Abstract
Chalcones are bioactive compounds obtained from either natural sources or synthetic procedures and widely used due to their several biological properties. The most common experimental methodology in obtaining these compounds is Claisen–Schmidt reaction, which is a particular type of aldolic condensation. In this work, we have synthesized 23 chalcones and by density functional theory (DFT) calculation, we have studied the difference in reactivity of the several benzaldehydes and their effects on the yield of this reaction. From molecular orbital descriptors were obtained two quantitative structure–reactivity relationship (QSRR) models based on Hansch’s analysis. The results of this study showed that, for the most benzaldehydes (15 of 23 compounds), their reactivity was correlated with LUMO energy and global Electrophilicity Index (ω) values, which are determined in the first step of Claisen–Schmidt condensation mechanism (nucleophilic addition). Likewise, for the smallest group of benzaldehydes, their reactivity was related to their HOMO and ΔL − H (LUMO − HOMO) energies, which were determined in the second step of the mechanism (trans-elimination). This is the first report of a QSRR model analyzing the yield of chalcone synthesis based on DFT methodology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chalcones (or 1,3-diaryl-2-propen-1-one, IUPAC name) are obtained from either natural sources or synthetic procedures and correspond to the main group of bioactive compounds because these have several pharmacological applications, such as anti-inflammatory, anti-pyretic, anti-fungal, anti-bacterial, anti-mutagenic, anti-leishmanial, anti-cancer, anti-HIV, and anti-oxidant properties (Anto et al. 1994; Kamal et al. 2011; Luo et al. 2012; Pilatova et al. 2010; Alegaon et al. 2014; Zhuang et al. 2017). These compounds can be synthesized through the condensation of acetophenone (1) with benzaldehyde (2) in the presence of a base or an acid to form the respective chalcone (4) with high chemoselectivity (see Scheme 1). This reaction is generally known as Claisen–Schmidt condensation, and therefore, this a specific type of aldolic condensation and in the reaction mechanism, an intermediary product known as aldol (3) is formed. To obtain bioactive chalcone derivatives, usually alkaline conditions are more appropriated and efficient. (Akansksha, 2007; Bhagat et al. 2006; Jeon et al. 2012; Li et al. 2010; Liu et al. 2011; Narender and Reddy 2007; Patil and Bhanage 2013; Petrov et al. 2008; Sivakumar et al. 2001; Sugamoto et al. 2011; Narender et al. 2011). Likewise, it is well known that the substitution patterns of both 1 and/or 2 are important to achieve in a successful way this reaction, since which can influence the yield of this transformation.
On the other hand, several computational methodologies have been used to study the mechanism of reactions. Some analysis of structural relationships such as quantitative structure–property relationships or quantitative structure–reactivity relationships (QSPR and QSRR, respectively) have emerged as a useful tool to find relevant physicochemical descriptors to get some insight into the underlying mechanisms of reaction. These mathematical models are based on Hansch’s analysis and on the assumption that structurally similar compounds have similar activities or properties (Yee and Wei 2012). The QSPR and QSRR can also be used in the analysis of structural descriptors that capture different constitutional, topological, geometrical, or electronic features of molecular structures in relation to properties of interest (Duchowicz and Castro 2009; Yee and Wei 2012). These molecular descriptors can be readily calculated through mathematical formulae derived from several theories, e.g., quantum mechanics and other similar theories, which have been proven to function quite well for the wide spectra of properties/activities (Duchowicz and Castro 2009). In this context, density functional theory (DFT) is an important method in designing bioactive compounds, obtaining satisfactory results based on accuracy and reliability (Carloni et al. 2006). Furthermore, this methodology has been used in understanding chemical system reactivity (De Vleeschouwer et al. 2007; Moens et al. 2007). In fact, through DFT, it is possible to calculate different reactivity descriptors, such as electronic chemical potential (μ), Pearson’s hardness and softness (η and S, respectively), and global Electrophilicity Index (ω), all of which led to understanding reactivity in organic molecules (Chermette 1999; Li et al. 2010).
Herein, a quantitative structure–reactivity relationship (QSRR) model was developed to explain the different yields observed in Claisen–Schmidt condensation to obtain several chalcones, in alkaline conditions. The uses of this methodology should be useful for other similar reactions.
Experimental
General
Melting Point was measured in Fischer Scientific apparatus. Infrared spectra were recorded in Buck Scientific M500. Recorded range 600 cm−1–4000 cm−1, all samples were registered on ATR system (attenuated total reflectance). 1H NMR, 13C NMR, 2D-HSQC and 2D-HMBC spectra were recorded on a Bruker Avance 400 Digital NMR spectrometer, operating at 400.13 MHz for 1H and 100.6 MHz for 13C, respectively. Chemical shifts are reported in δ (ppm downfield from the TMS resonance) and coupling constants (J) are given in Hz. GC–MS was carried out Agilent Technologies 6890 model with automatic ALS, mass detector HP MD 5973 in spitless mode.
Chemicals
To a stirred solution of 4-methoxyacetophenone (5) (250 mg, 2.08 mmol) and the appropriate benzaldehydes (6a–w; 2.29 mmol) in EtOH (2.5 mL) was added saturated ethanolic NaOH (20 mL). The mixture was stirred for 48 h at room temperature. After reaction time, the reaction mixture was neutralized with HCl 5% solution until pH ≈ 7 and extracted with ethyl acetate (3 × 50 mL). Later, the organic phase was dried, concentrated and purified by flash column chromatography using EtOAc: Hexane mix (0:1–1:1) as eluent, obtaining the corresponding chalcones (7a–w).
Computational details
All compounds considered in this study (acetophenone (5), benzaldehydes (6a–w) and aldol-intermediate structures), were subjected to the following computational procedure. Full unconstrained geometry optimizations of these compounds were carried out using Gaussian 03 program (Frisch et al. 2004). The most widely used exchange–correlation function suggested exchange potential by Becke with gradient-corrected correlation provided by Lee, Yang and Parr (B3LYP) with Gaussian basis set triple-Z 6-311G double-polarized and d orbital expansion was necesary for obtained chemical properties of all compounds. Optimized geometries were verified by frequency calculations and characterized as minimum structure (no imaginary frequency) in their potential energy surfaces. The reactivity descriptors: Mülliken’s Charge on carbon and oxygen of carbonyl benzaldehyde, highest-occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO), electronic chemical potential (µ), hardness (η), softness (S) and Electrophilicity Global Index (ω) were calculated with the following equations:
Hansch’s analysis
Hansch’s analysis was performed using multiple linear regressions, which has been attempted to relate the structural features of these benzaldehydes on the yield of reactions. In this analysis, different descriptors previously calculated were selected: HOMO and LUMO energies, energy difference between LUMO and HOMO (ΔL − H), electronic chemistry potential (µ), hardness (η), softness (S), global electrophilicity (ω), Mulliken’s charge (on CO and O of carbonyl groups) of benzaldehydes. Statistica 7.0 Package Software carried out model optimization.
Cross-validation QSRR model
Cross-validation of QSRR model was carried out by Golbraikh method (Golbraikh and Alexander 2002). Acceptable q 2 values are equal or greater than 0.5. q 2 values are obtained by the following formula:
where y obs is pYield observed, y calc is pYield calculated by QSPR model and y ave is pYield average of all compounds in the training set.
Results and discussion
Chemistry
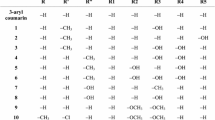
The preparation of the chalcones 7a–w is described in Scheme 2. 4-methoxyacetophenone (5) was treated with different benzaldehydes (6a–w) in ethanolic NaOH to give 23 chalcones (7a–w), with medium to excellent yields (40–98%).
The difference in the yields in this reaction is probably related to the substitution patterns of benzaldehydes, in terms of structural features and position of these groups. If considered 7a as a reference compound to evaluate the effect of chemical modifications over Claisen–Schmidt reaction, some tendency is possibly observed. One of these is the acidic hydrogen of hydroxyl–benzaldehydes (OH group) generated decreased yield (except 7q, 80% yield) (see Scheme 2). On the other hand, strong electron donor π groups on R3’ position (7g and 7h) decrease the chalcone yield, however, a few electronic donor as methyl group generate to increase the chalcone yield. Likewise, when the replacement of the hydrogen atom is in R4’ positions of benzaldehydes, is observed the best results for each group in particular (7j–l), except to 7n. In the case of the poly-substitution (7o–w), the behavior is unclear, because of the yield of this reaction depended on both, the position and feature of these groups. However, a Hansch’s analysis model gives an even more precise explanation of the yield differences of the studied compounds, which was developed in the next section.
The chemical structures of the target compounds were established based on their spectral properties (IR, 1H NMR, 13C NMR and MS, see the Experimental part and Supplementary Material). Infrared spectra of all obtained chalcones (7a–w) showed absorption peaks characteristic of conjugated carbonyl groups (υ = 1665 cm−1) by resonance effects (Silverstein et al. 2005). Furthermore, these peaks are characteristic of aromatic systems, with angle deformations of υ ≈ 1600, 1550, and 1500 cm−1.
The 1H-NMR spectra recorded in deuterated chloroform (CDCl3) of all compounds shows two coupled hydrogens with geometry trans to the down field (δ ≈ 7.88 and 7.56 ppm, J ≈ 15.6 Hz), corresponding to α and β hydrogens, respectively. 13C-NMR and 2D-HSQC spectra show two carbons correlated to hydrogens α (δ ≈ 122 ppm) and β (δ ≈ 143 ppm). Finally, with 2D-HMBC experiments, quaternary carbons that link the two aryl systems (see Scheme 3a) were determined. The EIMS of all compounds showed three key fragments: M+-15 (lost CH3 from methoxy group), M+-131 (lost cinnamic fragment from α carbonyl rupture), and M+-103 (lost styrene fragment from α carbonyl rupture) (see Scheme 3b).
Hansch’s analysis
Quantum chemical calculations are an attractive source for new molecular descriptors, which can express all of the electronic and geometric properties of molecules and their interactions. Indeed, many recent Hansch’s analysis (QSAR/QSRR/QSPR studies) have employed quantum chemical descriptors alone or in combination with conventional descriptors, such as atomic charges, orbital electron density, dipole moments, and polarity indices, among others (Karelson and Lobanov 1996).
There are several descriptors to understand chemical reactivity. The most widely used are that of the molecular orbital (MO), e.g., highest-occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), equivalent to Lewis base and acid, respectively (Ege 2000). HOMO and LUMO values can be used to calculate other reactivity descriptors, such as ΔLH (LUMO–HOMO), electronic chemical potential (µ), Pearson’s hardness and softness (η and S, respectively) and global Electrophilicity Index (ω). Electronic chemical potential (µ) is, at the physical level, electron donation capacity from HOMO to LUMO. Hardness can be understood as resistance to charge transfer. In addition, the global Electrophilicity Index (ω) is a concept of measure for energy stabilization, such as when systems receive additional electronic charges from underground (Lopez et al. 2013; Pearson 1993).
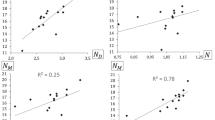
According to previously mentioned descriptors, the values of these parameters were calculated for benzaldehydes under investigation and those that elicited a statistical significance (p ≤ 0.05), then were correlated with the respective reaction yields (pYield). The descriptors considered were: Mülliken charge of the carbon in the carbonyl group (CO), LUMO, electronic chemical potential (µ), and global Electrophilicity Index (ω). However, when finished Hansch’s analysis statistical depuration, only LUMO and ω were the descriptors that can explain reactivity differences with 99.99% of certainity (p < 0.0001) (see Eq. 6 and Table 1 Supplementary Material).
The first model (Eq. 6) shows that Claisen–Schmidt condensation is related to LUMO and global Electrophilicity Index (ω) values of the benzaldehydes, which could be connected to their capacities to accept two electrons (Lopez et al. 2013; Parr et al. 1999; Pearson 1993). This point is pivotal in the first step in the Claisen–Schmidt reaction mechanism, which involves nucleophilic attack from enolate through benzaldehyde, to obtain the respective addition intermediate (see Scheme 4).
Furthermore, in optimizing this Hansch’s analysis model, eight outlier compounds with no significance in this model were identified. All outlier compounds have a common feature: OH or O-alkyl groups, in positions 2 and/or 4, which could influence the stereo-electronic properties of these benzaldehydes. For explaining different features of outlier compounds and chalcone yield relationship, all descriptors calculated were correlated with pYield again, found a significant correlation (p < 0.05) with HOMO, ΔLH, η, S and µ descriptors. Furthermore, a new Hansch’s analysis statistical depuration showed that HOMO and ΔLH are the only descriptors that can explain reactivity differences with 99.99% of certainity (p < 0.0001). In second, the Hanch’s analysis (outlier model), two non-correlated compounds were found (6q and 6t). Despite efforts by reactivity understanding of this compounds using Hansch’s analysis, into two models have no contribution statistical. Variables involved in the Hanch’s analysis (outlier model) were thus HOMO and ΔLH (see Eq. 7 and Table 2 supplementary material), which correspond to reactivity descriptors related to electron-donating capacity (Lopez et al. 2013; Pearson 1993).
Equation 7 shows that HOMO is the most important descriptor (up to four times more important than ΔLH), related to electron-donating capacity or the behavior as Lewis base of the outliner benzaldehydes (Ege 2000; Lopez et al. 2013; Pearson 1993). If Claisen–Schmidt reaction mechanism is considered, these electronic features are important for the second step of the reaction, the trans-elimination (see Scheme 5). To confirm this hypothesis, HOMO and ΔLH values of aldols were calculated and the values of these descriptors were correlated with the corresponding benzaldehydes, which shows r 2 ≥ 0.9. These results could indicate that in the Claisen–Schmidt reaction mechanism, the reactivity of outlier compounds was determined by the second step of the reaction, the trans-elimination (see Scheme 5).
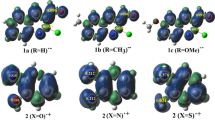
Figure 1 shows HOMO diagrams for aldol intermediates of 7a and 7c. Figure 1b demonstrates that 2′-OH substitution increases electronic population in the ring near OH-aldol, behavior not observed for aldol intermediate of 7a. This effect producing smaller stability on the addition intermediate, which can be related to the HOMO values in Hansch’s analysis outlier model (All HOMO plots of aldols outlier model are in Fig. 1 Supplementary Material).
Conclusions
In summary, a series of 23 chalcones were synthesized through Claisen–Schmidt condensation using alkaline media and structural characterization several spectroscopic techniques (IR, NMR, and MS). Through DFT calculus with B3LYP exchange-correlated basis set and 6-311G ++(d) calculus level, was possible obtained quantum descriptors for Hansch’s analysis. The Hansch’s analysis indicated that, for 15/23 compounds (65%), the yield to obtain the respective chalcone, were related to the nucleophilic attack from enolate to the carbonyl group of the respective benzaldehyde. On the other hand, for 6/23 compounds (26%), the yield of this reaction was determined for aldol dehydration process. In two models, developed descriptors were correlated with 99.9% certainty (p < 0.001). Finally, this is the first report of Hansch’s analysis use to explain different yields in Claisen–Schmidt reaction and would be used in other chemical reactions to explain the same experimental aspects.
References
Akansksha AK (2007) Zirconium chloride catalyzed efficient synthesis of 1,3-diaryl-2-propenones in solvent free conditions via aldol condensation. J Mol Catal A Chem 274:212–216. doi:10.1016/j.molcata.2007.05.016
Alegaon SG, Alagawadi KR, Vinod D, Unger B, Khatib NA (2014) Synthesis, pharmacophore modeling, and cytotoxic activity of 2-thioxothiazolidin-4-one derivatives. Med Chem Res 23:5160–5173. doi:10.1007/s00044-014-1087-9
Anto RJ, Kutaan G, Kuttan R, Sathyanarayana K, Rao MNA (1994) Tumor-reducing and antioxidant activities of sydnone-substituted chalcones. J Clin Biochem Nutr 17:73–80. doi:10.3164/jcbn.17.73
Bhagat S, Sharma R, Sawant D, Sharma L, Chakraborti A (2006) LiOH· H2O as a novel dual activation catalyst for highly efficient and easy synthesis of 1, 3-diaryl-2-propenones by Claisen–Schmidt condensation under mild conditions. J Mol Catal A Chem 244:20–24. doi:10.1016/j.molcata.2005.08.039
Carloni P, Alber F, Mannhold R, Kubinyi H, Folkers G (2006) Quantum medicinal chemistry, vol 17. Wiley-VCH, Alemania
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comp Chem 20:129–154. doi:10.1002/(Sici)1096-987x(19990115)20:1<129:Aid-Jcc13>3.0.Co;2-A
De Vleeschouwer F, Van Speybroeck V, Waroquier M, Geerlings P, De Proft F (2007) Electrophilicity and nucleophilicity index for radicals. Org Lett 9:2721–2724. doi:10.1021/ol071038k
Duchowicz PR, Castro EA (2009) QSPR studies on aqueous solubilities of drug-like compounds. Int J Mol Sci 10:2558–2577. doi:10.3390/ijms10062558
Ege S (2000) Reacciones Concertadas Química Orgánica Estructura y Reactividad, vol TOMO 1. Editorial Revertè, España, pp 1297–1356
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.02. Gaussian, Inc, Wallingford
Golbraikh A, Alexander T (2002) Beware of q2! J Mol Graph Model 20:269. doi:10.1016/S1093-3263(01)00123-1
Jeon JH, Kim SJ, Kim CG, Kim JK, Jun JG (2012) Synthesis of biologically active chalcones and their anti-inflammatory effects. Bull Korean Chem Soc 33:953–957. doi:10.5012/bkcs.2012.33.3.953
Kamal A, Prabhakar S, Janaki Ramaiah M, Venkat Reddy P, Ratna Reddy C, Mallareddy A, Shankaraiah N, Lakshmi Narayan Reddy T, Pushpavalli SN, Pal-Bhadra M (2011) Synthesis and anticancer activity of chalcone-pyrrolobenzodiazepine conjugates linked via 1,2,3-triazole ring side-armed with alkane spacers. Eur J Med Chem 46:3820–3831. doi:10.1016/j.ejmech.2011.05.050
Karelson M, Lobanov VS (1996) Quantum-chemical descriptor in QSAR/QSPR studies. Chem Rev 96:1027–1043. doi:10.1021/cr950202r
Li W, Xu K, Xu L, Hu J, Ma F, Guo Y (2010) Preparation of highly ordered mesoporous AlSBA-15–SO3H hybrid material for the catalytic synthesis of chalcone under solvent-free condition. Appl Surf Sci 256:3183–3190
Liu Z, Lee W, Kim SN, Yoon G, Cheon SH (2011) Design, synthesis, and evaluation of bromo-retrochalcone derivatives as protein tyrosine phosphatase 1B inhibitors. Bioorg Med Chem Lett 21:3755–3758. doi:10.1016/j.bmcl.2011.04.057
Lopez JM, Ensuncho AE, Robles JR (2013) Global and local reactivity descriptors for the design of new anticancer drugs based on cis-platinum(II). Quim Nova 36:1308–1317. doi:10.1590/S0100-40422013000900006
Luo Y, Song R, Li Y, Zhang S, Liu ZJ, Fu J, Zhu HL (2012) Design, synthesis, and biological evaluation of chalcone oxime derivatives as potential immunosuppressive agents. Bioorg Med Chem Lett 22:3039–3043. doi:10.1016/j.bmcl.2012.03.080
Moens J, Geerlings P, Roos G (2007) A conceptual DFT approach for the evaluation and interpretation of redox potentials. Chem Eur J 13:8174–8184. doi:10.1002/chem.200601896
Narender T, Reddy KP (2007) A simple and highly efficient method for the synthesis of chalcones by using borontrifluoride-etherate. Tetrahedron Lett 48:3177–3180. doi:10.1016/j.tetlet.2007.03.054
Narender T, Venkateswarlu K, Nayak BV, Sakar S (2011) A new chemical access for 3′-acetyl-4′-hydroxychalcones using borontrifluoride–etherate via a regioselective Claisen–Schmidt condensation and its application in the synthesis of chalcone hybrids. Tetrahedron Lett 52:5794–5798. doi:10.1016/j.tetlet.2011.08.120
Parr RG, Von Szentpaly L, Liu SB (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. doi:10.1021/Ja983494x
Patil AB, Bhanage BM (2013) Novel and green approach for the nanocrystalline magnesium oxide synthesis and its catalytic performance in Claisen–Schmidt condensation. Catal Commun 36:79–83. doi:10.1016/j.catcom.2013.03.012
Pearson RG (1993) The principle of maximum hardness. Acc Chem Res 26:250–255. doi:10.1021/Ar00029a004
Petrov O, Ivanova Y, Gerova M (2008) SOCl2/EtOH: catalytic system for synthesis of chalcones. Catal Commun 9:315–316. doi:10.1016/j.catcom.2007.06.013
Pilatova M, Varinska L, Perjesi P, Sarissky M, Mirossay L, Solar P, Ostro A, Mojzis J (2010) In vitro antiproliferative and antiangiogenic effects of synthetic chalcone analogues. Toxicol In Vitro 24:1347–1355. doi:10.1016/j.tiv.2010.04.013
Silverstein RM, Webster FX, Kiemle DJ (2005) Spectrometric identification of organic compounds, Seventh edn. Wiley, USA
Sivakumar PM, Prabhakar PK, Doble M (2001) Synthesis, antioxidant evaluation, and quantitative structure–activity relationship studies of chalcones. Med Chem Res 20:482–492. doi:10.1007/s00044-010-9342-1
Sugamoto K, Matsusita YI, Matsui K, Kurogi C, Matsui T (2011) Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 67:5346–5359. doi:10.1016/j.tet.2011.04.104
Yee LC, Wei YC (2012) Statistical modelling of molecular descriptors in QSAR/QSPR. In: Dehmer M, Varmuza K, Bonchev D (eds) Statistical modelling of molecular descriptors in QSAR/QSPR, vol 2. Wiley, Weinheim
Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z (2017) Chalcone: a privileged structure in medicinal chemistry. Chem Rev 117:7762–7810. doi:10.1021/acs.chemrev.7b00020
Acknowledgements
The authors thank the Dirección de Investigación y Postgrado (DGIP) of Universidad Técnica Federico Santa María; scientific initiation project 2015 (PIIC-MM), CONICYT Programa Formación de Capital Humano Avanzado 21130456; Proyecto VRIEA-PUCV “37.0/2017” and Dr. Guillermo Díaz Felming of Laboratorio de Espectroscopia Atómica y Molecular of Universidad de Playa Ancha.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mellado, M., Madrid, A., Martínez, Ú. et al. Hansch’s analysis application to chalcone synthesis by Claisen–Schmidt reaction based in DFT methodology. Chem. Pap. 72, 703–709 (2018). https://doi.org/10.1007/s11696-017-0316-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0316-3