Abstract
Background
Despite limited evidence about the efficacy and safety of anticoagulation in patients post bariatric surgery, both vitamin K antagonists (VKA) and direct-acting oral anticoagulants (DOACs) are commonly prescribed.
Aim
To evaluate plasma anti-Xa levels of DOACs in morbidly obese (MO) patients before and after a Roux-en-Y gastric bypass (RYGB) procedure.
Patients and Methods
Retrospective, cross-sectional, and longitudinal study of anti-Xa activity of apixaban or rivaroxaban in MO patients (N = 41).
Results
Preoperative analysis of plasma anti-Xa levels were within the normal range in patients using apixaban (n = 29; body mass index [BMI] 44.5 ± 5.1 kg/m2) as well as those using rivaroxaban (n = 12; BMI 42.6 ± 5.9 kg/m2). Postoperative anti-Xa levels of apixaban were all within the therapeutic range, whereas anti-Xa levels of rivaroxaban were subtherapeutic in nine out of 14 (64%) patients. Perioperative longitudinal follow-up in patients using apixaban (n = 18) showed no significant change in anti-Xa levels after RYGB.
Conclusion
Plasma anti-Xa levels of apixaban in MO patients remained within the therapeutic range up to a body weight of 144 kg. In patients using rivaroxaban, no statistically significant relation between anti-Xa levels and bodyweight was found. After RYGB, plasma anti-Xa levels of apixaban were unaffected, whereas plasma anti-Xa levels of rivaroxaban tended to become subtherapeutic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morbid obesity (MO; Body Mass Index [BMI] > 40 kg/m2, or > 35 kg/m2 with obesity-related health conditions) is associated with an increased risk of atrial fibrillation (AF) and venous thromboembolism (VTE). Compared to patients with a normal body weight (BMI 20 to 25 kg/m2), prevalence of AF increases by 4.7% for every 1 kg/m2 increase in BMI [1, 2]. The prevalence of VTE increases by 8% for every 1 kg/m2 increase in BMI [3]. Until recently, these conditions were usually treated with vitamin K antagonists (VKA). In the last decade, direct-acting oral anticoagulants (DOACs) are increasingly used as an alternative for VKA. The benefits of DOAC use compared to VKA are substantial: more predictable pharmacokinetics, fixed doses, and no need to perform blood coagulation tests at regular intervals [4, 5].
There are four registered DOACs in Europe: rivaroxaban, apixaban, edoxaban, and dabigatran, with rivaroxaban and apixaban being the most prescribed DOACs in the Netherlands. DOACs are at least as safe as VKA in patients with AF and in VTE and effectively reduce the occurrence of stroke and mortality. DOACs tend to have increased risk of major bleeding in the gastrointestinal tract compared to warfarin, whereas the risk of intracranial hemorrhage is significantly lower in patients using DOACs. Overall, the risk of major bleeding of DOACs compared to warfarin is equal [6, 7]. Compared to VKA, treatment of AF and VTE with DOACs showed comparable quality of life, higher treatment satisfaction, and lesser hospitalization [8]. As a result of these benefits, DOACs have become the first line of treatment in VTE and AF in the Netherlands.
Due to lack of evidence about the safety and efficacy of DOACs in post bariatric patients, VKAs are generally recommended as the drug of choice [9,10,11,12,13]. the foremost reason for VKA recommendation is the possibility of International Normalized Ratio (INR) monitoring although safety data on VKA in these patients are absent. Compared to other types of abdominal surgery, anticoagulation with VKA is associated with a higher risk for development of bleeding complications and all-cause readmissions rate in bariatric surgery [14]. A possible explanation could be that patients after bariatric surgery are prone to develop vitamin K deficiency, which may increase the risk of hemorrhagic events [7, 15, 16].
The efficacy and safety of DOACs in (morbidly) obese patients has been evaluated in nine large studies including a total of 20,000 patients with a body weight up to 120 kg, roughly equivalent to a BMI of 35–40 kg/m2 [17]. In contrast, evidence regarding the efficacy of DOACs in MO subjects (BMI > 40 kg/m2) is limited to five small case series, including a total of 379 patients [18,19,20,21,22]. Three of these case series included anti-Xa blood levels, measured preoperatively, in a total of 32 patients. On top of that, information about DOAC use after bariatric surgery is sparse [23, 24]. To the best of our knowledge, this is the largest study to date in which anti-Xa levels were monitored perioperatively and followed-up structurally in MO patients undergoing bariatric surgery.
Presently, a relatively large proportion of MO patients are being treated with DOACs instead of VKA [25]. In the Rijnstate Hospital in Arnhem (the Netherlands), about 1,200 bariatric procedures are performed yearly. This provided a unique opportunity to structure perioperative anticoagulation policy (i.e., continuation of DOAC use or switching to VKA) in MO patients, based on anti-Xa levels. This study is a retrospective evaluation of this anticoagulation approach in MO patients before and after bariatric surgery.
Patients and Methods
This retrospective, single-center study analyzed peak plasma anti-Xa activity in adult MO patients who underwent bariatric surgery. It was approved by the Institutional Medical Ethical Committee (Study Number: 2020–1637), and all patients gave their informed consent. It comprised an evaluation of a protocol that was developed to standardize perioperative anticoagulation policy in MO patients before and/or after bariatric surgery.
Included patients were over 18 years of age and had a BMI above 40 kg/m2 or over 35 kg/m2 with obesity-related comorbidities. All patients were using anticoagulation therapy and were scheduled for bariatric surgery (six patients underwent gastric sleeve, one underwent duodenal switch and the rest of the patients underwent Roux-en-Y gastric bypass (RYGB) procedure). Patients with gastrointestinal disease (e.g., coeliac disease, Crohn’s disease, ulcerative colitis) were excluded.
Several obesity related comorbidities were reported, such as hypertension, obstructive sleep apnea syndrome (OSAS), diabetes mellitus type 2, and hypercholesterolemia.
Bariatric Surgery Patients
Eligible patients were identified while using DOACs preoperatively through the surgeons’ screening program and were referred to the Department of Internal Medicine for advice on peri- and postoperative anticoagulation use. Patients scheduled for bariatric surgery were advised to discontinue their DOAC 2 days before surgery and to use low molecular weight heparin (nadroparin, 5700 IU anti-Xa/ml/day) for 2 days postoperatively, starting 8 h after surgery. DOACs were resumed on the third day after surgery and blood samples were taken 2 to 4 h after ingestion of the DOAC. Measurements of peak anti-Xa activity were performed either preoperatively (group A) or postoperatively (group B) or both (group C). Group C consisted of patients who had been on apixaban preoperatively and had chosen to continue this medication postoperatively. In this group, anti-Xa activity was measured 2 weeks after surgery. In addition, anti-Xa activity was also measured just before surgery (i.e., 2 days after the discontinuation of DOAC) in order to check whether the chosen interruption interval was long enough to ensure normalization of anti-Xa activity.
Patients After RYGB Procedure
Eligible postoperative patients who had undergone a RYGB were identified during follow-up visits after bariatric surgery. In our institution, a 5-year follow-up was standard procedure after RYGB. This included a medication check and a blood test to screen for mineral and vitamin deficiencies. Patients using DOACs were referred to the Department of Internal Medicine to discuss the appropriateness of DOAC-based anticoagulation after RYGB. During this consultation, all patients were informed about the uncertainties regarding the efficacy of DOACs in patients with a body weight above 120 kg. It was explained that these uncertainties are based on a lack of evidence, and that for reasons of safety VKA-based anticoagulation is generally recommended. Patients who, after receiving this information, did not want to switch to a VKA were allowed to continue their DOAC if the anti-Xa level was within the therapeutic range. Patients with subtherapeutic anti-Xa levels were advised to switch to VKA. Eventually, treatment was based on patient-shared decision-making. All patients wishing to continue DOAC-based anticoagulation were informed about the potential risks, and were instructed to seek immediate contact in case of signs of bleeding or thrombo-embolic events.
Assays
Compound-specific peak anti-Xa activity was measured three hours after DOAC ingestion. Normal values are as follows: 59–302 mcg/L for apixaban [26] and 177–361 mcg/L for rivaroxaban [27].
Apixaban and rivaroxaban anti-Xa activity was determined using a commercially available chromogenic anti-Xa Assay on a STA-R-Max analyzer at the Department of Clinical Chemistry of the Radboud University Medical Center (Nijmegen, The Netherlands). The assay was calibrated with commercially available apixaban and rivaroxaban calibrators.
Statistical Analysis
Relevant demographic and clinical characteristics were reported using descriptive statistics. Linear regression analysis was performed to examine the relationship between body weight and peak DOAC levels; assumptions for linear regression analysis were met. Findings with a p value < 0.05 were considered statistically significant. All analyses were conducted using IBM Statistical Package for Social Sciences (SPSS Inc., version 22.2. IBM corp. Chicago, IL, USA).
Results
A total of 52 patients (14 male and 38 female) were included in the study. Their BMI ranged from 35.6 to 57.6 kg/m2, with a mean of 44.3 ± 5.2 kg/m2.
With regard to obesity related comorbidities, hypertension (n = 23; 44.2%), obstructive sleep apnea syndrome (OSAS; n = 18; 34.6%), and diabetes mellitus type 2 (n = 16; 30.8%) were more prevalent than hypercholesterolemia (n = 3; 5.8%). Eight patients received levothyroxine replacement therapy for primary hypothyroidism; their plasma thyroid stimulating hormone (TSH) and thyroid hormone levels were within the normal range. All patients had a glomerular filtration rate measured above 60 ml/min/1.73m2 (CKD-EPI).
In total, preoperative anti-Xa level measurements were available for 33 patients, whereas postoperative anti-Xa level measurements were available for 37 patients. Within-patient longitudinal (i.e., preoperative and postoperative), measurement of anti-Xa levels was available in 18 cases.
Baseline characteristics of all patients are shown in Table 1.
Preoperative Anti-Xa Level Measurement
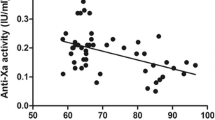
Preoperative anti-Xa activity was measured in 29 patients using apixaban and in 12 patients using rivaroxaban. Results are shown in Fig. 1 as a function of body weight. The median anti-Xa level of apixaban was 183.0mcg/L (total range 107–273mcg/L). All anti-Xa levels were above the lower limit of normal (LLN) for apixaban.
Preoperative plasma anti-Xa peak levels during apixaban use (n = 29) and rivaroxaban use (n = 12), in relation to body weight. The gray area reflects the therapeutic range of anti-Xa levels for apixaban and rivaroxaban. The bold line with the two parallel interrupted lines represents the mean linear regression line and its ± 2SD limits in the apixaban figure
Postoperative Anti-Xa Level Measurement
Post-RYGB anti-Xa levels were measured in 28 patients using apixaban and in 14 patients using rivaroxaban. Anti-Xa levels are shown in Fig. 2. In all patients on apixaban, anti-Xa levels were within the normal range. The inverse correlation with body weight observed in the preoperative patients was not present in this postoperative group. Subtherapeutic anti-Xa levels were found in 9 out of 14 (64%) patients using rivaroxaban.
Longitudinal Follow-Up
Longitudinal anti-Xa level measurements were available for 18 patients (34.6%) using apixaban. Anti-Xa levels did not change significantly (191.4 ± 65.3 mcg/L versus 198.7 ± 65.8 mcg/L; p = 0.68) between the preoperative and postoperative period (Fig. 3).
Preoperative Discontinuation
All patients discontinued apixaban 48 h before surgery, and anti-Xa levels were measured 2 to 6 h before surgery. All patients (N = 20) had undetectable anti-Xa levels (i.e., below 20 mcg/L). None of the patients had bleeding or thrombotic events perioperatively.
Discussion
This study provides novel data on plasma anti-Xa levels in MO patients using apixaban and rivaroxaban, as well as in post-bariatric patients. Our results show that plasma anti-Xa level measurements are predominantly within the therapeutic range in MO patients; they only reach subtherapeutic levels in MO patients using apixaban with a bodyweight above 144 kg. In patients using rivaroxaban, no statistically significant relation between plasma anti-Xa levels and bodyweight was found. After a RYGB procedure, plasma anti-Xa levels of apixaban were unaffected, whereas plasma anti-Xa levels during rivaroxaban use tended to become subtherapeutic. In this retrospective study, we collected the available data from patients with measured anti-Xa activity. Some patients started anticoagulation with a DOAC years after the surgery, and some patients had already DOAC before the surgery. We decided to include all patients to analyze DOAC effectivity in obese as well as post-bariatric patients. (Fig. 4).
The main concern about DOACs in patients who undergo bariatric surgery is that absorption might be jeopardized due to changes in the intestinal tract. DOAC pharmacokinetics and bioavailability depend on food volume, pH, transit time, gastrointestinal absorptive surface, and location of drug absorption. The only way to examine the absorption of DOACs is to measure plasma peak anti-Xa levels. Our results indicate that plasma anti-Xa levels in MO patients using apixaban were within the therapeutic range. These were not only within therapeutic range short after bariatric surgery, but remained so in the 20 patients who had a measurement after a 1-year follow-up (data not shown). Although numbers are small, our results suggest that apixaban may be safe for long-term use after bariatric surgery. However, larger patient groups with appropriate follow-up will be needed for a more firm conclusion.
In contrast, MO patients using rivaroxaban were more likely to have lower plasma anti-Xa levels in our study; in nine out of 14 patients (64.3%), anti-Xa levels were subtherapeutic. The difference between plasma anti-Xa levels of apixaban and rivaroxaban may be explained by drug-specific differences related to the impact of smaller meals on absorption and the anatomical changes due to surgery. Apixaban is predominantly absorbed in the distal intestine and is available as non-ionizable, film-coated, immediate-release tablets with pH independent aqueous solubility [11]. The Cmax of apixaban is achieved 3 h after oral tablet administration in healthy subjects, with no clinically relevant food effect [12]. Rivaroxaban, however, is predominantly absorbed in the proximal small intestine, which is bypassed by the RYGB procedure. Moreover, rivaroxaban requires ingestion with meals for optimal absorption. The lipophilicity and limited aqueous solubility of rivaroxaban increases its bioavailability by approximately 39% upon its administration with meals [10]. Hence, the smaller meals usually taken in patients after a RYGB procedure may also contribute to a poorer absorption [6].
We found 2 reports on the efficacy of rivaroxaban in bariatric patients [24, 29]. The first one is a case report, and the second paper includes 12 patients (six RYGB and six SG). The auteurs conclude that significant weight loss and altered anatomy after RYGB and SG procedures do not appear to affect the pharmacokinetics and pharmacodynamics of prophylactic rivaroxaban. It is obvious that more studies are needed to determine the efficacy of DOACS in bariatric surgery.
Therapeutic blood levels of DOACs are used as a surrogate to determine safety and thrombosis risk in patients with AF and/or VTE. Subtherapeutic plasma anti-Xa levels in patients with a high CHA2DS2‐VASc score are associated with more thrombotic events [28]. Therefore, measuring plasma anti-Xa levels after a RYGB procedure is essential. Our study provides the first arguments for safe usage of apixaban in MO patients who undergo bariatric surgery. Long-term follow-up of these patients is warranted to evaluate the clinical efficacy and safety based on clinical endpoints.
Despite the lack of VKA safety data in patients who undergo bariatric surgery, VKAs are often used as first choice in these patients. Patients undergoing bariatric surgery are more prone to bleeding when using anticoagulation (mainly VKA) [14]. In contrast to international guidelines that generally advise discontinuation of anticoagulation therapy 24 h before surgical interventions, we advised all MO patients to stop DOACs 48 hs before bariatric surgery to minimize bleeding complications. This approach resulted in undetectable plasma anti-Xa levels prior to surgery. We reported no bleeding complications or thrombotic events, which underlines the potential suitability of this protocol for MO patients who are scheduled for bariatric surgery.
This study has several strengths. It is one of the first studies structurally investigating the effect of DOAC use in MO patients undergoing bariatric surgery. The exact weight, above which the use of DOACs should be strongly discouraged, is yet to be determined, but our results suggest that the use of apixaban appears to be safe up to 144 kg. Furthermore, we performed perioperative measuring of anti-Xa blood levels to assess whether bariatric surgery was safe to undergo after a 2-day stop of apixaban treatment. In addition, in a limited number of patients, we repeated measurement of apixaban anti-Xa blood levels after 1 year of undergoing surgery to examine their stability. A limitation of this study is the small number of MO patients, in particular the MO patients using rivaroxaban. Moreover, long-term clinical data exceeding a 1-year follow-up with regard to postoperative complications possibly related to the specific anticoagulation regimen are not available yet. Confirmation of our findings by larger, prospective studies is warranted.
In conclusion, plasma anti-Xa levels of apixaban were likely to be within the normal range in MO patients with a body weight up to 144 kg. After RYGB, plasma anti-Xa levels of apixaban remained within the therapeutic range, whereas plasma anti-Xa levels of rivaroxaban became subtherapeutic in the majority of patients. For rivaroxaban, there was too few data to further elucidate a possible relationship with bodyweight. Therefore, future studies are needed to further elucidate and define the optimal anticoagulation policy for MO patients undergoing bariatric surgery.
References
Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev. 2019;8(1):28–36.
Ibáñez L, Sabaté M, Vidal X, et al. Incidence of direct oral anticoagulant use in patients with nonvalvular atrial fibrillation and characteristics of users in 6 European countries (2008–2015): a cross-national drug utilization study. Br J Clin Pharmacol. 2019;85(11):2524–39.
Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2(4):499–509.
Park J, Lee SR, Choi EK, et al. Effectiveness and safety of direct oral anticoagulant for secondary prevention in Asians with atrial fibrillation. J Clin Med. 2019;8(12).
Jong L, Koops M, Gout-Zwart JJ, et al. Trends in direct oral anticoagulant (DOAC) use: health benefits and patient preference. Neth J Med. 2018;76:426–30.
Chan N, Sobieraj-Teague M, Eikelboom JW. Direct oral anticoagulants: evidence and unresolved issues. Lancet. 2020;396(10264):1767–76.
Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62.
Ng DL, Gan GG, Chai CS, et al. Comparing quality of life and treatment satisfaction between patients on warfarin and direct oral anticoagulants: a cross-sectional study. Patient Prefer Adherence. 2019;13:1363–73.
Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467–507.
Kubitza D, Becka M, Zuehlsdorf M, et al. Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59–7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46(5):549–58.
Song Y, Wang X, Perlstein I, et al. Relative bioavailability of apixaban solution or crushed tablet formulations administered by mouth or nasogastric tube in healthy subjects. Clin Ther. 2015;37(8):1703–12.
Frost C, Wang J, Nepal S, et al. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75(2):476–87.
Cada DJ, Levien TL, Baker DE. Apixaban. Hosp Pharm. 2013;48(6):494–509.
Sharma G, Hanipah ZN, Aminian A, et al. Bariatric surgery in patients on chronic anticoagulation therapy. Obes Surg. 2018;28(8):2225–32.
Sherf-Dagan S, Goldenshluger A, Azran C, et al. Vitamin K–what is known regarding bariatric surgery patients: a systematic review. Surg Obes Relat Dis. 2019;15(8):1402–13.
Homan J, Ruinemans-Koerts J, Aarts EO. Management of vitamin K deficiency after biliopancreatic diversion with or without duodenal switch. Surg Obes Relat Dis. 2016;12(2):338–44.
Kido K, Lee JC, Hellwig T, Gulseth MP. Use of direct oral anticoagulants in morbidly obese patients. Pharmacotherapy. 2020;40(1):72–83.
Upreti VV, Wang J, Barrett YC, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76(6):908–16.
Choi Y, Kushnir M, Billett HH. Apixaban is safe and effective in morbidly obese patients: a retrospective analysis of 390 patients with BMI ≥40. Blood. 2017;130(Supplement 1):1105.
Safouris A, Demulder A, Triantafyllou N, et al. Rivaroxaban presents a better pharmacokinetic profile than dabigatran in an obese non-diabetic stroke patient. J Neurol Sci. 2014;346(1–2):366–7.
Kubitza D, Becka M, Zuehlsdorf M, et al. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59–7939) in healthy subjects. J Clin Pharmacol. 2007;47(2):218–26.
Patil T, Lebrecht M. A single center retrospective cohort study evaluating use of direct oral anticoagulants (DOACs) in morbidly obese veteran population. Thromb Res. 2020;192:124–30.
Rottenstreich A, Barkai A, Arad A, et al. The effect of bariatric surgery on direct-acting oral anticoagulant drug levels. Thromb Res. 2018;163:190–5.
Mahlmann A, Gehrisch S, Beyer-Westendorf J. Pharmacokinetics of rivaroxaban after bariatric surgery: a case report. J Thromb Thrombolysis. 2013;36(4):533–5.
Leven C, Hoffmann C, Roche C, et al. Impact of bariatric surgery on oral anticoagulants pharmacology, and consequences for clinical practice: a narrative review. Fundam Clin Pharmacol. 2021;35(1):53–61.
Byon W, Garonzik S, Boyd RA, et al. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019;58(10):1265–79.
Harder S. Pharmacokinetic and pharmacodynamic evaluation of rivaroxaban: considerations for the treatment of venous thromboembolism. Thromb J. 2014;12(1):22.
Testa S, Paoletti O, Legnani C, et al. Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16(5):842–8.
Kröll D, Nett PC, Borbély YM, et al. The effect of bariatric surgery on the direct oral anticoagulant rivaroxaban: the extension study. Surg Obes Relat Dis. 2018;14(12):1890–6.
Author information
Authors and Affiliations
Contributions
Thom Kok conceived and designed the analysis; collected the data; contributed data or analysis tools; performed the analysis; and wrote the paper. Hans de Boer conceived and designed the analysis; contributed data or analysis tools; performed the analysis; and wrote the paper. Houshang Monajemi conceived and designed the analysis; contributed data or analysis tools; performed the analysis; and wrote the paper. Marcel Hovens wrote the paper. Bart Witteman wrote the paper. Matthijs van Luin wrote the paper.
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
- There is no evidence of safe use for DOACs in morbid obesity (MO) and after bariatric surgery (BS).
- We performed a retrospective, cross-sectional, and longitudinal study of anti-Xa levels for apixaban and rivaroxaban for patients with MO and after BS.
- Despite small numbers, apixaban and rivaroxaban appear to be safe in MO, while apixaban shows in range anti-Xa levels after BS.
- Further investigation is needed, but apixaban could be a possible safe medication to use in this category.
Rights and permissions
About this article
Cite this article
Kok, T., de Boer, H., Witteman, B. et al. Anti-Xa Levels in Morbidly Obese Patients Using Apixaban or Rivaroxaban, Before and After Bariatric Surgery. OBES SURG 32, 607–614 (2022). https://doi.org/10.1007/s11695-021-05814-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05814-y