Abstract
Zinc phosphate (Zn3(PO4)2) nanocrystals were synthesized and used for making conducting polyaniline/nano-zinc phosphate composite by chemical oxidative method. The product was characterized by UV–visible absorption spectroscopy. The crystal structure, morphology and thermal stability of the product were studied by X-ray diffraction, scanning electron microscopy, transmission electron microscopy and thermo gravimetric analysis, respectively. The epoxy-based paint containing conducting polyaniline/nano-zinc phosphate composite pigment was applied on low-carbon steel samples. Corrosion protection performance of the painted low-carbon steel samples in 3.5 mass % sodium chloride solution was evaluated using electrochemical technique. Transmission electron microscopic image revealed the formation of core shell structure of the composite. Composite was found to be more thermally stable than the conducting polyaniline. The corrosion rate of conducting polyaniline/nano-zinc phosphate-painted low-carbon steel was found to be 5.1 × 10−4 mm per year, about 34 times lower than that of unpainted low-carbon steel and 10 times lower than that of epoxy nano-zinc phosphate paint-coated steel. The study reveals the possibility of using conducting polyaniline/nano-zinc phosphate as a pigment for corrosion protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Low-carbon steels corrode in most of the atmospheric environments when the relative humidity exceeds sixty per cent. Corrosion, being an electrochemical phenomenon, can be handled by the use of electrochemistry and conducting polymers (Ahmad and Mac Diarmid 1996). Within the class of conducting polymers, conducting polyaniline (PANI) occupies an important place due to its low cost, ease of synthesis and good stability (Trivedi 1997). Due to its capacity to be both oxidized and reduced, PANI can participate in various redox processes involved in corrosion of metals and alloys or it can affect them. As such, it has been the subject of numerous corrosion prevention studies conducted in last two decades. Many comprehensive review papers have been published containing detailed literature survey on corrosion protection of metals and alloys by conducting polymers including polyaniline (Tallman 1999; Tallman et al. 2002; Spinks et al. 2002; Gvozdenovic et al. 2012; Deshpande et al. 2014). Conducting polyaniline coating on metals or alloys can be obtained using electrochemical deposition technique or by applying chemically synthesized paints (Deshpande et al. 2001a, b). The coating protects the underlying steel either by barrier protection or by generation of electric field or by controlled inhibitor release mechanism or by anodic protection or by combination of these mechanisms. However, in the presence of relatively large defects in the coating as in the case of pitting or crevice corrosion, protection mechanisms become ineffective and the coating ceases to protect the steel. Thus, it should be kept in mind that while PANI always affects the corrosion behaviour, its effect is not essentially always be positive, albeit positive results predominate. One of the strategies, recently adopted, for improving the performance of conducting polymer coating is the use of nanocomposites (Deshpande and Sazou 2015). Radhakrishnan et al. (2009) synthesized conducting polyaniline/nano-TiO2 composite-based paint for corrosion protection of stainless steel and evaluated its corrosion resistance in 3.5 mass% NaCl by electrochemical methods. The open-circuit potential was found to shift with time from 0. 38 V SCE to more anodic side. The presence of nano-TiO2 was found to be crucial in the prevention of corrosion and the shift of open-circuit potential to anodic side. Al Dulami et al. (2011) used both TiO2 and SiO2 in acrylic to prepare conducting polyaniline-based paints. In this work, it was found that the acrylic paint containing SiO2 was more effective for corrosion protection of steel. However, there is evidence that amorphous nanoparticles such as SiO2 and TiO2 do not remain fixed in the composite and hence their use can be hazardous (Reijnders 2009).
This inspired us to look for alternatives to nano-metallic oxides for making conducting polyaniline/nanocomposite-based paints for corrosion prevention. We recently reported the corrosion protection performance of conducting polyaniline/multiwall carbon nanotubes composite-based paint on low-carbon steel. The corrosion rate of low-carbon steel coated with 1.5 mass% PANI-MW CNT-based paint in 3.5 mass% NaCl was found to be 0.037 mm per year, about 5.2 times lower than that of unpainted low-carbon steel and 3.6 times lower than that of epoxy-coated steel (Deshpande et al. 2013). Zinc phosphate, Zn3 (PO4)2, a well-known inorganic foundational material, is considered as an environmentally acceptable pigment and it had become the most commonly used corrosion inhibitor (Kalendova et al. 2006). It is anticipated, therefore, that conducting polyaniline/nano-zinc phosphate composite can be used as a corrosion inhibitor. To the best of our knowledge, there are no reports in the literature regarding the use of conducting polyaniline–nano-zinc phosphate composite as a pigment for making anti-corrosive paints. In the present work, therefore, nano-zinc phosphate crystals were synthesized and used in making conducting polyaniline/nano-zinc phosphate composite. Finally, conducting polyaniline/nano-zinc phosphate composite-based paint was prepared and the painted low-carbon steel samples were investigated in 3.5 mass% NaCl by potentiodynamic polarization.
Experimental
Synthesis of nano-zinc phosphate crystals
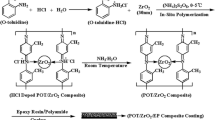
Nano-zinc phosphate crystals were synthesized as per the similar method described by Jin Ku Wang et al. (2011). Into 100 ml of distilled water, 3.8018 g of trisodium phosphate (0.1 M of Na3 PO4. 12 H2O) was added. This solution along with another solution containing 4.4622 g of zinc nitrate (0.1 M of Zn (NO3)2.6H2O) was added in 500 ml of distilled water. The reaction system was put in the ultrasonic machine-Equitron (Supplied by Medico Instrument Manufacturing Co, Mumbai, India) with continuous stirring until complete precipitate was formed. It was sonicated for 15 min and filtered. The powder was dried in a simple oven at 100 °C for 45 min and crushed in a mortar for 10 min. Finally, it was dried in a microwave oven to avoid agglomeration (The drying power was 360 W for 15 min). The nano-zinc phosphate crystals, so obtained, were subsequently used for making conducting polyaniline/nano-zinc phosphate composite.
Synthesis of conducting polyaniline/nano-zinc phosphate composite
Conducting polyaniline was synthesized by the addition of 18 ml of aniline in 1 M ortho-phosphoric acid using ammonium persulfate (25 grams) as catalyst by following the method of Deshpande et al. (2012). Aniline (AR grade supplied by Loba Chemicals, Colaba, Mumbai, 400 005, India) was double distilled prior to use. Ortho-phosphoric acid and ammonium persulphate (AR grade supplied by Loba Chemicals, Colaba, Mumbai, 400 005, India, 230AC, 75 W) were used without further purification. Two grams of nano-zinc phosphate crystals was added during chemical polymerisation of aniline in the ortho-phosphoric acid solution to get conducting polyaniline/nano-zinc phosphate composite.
Conducting polyaniline/nano-zinc phosphate composite paint preparation and its application
Conducting polyaniline-based paint was prepared by following the technique developed by Samui et al. (2003). Conducting PANI/nano-zinc phosphate composite (2 gm) as a pigment, 12 g of xylene, 8 g of titanium dioxide (TiO2) and 8 g of bis-(2-ethylhexyl) phthalate (dioctyl phthalate: DOP) were added to the solution of 70 g of epoxy resin (Araldite GY 250 supplied by Huntsman Advanced Materials (India) Pvt Ltd. Andheri (East), Mumbai, 400 093, India). Epoxy resins are commonly used in paint formulations for making anti-corrosive coatings. Araldite GY 250 is a universal purpose unmodified medium viscous epoxy resin based on bisphenol A. Its density is 1.17 g/cm3, epoxy index and epoxy equivalent is in the range of 5.30–5.54 eq/kg and 183–189 g/eq, respectively. The mixture was ball milled for 16 h (Ball Mill supplied by Indo German Industries, Daman, India. Drive motor: Crompton Make—2 HP, 1440 rpm, 415 V, 50 Hz). The purpose of adding titanium dioxide and dioctyl phthalate in epoxy resin is to improve viscosity and elastic properties of paint. The cross-linking of epoxy resin was achieved by addition of hardener (Aradur 140 supplied by Huntsman Advanced Materials (India) Pvt. Ltd. Andheri (East), Mumbai, 400 093, India). Xylene was used as a solvent for paint formulation. The paint was filtered through fine cotton and applied on the low-carbon steel samples (AISI 1015 supplied by Rajasthan steels, Pune, India) by film applicator to keep paint thickness ~60 µm uniform on the entire surface. Finally, the painted low-carbon steel samples were cured in air at ambient temperature for 24 h.
Characterisation of conducting polyaniline/nano-zinc phosphate composite
Ultraviolet–visible absorption study was carried out ex situ within the wavelength range of 200–1200 nm to study the conducting phases using micro-processor-controlled double-beam spectrophotometer (Model JASCO, V-630, Japan). X-ray diffraction (Bruker AXS DS Advance, Germany) studies were carried out to find the composition of the composite. Scanning electron microscopy using field emission scanning electron microscope (SIGMA HV) was conducted to examine morphology and distribution of zinc phosphate in the conducting polyaniline matrix at 100 KX magnification. Conducting polyaniline/nano-zinc phosphate composite was observed at 125 KX using Transmission electron microscope TECNAI G-20 U-Twin (FEI, Netherlands). The thermal stability of the composite was investigated using thermogravimetric analyser (TGA Instruments Universal 4000, Perkin Elmer). The sample was heated from ambient temperature to 900 °C at the rate of 20 °C/min in nitrogen atmosphere.
Corrosion studies
A corrosion cell having three-electrode geometry of paint-coated sample as working electrode (8 cm2), platinum as counterelectrode and saturated calomel electrode (SCE; pH Products, Hyderabad, India) as a reference electrode was used. The cell was coupled with Gamry reference system 1000 (Wilmington, USA) for electrochemical measurements. Tafel extrapolation method was used to determine corrosion rate of the coated samples. In this technique, the polarization curves are obtained by applying potential of ±250 mV with respect to open-circuit potential. Resulting Tafel plots contain anodic and cathodic branches. Gamry software was used to determine corrosion rate. All measurements were carried out five times to obtain good reproducibility of the results.
Results and discussion
Characterisation
Optical absorption spectra of the chemically synthesized conducting polyaniline and conducting polyaniline/nano-zinc phosphate composite in dimethyl sulfoxide are shown in Figs. 1 and 2, respectively.
The absorption bands, observed in case of conducting polyaniline at around 331.5 nm and 617 nm and the absorption bands observed in case of conducting polyaniline/nano-zinc phosphate, at around 330 nm and 630 nm can be attributed to π—π* transition of the benzenoid ring and π—n* transition of benzenoid to quinoid, respectively (Mostafaei and Zolriasatein 2012). It has been noted that the shapes of UV spectra of nanocomposites are similar to those of polyaniline and shifting in the bands is observed. This shift can be assigned to the addition of nano-zinc phosphate. XRD spectra of as-synthesized nano-zinc phosphate powder and conducting polyaniline/nano-zinc phosphate composite are shown in Figs. 3 and 4, respectively.
The peaks at 2Ө values of 9.65, 16.68, 17.79, 18.26, 19.38, 22.17, 25.67, 31.31, 35.67, 39.88, 46. 78, 47.54, 49.94, 56.41 and 61.07 were supposed to be originated from (200), (210), (040), (011), (230), (221), (241), (002), (171), 371, (521), (402), and 303 of nano-zinc phosphate crystal which are matched with the standard XRD data of JC-PDS file (Numbers: 33-1474). This analysis is in good agreement with the previous work (Jin Ku Wang et al. 2011). It also shows XRD of the nano-zinc phosphate/conducting polyaniline composite. The two main Bragg diffraction peaks of polyaniline appeared at angles of 2Ө = 19.3 and 2Ө = 25.7 in the spectrum. This is in good agreement with previous work (Mostafaei and Zolriasatein 2012). Scanning electron photomicrograph of the conducting polyaniline/nano-zinc phosphate-coated low-carbon steel is shown in Fig. 5.
The SEM photomicrograph reveals the entire coverage and uniformity in conducting polyaniline/nano-zinc phosphate-based paint coating on the steel surface. The particle size and surface morphology of the composite was investigated by Transmission electron microscopy. TEM images of the conducting polyaniline/nano-zinc phosphate composite and electron diffraction pattern of single conducting polyaniline/nano-zinc phosphate composite are shown in Figs. 6 and 7, respectively.
The nano-zinc phosphate particles were found to be spherical with an average size 35–45 nm and the nano-zinc phosphate/conducting polyaniline particles were found to be spherical with an average diameter 50–62 nm. An increase in size of the composite can be attributed to the formation of core–shell structure: the nano-zinc phosphate particle core surrounded by conducting polyaniline shell.
The TGA thermograms of the conducting polyaniline and conducting polyaniline/nano-zinc phosphate composite at the heating rate of 20 °C/min under nitrogen atmosphere are shown in Figs. 8 and 9, respectively. The maximum decomposition temperature (T m) was taken as the temperature corresponding to maximum of the peak obtained by the first-order derivative curve. The temperature of 10 wt% loss was taken as the degradation temperature.
As observed from the Figs. 8 and 9, degradation temperature of conducting polyaniline is 198 °C whereas that of the conducting polyaniline/nano-zinc phosphate composite is 247 °C. It reveals better thermal stability of conducting polyaniline/nano-zinc phosphate composite matrix than that of the conducting polyaniline matrix. Thus, it can be said that the presence of nano-zinc phosphate is beneficial for the thermal stability of the composite matrix, which in turn, affects the paint durability.
Potentiodynamic polarization studies
Tafel curves generated by scanning potential from E corr to 250 mV (cathodic/anodic) for unpainted low-carbon steel, epoxy-coated without pigment low-carbon steel and polyaniline/nano-zinc phosphate-painted low-carbon steel in 3.5 mass % NaCl solution are depicted in Figs. 10, 11 and 12.
The values of the corrosion potential (E corr), corrosion current densities (I corr) and corrosion rates obtained from Figs. 10, 11 and 12 are recorded in Table 1.
Corrosion potential increased from −731 mV for uncoated low-carbon steel and −553 mV in case of epoxy nano-zinc phosphate paint-coated steel to −530 mV for conducting polyaniline/nano-zinc phosphate-painted steel. It should also be noted the corrosion rate is substantially reduced due to the decrease in current density from 70.3 to 0.22 µ A cm−2. The corrosion rate of conducting polyaniline/nano-zinc phosphate-painted low-carbon steel is found to be 5.1 × 10 −4 mm per year which is about 34 times lower than that of unpainted low-carbon steel and 10 times lower than that of epoxy nano-zinc phosphate paint-coated steel. The shift of the potential in anodic direction, as observed in these experiments, is known as ennobling. However, in this case, if the coating is damaged, protection will no longer exist since the underlying metal would serve as anode due to its lower potential. Thus, ennobling is essential but not sufficient for corrosion protection. Wessling (1996) and Lu et al. (1995) demonstrated that when doped polyaniline is placed in contact with steel, the steel surface undergoes rapid oxidation to provide a layer of iron oxide at the polyaniline–steel interface. This is an anodic protection-combined effect of ennobling and passive film formation. In the present investigations, we propose that combination of protection mechanisms—barrier action due to epoxy-based paint, passivation due to conducting polyaniline and self healing effect due to release of phosphate ions at the coating defect—contributes to overall corrosion protection.
Conclusions
The conducting polyaniline/nano-zinc phosphate composite pigment is thermally more stable than conducting polyaniline. The corrosion rate of conducting polyaniline/nano-zinc phosphate-painted low-carbon steel is found to be 5.1 × 10−4 mm per year which is about 34 times lower than that of unpainted low-carbon steel and 10 times lower than that of epoxy nano-zinc phosphate paint-coated steel. The specialty of the conducting polyaniline/nano-zinc phosphate paint coating lies in corrosion protection of steel by simultaneously operating mechanisms, viz. enhanced barrier protection, anodic protection and release of inhibiting phosphate ions when in contact with corrosive media.
References
Ahmad N, Mac Diarmid AG (1996) Inhibition of corrosion of steels with the exploitation of conducting polymers. Synth Met 78:103–110. doi:10.1016/0379-6779(96)80109-3
Al Dulami AA, Hashim S, Khan MI (2011) Corrosion protection of carbon steel using polyaniline composite with inorganic pigments. Sains Malaysiana 40:762
Deshpande PP, Sazou D (2015) Corrosion protection of metals by intrinsically conducting polymers. CRC Press, Boca Raton, pp 159–183
Deshpande PP, Peshwe DR, Pathak SU (2001a) A note on electrochemical synthesis of conducting polyaniline films on low alloy steel. Trans Indian Inst Metals 54:179–183
Deshpande PP, Peshwe DR, Pathak SU (2001b) Corrosion control by an organic metal: a review. J Inst Eng (India) Series A 82:33–36
Deshpande PP, Vagge ST, Jagtap SP, Khairnar RS, More MA (2012) Conducting polyaniline based paints on low carbon steel for corrosion protection. Prot Metals Phys Chem Surf 48:356–360. doi:10.1134/S2070205112030069
Deshpande PP, Vathare SS, Vagge ST, Tomsik E, Stejskal J (2013) Conducting polyaniline/multiwall carbon nano tubes composite paints on low carbon steel for corrosion protection: electrochemical investigations. Chem Pap 67:1072–1078. doi:10.2478/s11696-012-0273-9
Deshpande PP, Jadhav NG, Gelling VJ, Sazou D (2014) Conducting polymers for corrosion protection: a review. J Coat Technol Res 11:473–494. doi:10.1007/s1998-014-9586-7
Gvozdenovic M, Jugovic B, Jambrec D, Stevanovic J, Grgur B (2012) Application of polyaniline in corrosion protection of metals. Zasta Materijala 53:353–360
Kalendova A, Kalenda P, Vesely D (2006) Comparison of the efficiency of inorganic non metal pigments with zinc powder in anti corrosion paints. Prog Org Coat 57:1–10. doi:10.1016/j.porgcoat.2006.05.015
Lu WK, Elsenbaumer RL, Wessling B (1995) Corrosion protection of mild steel by coating containing polyaniline. Synth Met 71:2163–2166
Mostafaei A, Zolriasatein A (2012) Synthesis and characterization of conducting polyaniline/nano composites containing Zn O nano rods. Prog Nat Sci Mater Int 22:273–280. doi:10.1016/j.pusc.2012.07.002
Radhakrishnan S, Siju CR, Mahanta D, Patil S, Madras G (2009) Conducting polyaniline- nano—TiO2 composites for smart corrosion resistant coatings. Electrochim Acta 54:1249–1254. doi:10.1016/j.electacta.2008.08.069
Reijnders L (2009) The release of TiO2 and SiO2 nano particles from nano composites. Polym Degrad Stab 94:873–876. doi:10.1016/j.polymdegradstab.2009.02.005
Samui AB, Patankar AS, Rangarajan J, Deb PC (2003) Study of polyaniline containing paint for corrosion prevention. Prog Org Coat 47:1–7. doi:10.1016/s0300-9440(02)00117-0
Spinks G, Dominis AJ, Wallace GG, Tallman DE (2002) Electro active conducting polymers for corrosion control. J Solid State Electrochem 6:85–100. doi:10.1007/s100080100211
Tallman DE (1999) Conducting polymers and corrosion: polyaniline on steel. Corrosion 55:779–786
Tallman DE, Spinks G, Dominis A, Wallace GG (2002) Electroactive conducting polymers for corrosion control. J Solid State Electrochem 6:73–84. doi:10.1007/s100080100212
Trivedi DC (1997) Polyanilines. In: Nalwa HS (ed) Hand book of organic conductive molecules and polymers. Wiley, Chichester, pp 506–566
Wang JD, Da L, Liu JK, Yang XH, He JL, Lu Y (2011) One step Preparation and characterization of zinc phosphate nano crystals with modified surface. Soft Nano Sci Lett 1:81–85. doi:10.4236/snl.2011.13015
Wessling B (1996) Corrosion prevention with an organic metal (polyaniline): surface ennobling, passivation, corrosion test results. Mater Corros 47:439–445
Acknowledgements
The author thanks Prof. N.B. Dhokey, Head, Department of Metallurgy and Materials Science, College of Engineering, Pune, 411005 (M.S.) India for providing facilities for the work and Prof. B.B. Ahuja, Director, College of Engineering, Pune, 411005 (M.S.) India for his encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deshpande, P.P., Bhopale, A.A., Mooss, V.A. et al. Conducting polyaniline/nano-zinc phosphate composite as a pigment for corrosion protection of low-carbon steel. Chem. Pap. 71, 189–197 (2017). https://doi.org/10.1007/s11696-016-0082-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0082-7