Abstract
Background
An aggravation in pre-existing sarcopenia or the onset of sarcopenia may occur in the scenario of extensive and fast weight loss in the initial months following bariatric surgery. The accurate identification of sarcopenia criteria and its metabolic repercussions is vital for its correct management. The aim of this study is to evaluate the correlation between the diagnosis criteria for sarcopenia and metabolic repercussions during the first 6 months following bariatric surgery.
Methods
A prospective single-center cohort study was conducted. Convenience sampling was performed among patients with severe obesity undergoing preoperative evaluation for bariatric surgery. Metabolic parameters, nutritional evaluation, and skeletal muscle evaluation were assessed before surgery and 6 months later.
Results
A total of 129 patients were selected, 62 participants were included in the final analysis. Mean age was 37.7 years and 88.4% of participants were women. Mean body mass index was 41.8 kg/m2 and 47.8% of patients were sedentary. Sleeve gastrectomy was performed in 41 patients and Roux-en-Y gastric bypass in 21 patients. Significant improvement regarding muscle strength and function after surgery was observed. Sarcopenia criteria were not met by any participant before and after surgery. Blood glucose and ferritin levels remained independently associated with change in muscle strength.
Conclusions
Functional evaluation methods did not reflect the reduction in skeletal muscle mass demonstrated in bioelectrical impedance analysis 6 months after bariatric surgery in comparison to the preoperative baseline. Improvement in muscle strength was followed by improvement in metabolic parameters.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
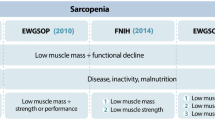
Sarcopenia is a condition defined by progressive and generalized impairment of skeletal muscle quantity and quality — strength and performance [1]. It involves muscle mass/fat ratio imbalance characteristic of aging, but also obesity. Obesity exacerbates sarcopenia as it increases the infiltration of fat into muscle and lowers physical function [1,2,3,4]. Sarcopenia is associated with unfavorable health outcomes, such as metabolic syndrome, cardiovascular disease, liver impairment, and osteoporosis [3,4,5,6,7,8,9,10,11]. Adverse outcomes also include functional and physical disability, decreased quality of life, and mortality [1, 2].
Bariatric surgery remains the most effective treatment for severe obesity and related comorbidities [12,13,14,15,16,17]. According to the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO), at least 830,000 patients underwent weight loss surgery throughout 61 countries in 2019 [18]. The increasing number of post-bariatric patients brings challenges in the evaluation of weight loss quality and its effects.
An aggravation in pre-existing sarcopenia or the onset of sarcopenia may occur in the scenario of extensive and fast weight loss in the initial months following bariatric surgery and its identification and metabolic effects are not well documented [19,20,21,22]. Although the impact of sarcopenia in patients after weight loss operations is still uncertain, an early and accurate identification of this outcome and its repercussions is vital for its correct management. The objective of this study is to evaluate the correlation between sarcopenia diagnosis criteria and metabolic repercussions during the first 6 months following bariatric surgery.
Methods
Subjects
A prospective single-center cohort study was conducted. Convenience sampling was performed among patients with severe obesity undergoing preoperative evaluation for bariatric surgery at the same obesity treatment referral center between September of 2017 and December of 2018. Criteria included: (1) Age between 18 and 65 years old; (2) Obesity with BMI ≥ 40 or BMI ≥ 35 associated with comorbid conditions and not responding to medical treatment for 2 years; (3) No psychological contraindication for surgery; (4) No previous bariatric surgery.
Sample size calculation was performed using WinPEPI (Programs for Epidemiologists for Windows) Version 11.43. Level of significance of 5%, power of 80% and minimal effect size of 0.37 standard deviations between pre- and postoperative assessments were considered to obtain a minimum of 60 patients. This sample size also sustains correlations of at least 0.4 between variables.
Time frame of 6 months was selected as it is the period of most accelerated and extensive weight loss, consequently the most vulnerable interval for occurrence of skeletal muscle mass variations. Early identification of sarcopenia parameters would also favor an early intervention for a better management in the long term.
Pre- and postoperative evaluation
Overall evaluation was performed before surgery and 6 months later.
Sarcopenia parameters were defined and measured according to European consensus recommendations [1]. Skeletal muscle mass was evaluated through bioelectrical impedance analysis (BIA) [1]. Skeletal muscle strength was evaluated through hand grip test (HG) Handgrip Strength (HGS) test [23]. Skeletal muscle performance was evaluated through Timed get-up and go test (TGUG) [24] and Gate Speed test (GS) [25, 26]. Metabolic and nutritional evaluations were determined by laboratory blood testing. Physical Activity Questionnaire (IPAQ) [27] was applied for sample characterization. Sarcopenia was determined according to the European consensus cutoff points: grip strength of < 27 kg for men and < 16 kg for women; GS test ≤ 0.8 m/s; and TUG test ≥ 20 s.
Bodyweight and height were measured using standard equipment. Body mass index (BMI) was calculated as weight/height2. Volume of lean body mass and fat and was estimated through BIA (InBody 720, Biospace, Seoul, Korea). HG test was evaluated with a Jamar dynamometer and the median of three measurements was considered. TGUG test is a measure of the time needed for the subject to stand up from a chair, walk for 3-m distance, turn around, return and sit down again. GS test is a measure of gait speed throughout a 4-min walk. IPAQ validated questionnaire evaluates the time each subject spends being physically active in the last 7 days — including work, house and yard routine, commute, and spare time for recreation, exercise or sport. Patients are classified in levels of activity that include irregularly active B (no activity), irregularly active a (physical activities for 150 min a week or 5 times a week), and active (physical activities for more than 150 min a week or more than 5 times a week). Laboratory testing parameters included the following biological markers: hemogram, fasting blood glucose, glycosylated hemoglobin (HbA1c), total cholesterol, triglyceride, LDL-cholesterol, HDL-cholesterol, total cholesterol, triglycerides, high density lipoprotein cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST), vitamin B12, folic acid, vitamin D, zinc, uric acid, ferritin and iron.
Surgical Procedures
All patients went laparoscopic sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) at the same obesity treatment center and according to patient and multidisciplinary team judgement. RYGB was preferably performed in patients who presented with metabolic syndrome or gastro-esophageal reflux disease.
Roux-en-Y gastric bypass included a 4.5-cm width gastric pouch with a 100-cm biliopancreatic limb and a 100-cm roux limb; gastrojejunostomy was 4.5-cm width and not calibrated. Sleeve Gastrectomy was performed at 3 cm from the pylorus using a 32F Fouchet calibration tube.
Statistical Analysis
Quantitative variables were expressed as mean and standard deviation and categorical variables as absolute and relative frequencies. Comparison of means was performed through Student’s t-test. Association between numerical variables was calculated through Pearson correlation coefficients. Generalized estimating equations (GEE) model with Bonferroni adjustment was used to compare variables between the two time points of assessment (before and after surgery), as the numbers in comparison were small. To control for confounding factors, linear regression model with backward extraction was run to identify factors independently associated with variation in muscle strength. Variables with p < 0.20 on bivariate analysis in association with change in handgrip strength were included in a multivariate linear regression model with backward extraction to identify factors independently associated with this outcome. Level of significance was set at 5% (p < 0.05). All analyses were performed using SPSS Version 21.0.
Ethical Considerations
Written informed consent was obtained from all individual participants included in the study.
The study protocol and the informed consent procedure were approved by the institutional ethics committee under the registration number 73910017.0.0000.5335.
Results
A total of 129 patients were selected. Fifty-seven patients were excluded — 21 patients refused to participate, 3 patients accidentally became pregnant, and 33 patients were lost to follow-up. A total of 62 participants were included in the final analysis and their characteristics are presented in Table 1. Mean age was 37.7 years and 88.4% of participants were female. Mean BMI was 41.8 kg/m2. SG was performed in 41 patients and RYGB was performed in 21 patients. According to IPAQ questionnaire, 47.8% of patients were sedentary.
Body composition assessment through BIA demonstrated an average weight loss of 31% — a mean 11.5 kg/m2 reduction in BMI (from 68.8 to 22.8% excess BMI). The average lean body mass and fat mass lost was 4 kg and 25 kg, respectively. The mean ratio of appendicular lean mass to height (kg/m2) was significantly reduced from 9 to 7.7. The mean body fat percentage was significantly reduced from 49.1 to 36.9% (Table 2).
Significant improvement was observed regarding muscle function evaluated by the TGUG test and gait speed test postoperatively. HG test results remained stable (Table 3). Sarcopenia criteria were not met by any participant during the 6-month follow-up period.
Significant decrease in fasting blood glucose was detected (mean — 13.3 mg/dL). Parameters such as total cholesterol and triglycerides were also significantly improved. Vitamin B12 and D levels increased significantly after surgery (Table 3).
Comparison of muscle strength and function scores, metabolic variables, and anthropometric parameters between surgical techniques revealed no significant difference (Table 4).
After adjustment by the multivariate model, change in blood glucose and ferritin levels remained independently associated with change in muscle strength, as shown in Tables 5 and 6. Percent weight loss was borderline significant after adjustment. For every 1 mg/dL reduction in blood glucose levels after surgery, there was an average increase of 0.08 units in handgrip strength after multivariate adjustment (Fig. 1). For every 1-unit reduction in ferritin levels after surgery, there was an average increase of 0.02 units in handgrip strength.
Discussion
Reduced skeletal muscle mass may be associated with the development of metabolic disease, such as type 2 diabetes. Likewise, skeletal muscle mass gain and strength is related with a lower risk of type 2 diabetes [28]. Although a reduction in skeletal muscle mass after surgery was detected in some studies [29, 30], as well as, in our sample, this muscle mass decrease did not affect muscle function and was not enough to independently affect blood glucose levels. In our study, loss of skeletal muscle mass did not present any correlation with metabolic factors worsening. Moreover, patients in this sample presented an increase in muscle strength after surgery, although their significantly lean body mass loss, an outcome that has been correlated with improvement in glucose metabolism disorders [31]. The inverse relation between muscle strength and type 2 diabetes presented a linear positive correlation that may be due to a greater peripheral insulin action.
Serum ferritin levels are an important marker of systemic inflammation and their values are directly correlated with other inflammatory markers levels, like HbA1c [32]. Serum ferritin also reflects body iron stores. It is suggested that increased serum ferritin levels in patients with obesity may be associated to a raise in fatty acids values and consequently, to an aggravation of insulin resistance due to iron metabolism imbalance [33]. A linear correlation between reduction in serum ferritin levels and increase in muscle strength was found in our analysis.
The contradictory association between loss of skeletal muscle mass, increase in muscle strength and improvement in metabolic parameters may strengthen the hypothesis that skeletal muscle tissue in the patient with severe obesity is of lower functional and metabolic quality. Young patients with severe obesity have a greater quantity of skeletal muscle mass than the one expected for their weight, yet evidence suggests it is of lower quality. Due to fat infiltration into muscle tissue, its function may be impaired. A prior study evidenced a negative correlation between muscle strength and the degree of fat infiltration in the muscle tissue [34]. These findings reinforce the importance of adequate interpretation of skeletal muscle mass evaluation parameters and diagnosis criteria for sarcopenia. An isolated measurement of skeletal muscle mass may induce a false diagnosis. Loss of muscle mass after bariatric surgery may be considered a metabolic adaptive process, since factors related to muscle quality (like strength) and metabolic syndrome markers show significant improvement after surgery.
The high rate of weight loss in the early postoperative period of our patients did not induce the development of sarcopenia that reinforces the safety of bariatric surgery as a weight loss strategy. Nevertheless, further studies on continuous loss of skeletal muscle mass must be conducted to evaluate the occurrence and long-term outcomes of this initial loss of lean body mass.
Limitations of this study included the inclusion of two different metabolic procedures; high dropout rate and loss of follow up; non-individual analysis of the group of patients with diabetes; the lack of direct markers of glucose intolerance; and general aspects of non-intervention studies, such as the lack of control over physical training and postoperative nutritional supplementation.
Conclusion
Functional evaluation methods (TGUG test, GS test and HG test) did not reflect the reduction in skeletal muscle mass demonstrated in BIA 6 months after bariatric surgery in comparison to the preoperative baseline. Improvement in muscle strength was followed by improvement in metabolic parameters.
References
Cruz-Jentoft AJ, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Lee J, et al. Associations of sarcopenia and sarcopenic obesity with metabolic syndrome considering both muscle mass and muscle strength. J Prev Med Public Health. 2016;49(1):35–44.
Mastino D, et al. Bariatric surgery outcomes in sarcopenic obesity. Obes Surg. 2016;26(10):2355–62.
Baumgartner RN, et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995–2004.
Atkins JL, et al. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62(2):253–60.
Batsis JA, et al. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68(9):1001–7.
Hara N, et al. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med. 2016;55(8):863–70.
Ma J, et al. Mid-adulthood cardiometabolic risk factor profiles of sarcopenic obesity. Obesity (Silver Spring). 2016;24(2):526–34.
Onoue Y, et al. A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int J Cardiol. 2016;215:301–6.
Hunter GR, et al. Sarcopenia and its implications for metabolic health. J Obes. 2019;2019:8031705.
Buchwald H, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Mamplekou E, et al. Psychological condition and quality of life in patients with morbid obesity before and after surgical weight loss. Obes Surg. 2005;15(8):1177–84.
Sjöström L, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65.
Reges O, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319(3):279–90.
Schiavon CA, et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (gastric bypass to treat obese patients with steady hypertension). Circulation. 2018;137(11):1132–42.
Billeter AT, et al. Meta-analysis of metabolic surgery versus medical treatment for macrovascular complications and mortality in patients with type 2 diabetes. Surg Obes Relat Dis. 2019;15(7):1197–210.
Ramos A, et al. Fifth IFSO Global Registry Report. 2019, Dendrite clinical systems ltd, Henley-on-Thames, RG9 1AY, UK 2014.
Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond). 2007;31(5):743–50.
Voican CS, et al. Predictive score of sarcopenia occurrence one year after bariatric surgery in severely obese patients. PLoS One. 2018;13(5):e0197248.
Heshka S, et al. Resting energy expenditure and organ-tissue body composition 5 years after bariatric surgery. Obes Surg. 2020;30(2):587–94.
Mohapatra S, Gangadharan K, Pitchumoni CS. Malnutrition in obesity before and after bariatric surgery. Dis Mon. 2020;66(2):100866.
Leong DP, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–73.
Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8.
Studenski S, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8.
Bianchi L, et al. The predictive value of the EWGSOP definition of sarcopenia: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2016;71(2):259–64.
Lee PH, et al. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115.
Yeung CHC, et al. Lean mass, grip strength and risk of type 2 diabetes: a bi-directional Mendelian randomisation study. Diabetologia. 2019;62(5):789–99.
Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med. 2016;31(6):1054–60.
Johnson Stoklossa CA, et al. Prevalence of sarcopenic obesity in adults with class II/III obesity using different diagnostic criteria. J Nutr Metab. 2017;2017:7307618.
Holten MK, et al. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53(2):294–305.
Elimam H, Abdulla AM, Taha IM. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13(1):800–4.
Ryan BJ, et al. Plasma ferritin concentration is positively associated with in vivo fatty acid mobilization and insulin resistance in obese women. Exp Physiol. 2018;103(11):1443–7.
Inhuber S, et al. Associations of thigh muscle fat infiltration with isometric strength measurements based on chemical shift encoding-based water-fat magnetic resonance imaging. Eur Radiol Exp. 2019;3(1):45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study protocol and the informed consent procedure were approved by the institutional ethics committee under the registration number 73910017.0.0000.5335.
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Reduction in skeletal muscle mass was detected after surgery.
• Function evaluation methods did not reflect the reduction in skeletal muscle mass.
• Improvement in muscle strength was followed by improvement in metabolic parameters.
Rights and permissions
About this article
Cite this article
Coral, R.V., Bigolin, A.V., Machry, M.C. et al. Improvement in Muscle Strength and Metabolic Parameters Despite Muscle Mass Loss in the Initial Six Months After Bariatric Surgery. OBES SURG 31, 4485–4491 (2021). https://doi.org/10.1007/s11695-021-05634-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05634-0