Abstract
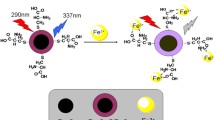
An off–on colorimetric and fluorescent probe N1, N4-bis (2-(3′, 6′-bis(diethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl) ethyl) terephthalamide (Rp) for Fe3+ was designed and synthesized, which could selectively and sensitively respond to Fe3+ among other tested metal cations in THF–H2O solution (v/v = 4/6, pH = 7.2) accompanied with a remarkable fluorescence enhancement and an obvious color change visible to “naked-eye”. The fluorescence intensity of the probe Rp showed a good linearity with the concentration of Fe3+ covering from 0.1 to 26 μM and the detection limit was 0.0231 μM. Meanwhile, the binding mode between Rp and Fe3+ was found to be a 1:2 stoichiometric formation. Moreover, the complexation of Rp–Fe3+ could be broken by carbonate, so this system might be utilized as a carbonate probe in the future. Most importantly, the probe Rp was not only applied to monitor Fe3+ level in human blood serum and water samples, but also was used for fluorescence imaging of Fe3+ in living cell.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The design and development of probes for the detection of heavy and transition metal ions have attracted more and more attention nowadays (Sahana et al. 2018; Zhou et al. 2018; Liao et al. 2017; Zheng et al. 2017), which is not only because of their inherent excellent properties including operational simplicity, low cost and real-time monitoring, but also their advantages of significant application in biological and environmental systems (Li et al. 2015; Ucar et al. 2017; Zhu et al. 2017; Gu et al. 2017; Karimi et al. 2016). Among these metal ions, Fe3+ is one of the important targets to be monitored in the biological and environmental matrices. On the one hand, the deficiency or overload of Fe3+ in human and animal bodies can result in various disorders and harmful impact on the health of living body subsequently (Gao et al. 2015; Chen et al. 2013; Carlson et al. 2008). For another, Fe3+ as one of the environmental contaminants has serious effects on the yield and quality of crops via various people’s activities (Jolly 2013; Hofmann et al. 2015). It is known that Fe3+ is easily soluble in water and the environment of the organism is an aqueous solution, but most of the reported Fe3+ probes are only used in the detection system with low water content (Liu et al. 2014; Kagit et al. 2014). And though there is a popular belief that turn-on probes are more efficient, most of the reported probes for Fe3+ undergo a fluorescence quenching due to the paramagnetic nature of Fe3+ (Fu et al. 2013; Wang et al. 2016). Hence, it is significant and challenging to develop turn-on fluorescent probes for Fe3+ which can be used in the system with high water content to meet the needs of biological and environmental application.
Among the probes family, the rhodamine framework is used as a platform to construct colorimetric and turn-on fluorescent probes for detecting ions and has attracted considerable interests because of its excellent photophysical properties with large molar extinction coefficients and high fluorescence quantum yields (Mandal et al. 2013; Jiao et al. 2016; Puangploy et al. 2014; Ramette and Sandell 1956). In addition, the rhodamine derivatives undergo the form’s conversion from spirolactam structure (nonfluorescence) to ring-opening amide form (fluorescence) once interacting with specific metal ions (Zhang et al. 2015; Kim et al. 2008) or some solvents (Sekar and Kothavale 2016) or protons (Xu et al. 2017). However, most of the reported probes based on rhodamine for Fe3+ still have some shortcomings in practical application, such as relatively narrow detection range (Liu et al. 2014), higher detection limit (Liu et al. 2014; Ji et al. 2013; Liu et al. 2015) and without application in biological systems (Sivaraman et al. 2014; Hu et al. 2011). Moreover, the reversibility of a fluorescent probe is an important feature because this feature can reduce the cost of analysis, but only a few rhodamine-based Fe3+ probes are reversible (Lee et al. 2015; Zhang et al. 2013; Sun et al. 2015). It is believed that bis-(rhodamine)-based probes with appropriate binding sites for metal ions would have better sensitivity and selectivity than conventional mono-rhodamine-based probes (Yan et al. 2015; Chereddy and Thennarasu 2011). On the other hand, increasing the conjugation or rigidity of the probe will lead to the changes in the optical properties and responsiveness (Weerasinghe et al. 2010; Chen et al. 2012; Luo et al. 2016).
Based on the above studies, herein, a bis-(rhodamine)-based colorimetric and fluorescent probe Rp for Fe3+ was reported by the means of changing the structure of the probe to ameliorate the characteristic of flexibility and rigidity and increase the conjugated structure of the probe. As expected, the Rp exhibited an excellent selectivity and sensitivity toward Fe3+ by forming a 1:2 complex in a high water content detection system. Meantime, the Rp–Fe3+ complex was broken in the presence of carbonate, so it had the value for detecting carbonate. Most importantly, the probe Rp was used to analyze Fe3+ in human blood serum and water samples and image Fe3+ in living SGC-7901 cells.
Experimental
Materials and apparatus
All the reagents were analytical grade and used without further purification unless otherwise noted. The pH was measured using a Model pHs-3C meter (Shanghai, China). The melting points were acquired from a X-4 microscopic melting point apparatus (Shanghai, China). NMR spectra were determined by a Bruker 400 MHz spectrophotometer with CDCl3 as solvent. HRMS spectra were recorded using a Thermo Fisher Scientific LTQ FT Ultramass spectrometer. UV–Vis spectra were obtained from a Hitachi U-3900 spectrophotometer (Tokyo, Japan). Fluorescence spectra were measured at λex = 550 nm on a Hitachi F-7000 spectrofluorimeter (Tokyo, Japan) with a 10 mm quartz cuvette. The fluorescence image experiments were performed on a Nikon TE2000 inverted fluorescence microscope.
UV–Vis absorption and fluorescence measurement
Stock solutions (1.0 × 10−3–1.0 × 10−2 mol/L) of Li+, Na+, K+, Ag+, Ni2+, Mn2+, Mg2+, Pb2+, Ca2+, Ba2+, Zn2+, Cu2+, Cd2+, Co2+, Hg2+, Fe2+, Fe3+, Al3+ and Cr3+ were prepared by dissolving their nitrate or chloride salts into double-distilled water. Stock solution (1.0 × 10−3 mol/L) of Rp was prepared by dissolving the appropriate amount of Rp into DMSO, DMF or THF. 0.1 mL of the Rp stock solution (1.0 × 10−3 mol/L) and the appropriate amount of the ion stock solution were added into a 10 mL brown volumetric flask, and diluted to 10 mL with DMSO/Tris–HCl, DMF/Tris–HCl or THF/Tris–HCl aqueous buffer solutions, respectively. After waiting for 20 min, the spectral data were recorded. Fluorescence measurements were carried out with excitation wavelength of 550 nm and excitation and emission slit width of 5 nm.
Testing Fe3+ in the reference materials or samples
The reference materials: GBW(E)080915, GBW(E)080916 and GBW(E)080917 were purchased from Beijing Bohui Innovation Optoelectronic Technology Co., Ltd. (Beijing, China). The human blood serum was treated according to the reported method (Olson and Hamlin 1969). After 4 mL 20% trichloroacetic acid (TCA) was added into the blood serum (4 mL), the mixture was stirred and heated to 90 °C for 15 min. Then, the mixture was cooled into room temperature, sonicated for 2 min and centrifuged for 10 min at 10,000 rpm and then the supernatant was collected for analysis. The water samples were collected locally and filtrated through a 0.45 μm membrane before used. The concentration of Fe3+ in the reference materials, water sample or human blood serum was determined by the fluorescence intensity and the linear relationship between the fluorescence intensity of Rp–Fe3+ solution and Fe3+ concentration.
Fluorescence imaging
Gastric carcinoma cell lines (SGC-7901) were cultured with 10% (v/v) fetal bovine serum (FBS) in Dulbecco’s Modified Eagle Medium (DMEM) at 37 °C in an atmosphere of 5% CO2 for 24 h. Immediately the SGC-7901 cells were incubated with 10 μM Rp at 37 °C for 30 min, washed three times with phosphate buffered saline (PBS) and then imaged. Following, the cells were cultured with 50 μM FeCl3 for another 30 min, washed three times with phosphate buffered saline (PBS) and imaged.
Synthesis of the probe Rp
The synthetic route of the probe Rp was shown in Scheme 1. 2-(2-aminoethyl)-3′, 6′-bis(diethylamino)spiro[isoindoline-1,9′-xanthen]-3-one (1) was easily synthesized according to the procedure in the literature (Zhang et al. 2007). N1, N4-bis(2-(3′,6′-bis(diethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl)ethyl)terephthalamide (Rp) was synthesized according to the following procedure: The terephthaloyl chloride (0.1 g, 0.49 mmol) dissolved in the dry dichloromethane (10 mL) was added into the mixed solution of compound 1 (0.58 g, 1.19 mmol) and triethylamine (400 μL, 3.0 mmol) in dry dichloromethane (25 mL) with vigorously stirring at 0 °C. After adding, the solution was stirred for 8 h at 25 °C. Then, the reaction solution was washed three times with water and the organic layer was dried over anhydrous Na2SO4, and the crude solid was obtained by removing the organic solvent under reduced pressure. After purified by silica gel column chromatography (methanol/dichloromethane = 1/9, v/v), 0.42 g white solid (Rp) was obtained in 79% yield, m.p. > 320 °C. 1H NMR (400 MHz, CDCl3), δ (ppm): 8.25 (s, 2H), 7.93–7.96 (m, 6H), 7.42–7.45 (m, 4H), 7.05–7.08 (m, 2H), 6.42 (d, 4H, J = 8.85 Hz), 6.35–6.36 (m, 4H), 6.24 (d, 2H, J = 2.26 Hz), 6.22 (d, 2H, J = 2.25 Hz), 3.40–3.42 (m, 4H), 3.29 (q, 16H, J = 7.12 Hz), 3.20 (m, 4H), 1.14 (t, 24H, J = 7.07 Hz); 13C NMR (100 MHz, CDCl3), δ (ppm): 170.4, 166.4, 153.9, 153.3, 149.0, 136.6, 132.8, 130.4, 128.4, 128.2, 127.4, 123.9, 123.0, 108.4, 104.6, 97.8, 65.9, 44.4, 41.9, 40.0, 12.6; HRMS (MALDI) m/z calcd for [C68H74O6N8 + H]+: 1099.5818, found: 1099.5824.
Results and discussion
The effect of different detection media on the fluorescence spectral measurement of Fe3+ was investigated (Fig. 1). The free Rp hardly exhibited fluorescence in DMSO–water mixtures, DMF–water mixtures, or THF–water mixtures. But upon adding same amount of Fe3+ (5.0 equiv., 5.0 equiv. of Fe3+ or other metal ions were used in the all physical characteristics experiments of Rp to ensure that Rp with spirolactam structure could convert to ring-opening amide form as far as possible and the fluorescence intensity could reach the maximum value) into the Rp solutions with different detection media, respectively, the fluorescence intensity of all the solutions increased obviously, particularly in THF–water mixed solution. Furthermore, the content of water in the media had effect on the fluorescence intensity. The fluorescence intensity increased sharply and then decreased gradually along with the decrease of water content in the detection media. Interestingly, when the ratio of THF/water (v/v) was 4:6, the fluorescence intensity reached the maximum. The results showed that Rp exhibited a more sensitively response to Fe3+ in THF–water (v/v = 4:6) and the water content of the test media was distinctly higher than that of some reported Fe3+ fluorescent probes (Liu et al. 2014, 2015; Xu et al. 2017). And the solubility of Rp in THF/water solution was examined by adding 10 mL THF/water solution with different water content into Rp (0.0110 g, 0.01 mmol). The Rp was easily dissolved in THF, but in THF–water mixed solution, the Rp could not be dissolved completely once the ratio of THF/water (v/v) was lower than 7:13. It is well known that ions have better solubility in water than that in organic solvent and it is just the aqueous system in cells. Therefore, the probe Rp was more suitable for the detection of Fe3+ in biological systems and real samples.
The pH effect on the fluorescence intensity of Rp and Rp–Fe3+ was investigated (Fig. 2). Without Fe3+, the probe Rp with spirolactam structure was colorless and exhibited no fluorescence in the pH range of 4.0–14.0. However, when pH value was less than 4.0, an obvious fluorescence appeared due to the formation of merocyanine open structure induced by strong protonation. Once Fe3+ (5.0 equiv.) was added, ring-opening forms induced by Fe3+ occured with an obvious fluorescence enhancement in the pH range of 2.0–10.0, especially 4.0–9.0. The fluorescence intensity decreased sharply once the pH value was higher than 9.0, which could be ascribed to the formation of insoluble hydroxide. In consideration of the physiological condition, pH value was adjusted to 7.2 for the following experiments.
The effect of reaction time on the response of Rp to Fe3+ was studied (Fig. 3). Interestingly, when 5.0 equiv. Fe3+ was added into the solution of Rp (10 μM) in THF–water (v/v = 4/6, pH = 7.2), the color of the solution changed from colorless to pale red within few minutes, then gradually changed to red and remained unchanged after 20 min. Additionally, the fluorescence intensity of Rp–Fe3+ increased within few minutes and reached a maximum value at 20 min and then remained almost constant, while the fluorescence intensity of blank solution almost unchanged during the test time. Therefore, the spectral properties were measured after waiting for 20 min in subsequent experiments.
Selectivity is an important indicator to evaluate the performance of a fluorescent probe. Therefore, the UV–Vis absorption and fluorescence spectra of Rp with various metal ions were explored under the same condition by adding various metal ions (Li+, Na+, K+, Ag+, Ni2+, Mn2+, Mg2+, Pb2+, Ca2+, Ba2+, Zn2+, Cu2+, Cd2+, Co2+, Hg2+, Fe2+, Fe3+, Al3+, Cr3+) into the solution of Rp (10 μM) in THF–water (v/v = 4/6, pH = 7.2) (Fig. 4). After adding (5.0 equiv.) Fe3+ into the Rp solution, a strong absorption peak at 564 nm and fluorescence emission at 584 nm appeared, whereas the other tested metal ions did not produce an obvious response to Rp except for Hg2+ and Al3+, which only resulted in a little absorption peak and slight fluorescence emission.
Meanwhile, the colorimetric and fluorescent properties of Rp toward various metal ions in THF–water (v/v = 4/6, pH = 7.2) were studied under irradiation with daylight and a UV lamp (365 nm), respectively (Fig. 5). The free Rp solution was colorless and non-fluorescent. In the presence of Fe3+, the Rp solution absorbed daylight and became red (the upper part of Fig. 5) and a significant red fluorescence was observed (the lower part of Fig. 5), whereas the other tested metal ions did not induce the changes except for Al3+ and Hg2+, which only resulted in a little bit pink color and slight fluorescence emission.
Competitive experiments were performed to evaluate the possible interferences of other coexistent metal ions on the detection of Rp to Fe3+ (Fig. 6). As expected, other tested competitive metal ions hardly affected the recognition of Rp to Fe3+ even though their concentration were twice that of Fe3+ except for Hg2+ and Al3+, which induced the fluorescence intensity of the Rp and Fe3+ solution only to increase by 1.49 and 0.89%, respectively. These results indicated that the probe Rp could be employed to detect Fe3+ with a high selectivity.
The fluorescence intensity of Rp (10 μM) in the presence of various interfering ions (10 equiv.) and coexistence with Fe3+ (5.0 equiv.) in THF–water (v/v = 4/6, pH = 7.2). The black bars represent the fluorescence response of Rp and competing ions: 1, blank; 2, Li+; 3, Na+; 4, K+; 5, Mg2+; 6, Ca2+; 7, Zn2+; 8, Pb2+; 9, Cu2+; 10, Cd2+; 11, Ba2+; 12, Ni2+; 13, Mn2+; 14, Ag+; 15, Fe2+; 16, Cr3+; 17, Co2+; 18, Hg2+; 19, Al3+; 20, Fe3+. The red bars represent the subsequent addition of Fe3+ (5.0 equiv.) to the above solutions (λex = 550 nm, λem = 584 nm)
UV–Vis titration experiments were conducted by adding various concentrations of Fe3+ into the Rp solution in THF–water (v/v = 4/6, pH = 7.2) (Fig. 7). Once Fe3+ was added, a distinct absorption band at 564 nm occured and enhanced with gradual addition of Fe3+. When the concentration of Fe3+ was greater than 2.6 equiv. (excess), the absorbance did not increase further and became saturated (Fig. 7 inset).
The fluorescence sensing behavior of Rp for Fe3+ was explored by fluorescence titration in THF–water (v/v = 4/6, pH = 7.2) (Fig. 8). A significantly enhanced fluorescence signal at 584 nm could be detected when Fe3+ was added. Besides, the fluorescence intensity at 584 nm enhanced gradually with the increasing concentration of Fe3+. The results indicated that the fluorescence intensity of Rp–Fe3+ showed a good linearity with the concentration of Fe3+ covering from 0.1 to 26 μM. The linear regression equation was F = 20.75535 + 106.49896C and R2 was 0.9925. The detection limit was as low as 0.0231 μM calculated from \(3\delta /k\)(where δ was the blank solution standard deviation and k was the slope of the calibration curve) (Goswami et al. 2013). These results implied that Rp could be used as a sensitive probe for the detection of Fe3+.
To investigate the binding mode between Rp and Fe3+, the Job’s plot analysis was adopted according to the absorbance of Rp at different Fe3+ mole fraction in THF–water (v/v = 4/6, pH = 7.2) (Fig. 9). The absorption intensity of Rp came to maximum when the mole fraction of Fe3+ was around 0.67, which revealed that Rp and Fe3+ complexed at a stoichiometric ratio of 1:2.
The association constant K of Rp with Fe3+ was determined by the Benesi–Hildebrand equation (Wang and Yu 2007): \((I_{\hbox{max} } - I_{0} )/(I - I_{0} ) = 1 + (1/K) (1/[M^{n} ])\), where I was the measured absorption intensity, I0 was the absorption intensity of the free Rp, Imax was the absorption intensity of the Rp with saturated Fe3+, [Fe3+] was Fe3+ concentrations. Based on the 1:2 binding mode (n = 2), a plot of (Imax − I0)/(I − I0) vs. 1/[Fe3+]2 yielded the apparent binding constant value as 6.54 × 109 M−2 (R2 = 0.991) (Fig. 10). And the Benesi–Hildebrand plot’s excellent linearity further demonstrated the fact that the probe Rp responded to Fe3+ by the 1:2 binding mode. It’s interesting that the complex ratio of Rp to Fe3+ was distinct from that of the probes reported by Luo et al. (2016) or Weerasinghe et al. (2010) in which the complex ratio was 1:1, though they have similar molecular structure. Compared with the present work, the probe reported by Weerasinghe et al. (2010) had a higher rigidity and the probe in our group’s previous work (Luo et al. 2016) had a higher flexibility. That is to say, in this work, the rigidity and flexibility of Rp fell in between them, leading to the different spatial orientation of coordination atoms and thus the binding mode of Rp–Fe3+ was different.
The binding mode of Rp and Fe3+ was further explored using DFT calculations with the B3LYP method, where 6-31+G (d, p) used as the basis set (Bao et al. 2014; Yan et al. 2017). The stoichiometry ratio of Rp–Fe3+ was found to be 1:2 based on the Job’s plot and Benesi–Hildebrand plot and this Rp–Fe3+ complex with 1:2 binding mode was modelled by DFT calculations. At the same time, the probe Rp and the Rp–Fe3+ complex with 1:1 binding mode were also modelled as a comparison model. In the optimized geometries model of the Rp–Fe3+ complex with 1:2 binding mode (Fig. 11), the oxygen atoms and nitrogen atoms on each side of terephthaloyl were oriented in coordination with one Fe3+, that is, Rp and Fe3+ were coordinated by the ratio of 1:2. The spatial distributions and the energy gap (ΔE) of the HOMO and LUMO of these three structures were also obtained from the DFT calculations (Fig. 12). For Rp and the Rp–Fe3+ complex with 1:1 binding mode, the energy gap (ΔE) between the ELUMO and EHOMO were 92.3757 and 23.5861 kcal/mol, respectively. However, for the Rp–Fe3+ complex with 1:2 binding mode, the energy gap (ΔE) was 3.2944 kcal/mol, which was far less than that of Rp or the Rp–Fe3+ complex with 1:1 binding mode. These results indicated that the Rp–Fe3+ complex with 1:2 binding mode could converge to a more stable state than that of the Rp–Fe3+ complex with 1:1 binding mode and could play down the energy gap and stabilize the system.
The fluorescence changes of Rp–Fe3+ complex were investigated in the presence of various small anions, such as CO32−, HPO42−, SO42−, F−, Cl−, Br−, I−, HCO3−, NO3−, CH3COO− and H2PO4−. In the presence of carbonate, the fluorescence intensity of Rp–Fe3+ was obviously quenched and hydrogen phosphate resulted in slight fluorescence quenching, while other tested anions exhibited no significant influence. The quenching may be ascribed to the high alkalinity and large hydration-free energy of carbonate which produced more OH− and disrupted the complexation of Rp with Fe3+ (He et al. 2015). Subsequently, a reversible experiment of the probe Rp was also performed by alternating addition of Fe3+ and Na2CO3 into the Rp solution (Fig. 13). Upon addition of Fe3+ (5.0 equiv.), the solution of Rp (10 μM) transmitted strong fluorescence and noticeable color. Then, the addition of Na2CO3 (20 equiv.) into the mixed solution of Rp and Fe3+ resulted in fluorescence quenching and color fading, followed by adding Fe3+ (10 equiv.) into the mixed solution, the color and the fluorescence appeared again (Fig. 13 inset). It was interesting that in the reversible experiment of our group’s previous work (Luo et al. 2016), CO32− was 5.0 equivalent of Fe3+, which was more than that of the present work, and the fluorescence intensity of the probe-Fe3+ complex just decreased in some degree. Thus, this finding suggested that the complex of Rp–Fe3+ had potential application value in the detection of carbonate.
Based on the above results, the possible binding mode of Rp and Fe3+ was proposed in Scheme 2. The probe Rp interacted with Fe3+ in a 1:2 stoichiometry with fluorescence enhancement and color change. Then, the addition of carbonate disrupted the chelation of Rp–Fe3+, resulting in the reversible re-formation of the non-fluorescence spirolactam.
To evaluate the accuracy of the developed analytical method based on the probe Rp for detecting Fe3+, three certified reference materials (CRMs) (GBW (E) 080915, GBW(E) 080916, and GBW(E) 080917) were used. The test results agreed well with the standard values (Table 1). Furthermore, the applicability of the probe Rp in human blood serum and water samples was also conducted using standard addition method. As presented in Table 2, the concentration of Fe3+ in the serum was found to be 22.30 ± 0.10 µM, which coincided well with the value (21.1 ± 0.8 µM) obtained from human blood serum in previous study (Long et al. 2014). The recoveries of Fe3+ were in the range from 99.1 to 100.6% in human blood serum and 98.0 to 99.2% in waste water with good R.S.D. The results suggested that Rp had the potential application for quantitative detection of Fe3+ in real samples.
Cell imaging experiments were carried out to test the application of Rp in living cells (Fig. 14). After SGC-7901 cells were cultured with Rp (10 µM) for 30 min and washed with phosphate buffered saline (PBS), no intracellular fluorescence signal could be observed (Fig. 14b), which was probably because the intracellular concentration of Fe3+ was too low to reach the detection limit of Rp. When the cells were cultivated with FeCl3 (50 µM) for another 30 min and washed with phosphate buffered saline (PBS), some of the Fe3+ entered the cell. At this time, the intracellular concentration of Fe3+ was higher than the detection limit of Rp and thus a bright intracellular fluorescence appeared (Fig. 14d). The results demonstrated that the Rp had cell permeability and could be applied to image Fe3+ in SGC-7901 cells.
Fluorescence images of SGC-7901 cells. a bright-field image: cells stained by Rp (10 µM) for 30 min; b fluorescence image of “a”; c merged images of “a” and “b”; d bright-field image: cells stained with Rp (10 µM) for 30 min and then incubated with Fe3+ (50 µM) for another 30 min; e fluorescence image of “c”; f merged images of “d” and “e”
The comparison of the present probe Rp over some other pioneering fluorescent probes was listed in Table 3 (Liu et al. 2014; Wang et al. 2015; Liu et al. 2015; Luo et al. 2016; Wang et al. 2014; Zhou et al. 2017; Xu et al. 2017). The previous reported Fe3+ fluorescent probes had higher ratio of organic solvent in the detection system or higher detection limit, or the probes were not used in biological, environmental sample or cell imaging. Therefore, the present probe Rp had broader applications and higher potential value.
Conclusions
In conclusion, a colorimetric and fluorescent probe Rp for Fe3+ was developed, which showed a highly selective response to Fe3+ in THF–water (v/v = 4/6, pH = 7.2) with a low detection limit of 0.0231 μM. The complexation of Rp with Fe3+ could be broken by carbonate, so this system had a potential application in the detection of carbonate. In addition, the binding mode of Rp–Fe3+ was proposed and further verified by DFT calculation. Most importantly, the fluorescence imaging of intracellular Fe3+ in SGC-7901 cells and the determination of Fe3+ in human blood serum and water samples indicated that the probe Rp had practical application value in biological and environmental systems.
References
Bao X, Shi J, Nie X et al (2014) A new rhodamine B-based ‘on–off’ chemical sensor with high selectivity and sensitivity toward Fe3+ and its imaging in living cells. Bioorganic Med Chem 22:4826–4835. https://doi.org/10.1016/j.bmc.2014.06.054
Carlson ES, Magid R, Petryk A et al (2008) Iron deficiency alters expression of genes implicated in Alzheimer disease pathogenesis. Brain Res 1237:75–83. https://doi.org/10.1016/j.brainres.2008.07.109
Chen X, Hong H, Rui H et al (2012) A new bis(rhodamine)-based fluorescent chemosensor for Fe3+. J Fluoresc 22:789–794. https://doi.org/10.1007/s10895-011-1022-0
Chen WD, Gong WT, Ye ZQ et al (2013) FRET-based ratiometric fluorescent probes for selective Fe3+ sensing and their applications in mitochondria. Dalton Trans 42:10093–10099. https://doi.org/10.1039/c3dt50832c
Chereddy NR, Thennarasu S (2011) Synthesis of a highly selective bis-rhodamine chemosensor for naked-eye detection of Cu2+ ions and its application in bio-imaging. Dyes Pigm 91:378–382. https://doi.org/10.1016/j.dyepig.2011.04.016
Fu L, Mei J, Zhang JT et al (2013) Selective and sensitive fluorescent turn-off chemosensors for Fe3+. Luminescence 28:602–606. https://doi.org/10.1002/bio.2406
Gao M, Xie P, Wang L et al (2015) A new optical sensor for Al3+/Fe3+, based on PET and chelation-enhanced fluorescence. Res Chem Intermed 41:9673–9685. https://doi.org/10.1007/s11164-015-1956-y
Goswami S, Aich K, Das S et al (2013) A highly selective and sensitive probe for colorimetric and fluorogenic detection of Cd2+ in aqueous media. Analyst 138:1903–1907. https://doi.org/10.1039/c3an36884j
Gu B, Huang L, Su W et al (2017) A benzothiazole-based fluorescent probe for distinguishing and bioimaging of Hg2+ and Cu2+. Anal Chim Acta 954:97–104. https://doi.org/10.1016/j.aca.2016.11.0
He L, Liu C, Xin JH (2015) A novel turn-on colorimetric and fluorescent sensor for Fe3+ and Al3+ with solvent-dependent binding properties and its sequential response to carbonate. Sens Actuators B Chem 213:181–187. https://doi.org/10.1016/j.snb.2015.02.060
Hofmann J, Watson V, Scharaw B (2015) Groundwater quality under stress: contaminants in the Kharaa River basin (Mongolia). Environ Earth Sci 73:629–648. https://doi.org/10.1007/s12665-014-3148-2
Hu ZQ, Feng YC, Huang HQ et al (2011) Fe3+-selective fluorescent probe based on Rhodamine B and its application in bioimaging. Sens Actuators B Chem 156:428–432. https://doi.org/10.1016/j.snb.2011.04.075
Ji S, Meng X, Ye W et al (2013) A rhodamine-based “turn-on” fluorescent probe for Fe3+ in aqueous solution. Dalton Trans 43:1583–1588. https://doi.org/10.1039/c3dt52422a
Jiao Y, Zhang L, Zhou P (2016) A rhodamine B-based fluorescent sensor toward highly selective mercury(II) ions detection. Talanta 150:14–19. https://doi.org/10.1016/j.talanta.2015.11.065
Jolly YN (2013) Transfer of metals from soil to vegetables and possible health risk assessment. Springerplus 2:385–392. https://doi.org/10.1186/2193-1801-2-385
Kagit R, Yildirim M, Ozay O et al (2014) Phosphazene based multicentered naked-eye fluorescent sensor with high selectivity for Fe3+ ions. Inorg Chem 53:2144–2151. https://doi.org/10.1021/ic402783x
Karimi M, Badieia A, Ziarani GM (2016) Fluorescence-enhanced optical sensor for detection of Al3+ in water based on functionalised nanoporous silica type SBA-15. Chem Pap 70:1431–1438. https://doi.org/10.1515/chempap-2016-0079
Kim HN, Lee MH, Kim HJ et al (2008) A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem Soc Rev 37:1465–1472. https://doi.org/10.1039/B802497A
Lee S, Rao BA, Son YA (2015) A highly selective fluorescent chemosensor for Hg2+ based on a squaraine-bis(rhodamine-B) derivative: part II. Sens Actuators B Chem 210:519–532. https://doi.org/10.1016/j.snb.2015.01.008
Li C, Xiang K, Liu Y et al (2015) A novel colorimetric chemosensor for Cu2+ with high selectivity and sensitivity based on Rhodamine B. Res Chem Intermed 41:10169–10180. https://doi.org/10.1007/s11164-015-2024-3
Liao Z, Liu Y, Han SF et al (2017) A novel acylhydrazone-based derivative as dual-mode chemosensor for Al3+, Zn2+ and Fe3+ and its applications in cell imaging. Sens Actuators B Chem 244:914–921. https://doi.org/10.1016/j.snb.2017.01.074
Liu H, Wan X, Gu L et al (2014) Easily accessible ferric ion chemosensor based on rhodamine derivative and its reversible OFF–ON fluorescence response. Tetrahedron 70:7527–7533. https://doi.org/10.1016/j.tet.2014.08.008
Liu Y, Xu Z, Wang J et al (2015) Two new rhodamine-based fluorescent chemosensors for Fe3+ in aqueous solution. Lumin J Biol Chem Lumin 29:945–951. https://doi.org/10.1002/bio.2648
Long L, Zhou L, Wang L et al (2014) A ratiometric fluorescent probe for iron(III) and its application for detection of iron(III) in human blood serum. Anal Chim Acta 812:145–151. https://doi.org/10.1016/j.aca.2013.12.024
Luo A, Wang H, Wang Y et al (2016) A novel colorimetric and turn-on fluorescent chemosensor for iron(III) ion detection and its application to cellular imaging. Spectrochim Acta Part A Mol Biomol Spectrosc 168:37–44. https://doi.org/10.1016/j.saa.2016.05.048
Mandal S, Banerjee A, Lohar S et al (2013) Selective sensing of Hg2+ using rhodamine-thiophene conjugate: red light emission and visual detection of intracellular Hg2+ at nanomolar level. J Hazard Mater 261:198–205. https://doi.org/10.1016/j.jhazmat.2013.07.026
Olson AD, Hamlin WB (1969) A new method for serum iron and total iron-binding capacity by atomic absorption spectrophotometry. Clin Chem 15:438–444
Puangploy P, Smanmoo S, Surareungchai W (2014) A new rhodamine derivative-based chemosensor for highly selective and sensitive determination of Cu2+. Sens Actuators B Chem 193:679–686. https://doi.org/10.1016/j.snb.2013.12.037
Ramette RW, Sandell EB (1956) Rhodamine B Equilibria. J Am Chem Soc 78:4872–4878. https://doi.org/10.1021/ja01600a017
Sahana S, Mishra G, Sivakumar S et al (2018) Rhodamine-cyclohexane diamine based “turn-on” fluorescence chemosensor for Cr3+: photophysical & confocal cell imaging studies. J Photochem Photobiol A 351:42–49. https://doi.org/10.1016/j.jphotochem.2017.10.004
Sekar N, Kothavale S (2016) Novel triphenylamine based rhodamine derivatives: synthesis, characterization, photophysical properties and viscosity sensitivity. Rsc Adv 6:100271–100280. https://doi.org/10.1039/c6ra19899f
Sivaraman G, Sathiyaraja V, Chellappa D (2014) Turn-on fluorogenic and chromogenic detection of Fe(III) and its application in living cell imaging. J Lumin 145:480–485. https://doi.org/10.1016/j.jlumin.2013.08.018
Sun Z, Li H, Guo D et al (2015) A novel piperazine-bis(rhodamine-B)-based chemosensor for highly sensitive and selective naked-eye detection of Cu2+ and its application as an INHIBIT logic device. J Lumin 167:156–162. https://doi.org/10.1016/j.jlumin.2015.06.018
Ucar A, Findik M, Bingol H et al (2017) Organometallic chiral Schiff base for enantio-selective fluorescent recognition of methionine. Chem Pap 71:1855–1862. https://doi.org/10.1007/s11696-017-0179-7
Wang R, Yu Z (2007) Validity and reliability of Benesi–Hildebrand method. Acta Phys Chim Sin 23:1353–1359. https://doi.org/10.1016/S1872-1508(07)60071-0
Wang C, Zhang D, Huang X et al (2014) A fluorescence ratiometric chemosensor for Fe3+ based on TBET and its application in living cells. Talanta 128:69–74. https://doi.org/10.1016/j.talanta.2014.03.073
Wang C, Liu Y, Cheng J et al (2015) Efficient FRET-based fluorescent ratiometric chemosensors for Fe3+ and its application in living cells. J Lumin 157:143–148. https://doi.org/10.1016/j.jlumin.2014.08.039
Wang F, Hao Q, Zhang Y et al (2016) Fluorescence quenchometric method for determination of ferric ion using boron-doped carbon dots. Microchim Acta 183:273–279. https://doi.org/10.1007/s00604-015-1650-1
Weerasinghe AJ, Schmiesing C, Varaganti S et al (2010) Single- and multiphoton turn-on fluorescent Fe3+ sensors based on bis(rhodamine). J Phys Chem B 114:9413–9419. https://doi.org/10.1021/jp1034568
Xu H, Ding H, Li G et al (2017) A highly selective fluorescent chemosensor for Fe3+ based on a new diarylethene with a rhodamine 6G unit. Rsc Adv 7:29827–29834. https://doi.org/10.1039/c7ra04728b
Yan F, Zheng T, Guo S et al (2015) New fluorescence probe for Fe(3+) with bis-rhodamine and its application as a molecular logic gate. Spectrochim Acta Part A Mol Biomol Spectrosc 151:881–887. https://doi.org/10.1016/j.saa.2015.07.033
Yan L, Li R, Ma F et al (2017) A simple salicylaldehyde-based fluorescent “turn on” probe for selective detection of Zn2+ in water solution and its application in living cells imaging. Anal Methods 9:1119–1124. https://doi.org/10.1039/x0xx00000x
Zhang X, Shiraishi Y, Hirai T (2007) Cu(II)-selective green fluorescence of a rhodamine-diacetic acid conjugate. Org Lett 9:5039–5042. https://doi.org/10.1021/ol7022714
Zhang X, Huang XJ, Zhu ZJ (2013) A reversible Hg(II)-selective fluorescent chemosensor based on a thioether linked bis-rhodamine. Rsc Adv 3:24891–24895. https://doi.org/10.1039/C3RA43675F
Zhang D, Ma Y, An R (2015) New colorimetric chemosensor based on rhodamine hydrazide to detect Cu2+ ions by naked eye. Res Chem Intermed 41:5059–5069. https://doi.org/10.1007/s11164-014-1588-1595
Zheng S, Wang H, Hu Q et al (2017) “Turn-On” fluorescent chemosensor based on β-diketone for detecting Th4+ ions in Aqueous Solution and application in living cell imaging. Sensors & Actuators B Chemical 253:766–772. https://doi.org/10.1016/j.snb.2017.06.178
Zhou T, Chen X, Hua Q et al (2017) Synthesis and evaluation of a new furfuran-based rhodamine B fluorescent chemosensor for selective detection of Fe3+ and its application in living-cell imaging. Sens Actuators B Chem 253:292–301. https://doi.org/10.1016/j.snb.2017.06.134
Zhou F, Wang H, Liu P et al (2018) A highly selective and sensitive turn-on probe for aluminum(III) based on quinoline Schiff’s base and its cell imaging. Spectrochim Acta Part A Mol Biomol Spectrosc 190:104–110. https://doi.org/10.1016/j.saa.2017.09.007
Zhu D, Ren A, He X et al (2017) A novel ratiometric fluorescent probe for selective and sensitive detection of Cu2+ in complete aqueous solution. Sens Actuators B Chem 252:134–141. https://doi.org/10.1016/j.snb.2017.05.141
Acknowledgements
This work was supported by the National Natural Science Foundation of China (11175080), the Natural Science Foundation of Hunan Province, China (2016JJ5003), the Scientific Research Fund of the Hunan Provincial Education Department (12A117).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, P., Luo, A., Wang, Y. et al. A bis-(rhodamine)-based off–on colorimetric and fluorescent probe for Fe3+ ion detection in serum and bioimaging. Chem. Pap. 72, 2575–2587 (2018). https://doi.org/10.1007/s11696-018-0492-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0492-9