EO was significantly more potent than nanogel at concentrations of 125, 250, and 500 µg/mL (P value 0.0016, 0.0010, and 0.0241). IC50 values of the nanogel and the EO were 584 (282–1046) µg/mL and 394 (153–1033) µg/mL. Moreover, the blank gel and nanofibers did not significantly affect the growth of E. coli and S. aureus.
Discussions
In the current study, M. communis EO was used as the active agent. Its chemical compositions were investigated using GC–MS analysis with fine major compounds α-pinene, 1,8-cineole, linalool, linalool acetate, and geranyl acetate. Alpha-pinene is identified as the major compound; this finding (30%) is consistent with the literature (30–36) (Hsouna et al. 2019; Sen et al. 2020). Alpha-pinene is a bicyclic monoterpene (C5H8)n with a wide range of biological activities, including bronchodilator, antioxidant, and antimicrobial activities (Aydin et al. 2013; Nissen et al. 2010).
EOs as natural medicine have advantages such as biocompatibility and biodegradability. However, there are some challenges about the EOs, such as low water solubility and high volatility, and their efficacy should be improved (Herman and Herman 2015; Langeveld et al. 2014). Preparing nanostructures containing EO (nanoemulsions, nanofibers, polymeric nanoparticles, and lipidic nanocarriers) is a promising approach to meet the challenges (Bilia et al. 2014). In this study, an oil in water nanoemulsion (droplet size 179 ± 7 nm) containing the EO was first prepared. Nanoemulsions are a dispersion of two immiscible liquids by using amphiphilic material called surfactant (Jiang et al. 2013; Mason et al. 2006). After that, the nanoemulsion was gelified by adding a semi-synthetic thickening agent (carboxymethylcellulose). The topical application of nanogels is easier than nanoemulsions, and the EO remains stable for a longer period. To the authors’ best knowledge, M. communis EO or its nanogel delivery system has not been investigated yet on the A-375 cell line. The current study investigated the anticancer and antioxidant activities of the EO and nanogel. Interestingly, the potency of the nanogel against A-375 cells was fourth-folds more than the EO. Besides, significant antioxidant activities of nanogel compared to the EO were also observed. Antioxidants are substances that inhibit free radicals from raising the chance of several diseases (cancer, cardiovascular disease, and aging) (Antolovich et al. 2002). Cancer cells have downregulated gap junctions and become ready for metastasis. Besides, their membrane pores are wider than normal cells for obtaining nutrients molecules (Leithe et al. 2006; Ruch 2020). Weak lymphatic system with large gaps are two important factors in inactive nano drug delivery systems; nanostructures easily enter to cancer cell and do not allowed to exit from cells, so their efficacy are improved (Adepu and Ramakrishna 2021; Zhang and Lu 2014).
Large amounts of EO droplets could be loaded into one nanostructure; they prepare nanotanks containing EO (de Matos et al. 2019; Nazzaro et al. 2013). On the other hand, the entry of nanotanks into bacteria cell walls and membranes is difficult due to a regular cell wall structure (Ploux et al. 2010; Zhang and Rock 2008). However, the entry of dissolved EO in the culture media is easier than nanotanks. In the current study, the potency of the EO at four concentrations (62.5, 125, 250, and 500 µg/mL) is more than the nanogel. While at the 1000 µg/mL concentration (i.e., no-diluted nanogel), the potency of the nanogel is more than the EO. In addition, the role of the bacterial outer membrane in the gram-negative strains (E. coli) was significant; the EOs’ obtained IC50 (4547 µg/mL) 11 folds more than S. aureus (394 µg/mL). Moreover, the IC50 value of the nanogel against both strains was the same; however, the final efficacy (reduced growth) of the nanogel against S. aureus (~ 80%) was more than E. coli (62%).
As mentioned above, we prepared a natural nanogel with topical delivery (anti-melanoma and antibacterial). However, lesions must be covered after treatment; thus, PEO-Gel nanofibers is proposed as a dressing. Furthermore, an appropriate dressing should be biocompatible, biodegradable, and prevent entry from environmental pathogens (Barnea et al. 2010; Kamoun et al. 2017). Electrospun nanofibers with various polymers as precursors, controllable pore size, and excessive surface-to-volume ratio have been widely used in protective clothing, tissue engineering, functional materials, and energy storage (Abrigo et al. 2014; Xue et al. 2019). Gelatin is a natural hydrophilic polymer that is generally extracted from collagen. It has been widely used in the pharmacology industry due to its excellent biocompatibility, low immunological activity, and controllable physical parameters (Dille et al. 2015). However, it is impossible to be electrospun alone (Huang et al. 2004; Ki et al. 2005). PEO was thus added to Gel in the current study to meet this challenge. It is a nonionic hydrophilic polymer with high molecular weight and proper physical strength (Chiappetta and Sosnik 2007; Pinto et al. 2003). As results shows, the prepared PEO-Gel fibers were at the nanometer scale and therefore can prevent the entry of environmental pathogens. Besides, they did not affect cell viability and bacterial growth, so as a neutral dressing could be used. In the current study, a comprehensive comparsion was performed amongst biological activities of the M. communis EO and its nanogel dosage form. However, the comparison could also be made with alpha pinene (the main compound) and its nanogel dosage form.
Conclusions
The study evaluated the antioxidant, anticancer, and antibacterial activities of M. communis EO and its nanogel dosage form; the efficacy of nanogel was significantly more potent than the EO. Moreover, PEO-Gel electrospun nanofibers without any effect on A-375 melanoma cells, E. coli, and S. aureus were proposed as dressing after treatment for in vivo studies.
Data availability
Ok.
References
Abedinpour N, Ghanbariasad A, Taghinezhad A, Osanloo M (2021) Preparation of nanoemulsions of mentha piperita essential oil and investigation of their cytotoxic effect on human breast cancer lines. BioNanoScience 11:428–436. https://doi.org/10.1007/s12668-021-00827-4
Abrigo M, McArthur SL, Kingshott P (2014) Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol Biosci 14:772–792
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, vol 456. Allured publishing corporation, Carol Stream, IL
Adepu S, Ramakrishna S (2021) Controlled drug delivery systems: current status and future directions. Molecules (basel, Switzerland) 26:5905. https://doi.org/10.3390/molecules26195905
Aleksic V, Knezevic P (2014) Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol Res 169:240–254
Alipour G, Dashti S, Hosseinzadeh H (2014) Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytother Res 28:1125–1136
Anilkumar K, Jinisha B, Manoj M, Jayalekshmi S (2017) Poly (ethylene oxide)(PEO)–Poly (vinyl pyrrolidone)(PVP) blend polymer based solid electrolyte membranes for developing solid state magnesium ion cells. Eur Polym J 89:249–262
Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K (2002) Methods for testing antioxidant activity. Analyst 127:183–198
Avazmohammadi R, Castañeda PP (2015) The rheology of non-dilute dispersions of highly deformable viscoelastic particles in Newtonian fluids. J Fluid Mech 763:386–432. https://doi.org/10.1017/jfm.2014.687
Aydin E, Türkez H, Geyikoğlu F (2013) Antioxidative, anticancer and genotoxic properties of α-pinene on N2a neuroblastoma cells. Biologia 68:1004–1009. https://doi.org/10.2478/s11756-013-0230-2
Barnea Y, Weiss J, Gur E (2010) A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther Clin Risk Manag 6:21–27
Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC (2014) Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Evid Based Complement Alternat Med 2014:651593
Bird RB, Armstrong RC, Hassager O (1987) Dynamics of polymeric liquids. Vol. 1: fluid mechanics
Cai L, Shi H, Cao A, Jia J (2019) Characterization of gelatin/chitosan ploymer films integrated with docosahexaenoic acids fabricated by different methods. Sci Rep 9:1–11
Chiappetta DA, Sosnik A (2007) Poly (ethylene oxide)–poly (propylene oxide) block copolymer micelles as drug delivery agents: improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharm 66:303–317
de Matos SP, Lucca LG, Koester LS (2019) Essential oils in nanostructured systems: challenges in preparation and analytical methods. Talanta 195:204–214
Dille M, Draget K, Hattrem M (2015) Norwegian University of Science and Technology (NTNU), Trondheim, Norway. Modifying Food Texture: Novel Ingredients and Processing Techniques, 183.
Domingues B, Lopes JM, Soares P, Pópulo H (2018) Melanoma treatment in review. ImmunoTargets Ther 7:35
Esmaili F, Sanei-Dehkordi A, Amoozegar F, Osanloo M (2021) A review on the use of essential oil-based nanoformulations in control of mosquitoes. Biointerface Res Appl Chem 11:12516–12529
Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR (2005) Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 40:100–107
Ghanbariasad A, Valizadeh A, Noorpisheh Ghadimi S, Fereidouni Z, Osanloo M (2021) Nanoformulating Cinnamomum zeylanicum essential oil with an extreme effect on Leishmania tropica and Leishmania major. J Drug Deliv Sci Technol (Accepted manuscript)
Guo J, Xu S, Qin Y, Li Y, Lin X, He C, Dai S (2020) The temperature influence on the phase behavior of ionic liquid based aqueous two-phase systems and its extraction efficiency of 2-chlorophenol. Fluid Phase Equilib 506:112394. https://doi.org/10.1016/j.fluid.2019.112394
Hardy KM, Kirschmann DA, Seftor EA, Margaryan NV, Postovit L-M, Strizzi L, Hendrix MJ (2010) Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer Res 70:10340–10350
Herman A, Herman AP (2015) Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: a review. J Pharm Pharmacol 67:473–485
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S (2014) Drug resistance in cancer: an overview. Cancers (basel) 6:1769–1792
Hsouna AB, Dhibi S, Dhifi W, Mnif W, Hfaiedh N (2019) Chemical composition and hepatoprotective effect of essential oil from Myrtus communis L. flowers against CCL 4-induced acute hepatotoxicity in rats. RSC Adv 9:3777–3787
Huang Z-M, Zhang Y, Ramakrishna S, Lim C (2004) Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 45:5361–5368
Hussain A, Samad A, Singh S, Ahsan M, Haque M, Faruk A, Ahmed F (2016) Nanoemulsion gel-based topical delivery of an antifungal drug: in vitro activity and in vivo evaluation. Drug Deliv 23:642–657
Jiang S-P, He S-N, Li Y-L, Feng D-L, Lu X-Y, Du Y-Z, Yu H-Y, Hu F-Q, Yuan H (2013) Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int J Nanomed 8:3141–3150
Kamoun EA, Kenawy E-RS, Chen X (2017) A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res 8:217–233. https://doi.org/10.1016/j.jare.2017.01.005
Ki CS, Baek DH, Gang KD, Lee KH, Um IC, Park YH (2005) Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer 46:5094–5102
Kuppan P, Sethuraman S, Krishnan UM (2013) PCL and PCL-gelatin nanofibers as esophageal tissue scaffolds: optimization, characterization and cell-matrix interactions. J Biomed Nanotechnol 9:1540–1555
Kwack J, Masud A (2014) A stabilized mixed finite element method for shear-rate dependent non-Newtonian fluids: 3D benchmark problems and application to blood flow in bifurcating arteries. Comput Mech 53:751–776. https://doi.org/10.1007/s00466-013-0928-6
Langeveld WT, Veldhuizen EJ, Burt SA (2014) Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol 40:76–94
Leithe E, Sirnes S, Omori Y, Rivedal E (2006) Downregulation of gap junctions in cancer cells. Crit Rev Oncog 12:225–256. https://doi.org/10.1615/critrevoncog.v12.i3-4.30
Longley D, Johnston P (2005) Molecular mechanisms of drug resistance. J Pathol 205:275–292
Maksimović-Ivanić D, Bulatović M, Edeler D, Bensing C, Golić I, Korać A, Kaluđerović GN, Mijatović S (2019) The interaction between SBA-15 derivative loaded with Ph3Sn(CH2)6Oh and human melanoma A375 cell line: uptake and stem phenotype loss. J Biol Inorg Chem 24:223–234
Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM (2006) Nanoemulsions: formation, structure, and physical properties. J Phys Condens Matter 18:R635
Massi D, Brusa D, Merelli B, Ciano M, Audrito V, Serra S, Buonincontri R, Baroni G, Nassini R, Minocci D (2014) PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol 25:2433–2442
Merk M, Chirikian O, Adlhart C (2021) 3D PCL/gelatin/genipin nanofiber sponge as scaffold for regenerative medicine. Materials 14:2006
Messaoud C, Zaouali Y, Salah AB, Khoudja M, Boussaid M (2005) Myrtus communis in Tunisia: variability of the essential oil composition in natural populations. Flavour Fragr J 20:577–582
Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Tang AW, Phung TO, Spellberg B (2005) Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 352:1445–1453
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V (2013) Effect of essential oils on pathogenic bacteria. Pharmaceuticals (basel) 6:1451–1474. https://doi.org/10.3390/ph6121451
Nissen L, Zatta A, Stefanini I, Grandi S, Sgorbati B, Biavati B, Monti A (2010) Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 81:413–419. https://doi.org/10.1016/j.fitote.2009.11.010
Osanloo M, Abdollahi A, Valizadeh A, Abedinpour N (2020a) Antibacterial potential of essential oils of Zataria multiflora and Mentha piperita, micro-and nano-formulated forms. Iran J Microbiol 12:43–51. https://doi.org/10.18502/ijm.v12i1.2517
Osanloo M, Arish J, Sereshti H (2020b) Developed methods for the preparation of electrospun nanofibers containing plant-derived oil or essential oil: a systematic review. Polym Bull 77:6085–6104
Percival SL, Williams DW (2014) Chapter Six—Escherichia coli. In: Percival SL, Yates MV, Williams DW, Chalmers RM, Gray NF (eds) Microbiology of waterborne diseases (second edition). Academic Press, London, pp 89–117
Pinto N, Johnson A Jr, MacDiarmid A, Mueller C, Theofylaktos N, Robinson D, Miranda F (2003) Electrospun polyaniline/polyethylene oxide nanofiber field-effect transistor. Appl Phys Lett 83:4244–4246
Ploux L, Ponche A, Anselme K (2010) Bacteria/material interfaces: role of the material and cell wall properties. J Adhes Sci Technol 24:2165–2201
Qasemi H, Fereidouni Z, Karimi J, Abdollahi A, Zarenezhad E, Rasti F, Osanloo M (2021) Promising antibacterial effect of impregnated nanofiber mats with a green nanogel against clinical and standard strains of Pseudomonas aeruginosa and Staphylococcus aureus. J Drug Deliv Sci Technol 66:102844
Ruch R (2020) Gap junctions and connexins in cancer formation, progression, and therapy. Cancers (basel) 12:3307. https://doi.org/10.3390/cancers12113307
Sen A, Kurkçuoglu M, Yıldırım A, Dogan A, Bitis L, Baser KHC (2020) Chemical and biological profiles of essential oil from different parts of Myrtus communis L. subsp. communis from Turkey. Agric Conspec Sci 85:71–78
Standards NIo, Technology (2008) Mass spectral library (NIST/EPA/NIH). National Institute of Standards and Technology Gaithersburg, MD
Valizadeh A, Khaleghi AA, Roozitalab G, Osanloo M (2021) High anticancer efficacy of solid lipid nanoparticles containing Zataria multiflora essential oil against breast cancer and melanoma cell lines. BMC Pharmacol Toxicol 22:52. https://doi.org/10.1186/s40360-021-00523-9
Vincent KM, Postovit LM (2017) Investigating the utility of human melanoma cell lines as tumour models. Oncotarget 8:10498–10509. https://doi.org/10.18632/oncotarget.14443
Wanke C, Sears CL (2008) Escherichia coli. In: Heggenhougen HK (ed) International encyclopedia of public health. Academic Press, Oxford, pp 452–459
WHO WHO (2021) Cancer
Xue J, Wu T, Dai Y, Xia Y (2019) Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev 119:5298–5415. https://doi.org/10.1021/acs.chemrev.8b00593
Zare Y, Park SP, Rhee KY (2019) Analysis of complex viscosity and shear thinning behavior in poly (lactic acid)/poly (ethylene oxide)/carbon nanotubes biosensor based on Carreau-Yasuda model. Results Phys 13:102245
Zhang YS, Khademhosseini A (2017) Advances in engineering hydrogels. Science 356:eaaf3627. https://doi.org/10.1126/science.aaf3627
Zhang X-Y, Lu W-Y (2014) Recent advances in lymphatic targeted drug delivery system for tumor metastasis. Cancer Biol Med 11:247–254. https://doi.org/10.7497/j.issn.2095-3941.2014.04.003
Zhang Y-M, Rock CO (2008) Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233
Zhou Y, Qi P, Zhao Z, Liu Q, Li Z (2014) Fabrication and characterization of fibrous HAP/PVP/PEO composites prepared by sol-electrospinning. RSC Adv 4:16731–16738
Zhou L, Dai S, Xu S, She Y, Li Y, Leveneur S, Qin Y (2021) Piezoelectric effect synergistically enhances the performance of Ti32-oxo-cluster/BaTiO3/CuS p-n heterojunction photocatalytic degradation of pollutants. Appl Catal B 291:120019. https://doi.org/10.1016/j.apcatb.2021.120019
Funding
Fasa University of Medical Sciences supported this study, Grant No. 400178.
Author information
Authors and Affiliations
Contributions
GhR prepared the nanogel and nanofibers and performed MTT and DPPH assays. GhR and YY wrote the MS. AA performed the antibacterial tests. MS interpreted ATR-FTIR. FR contributed to the preparation of nanogel and MTT assay. MO design of the study, statistical analysis, and revised the MS. All authors contributed to drafting MS and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
Researchers have no conflict of interest in this study.
Ethical approval
This study has been ethically approved by the Fasa University of Medical Sciences’ ethical committee, IR.FUMS.REC.1400.129.
Inform consent
This research did not involve human study; thus, no constant form was used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roozitalab, G., Yousefpoor, Y., Abdollahi, A. et al. Antioxidative, anticancer, and antibacterial activities of a nanoemulsion-based gel containing Myrtus communis L. essential oil. Chem. Pap. 76, 4261–4271 (2022). https://doi.org/10.1007/s11696-022-02185-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02185-1