Abstract
This systematic review synthesized research evaluating the relationship between genetic predictors and weight loss after bariatric surgery. Fifty-seven studies were identified that examined single genes or genetic risk scores. Uncoupling protein (UCP) rs660339 was associated with excess weight loss after surgery in 4 of 6 studies. The most commonly assessed genes were fat mass and obesity–associated (FTO) gene (n = 10) and melanocortin-4 receptor (MC4R) (n = 14). Both were inconsistently related to weight loss. Genetic risk scores predicted weight loss in 6 of 7 studies. This evidence suggests the potential of using genetic variants and genetic risk scores to predict the amount of weight loss anticipated after bariatric surgery and identify patients who may be at risk for suboptimal weight reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the well-established use of bariatric surgery to treat obesity, significant interindividual variability has been noted in short-term and long-term weight loss [1, 2]. Patients generally achieve a maximum weight loss 1–2 years after surgery [3]. On average, patients who have sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) achieve a 25% or greater reduction of their initial weight within the first year [4]. However, there is a wide interindividual response to the various types of bariatric surgery, particularly during longer-term follow-up (>2 years). For example, in the Longitudinal Assessment of Bariatric Surgery-2 study, there were 6 distinct patterns of weight change identified in response to surgical treatments [2]. Genetic factors may help to explain these interindividual differences.
Family, twin, and adoption studies have demonstrated heritability estimates of 20–90% for body mass index (BMI) [5]. Multiple obesity-predisposing genetic loci have also been identified. For example, the fat mass and obesity–associated (FTO) gene has a well-established role in predisposing one to childhood or adult obesity [6]. Hundreds of additional genetic loci have been established in relation to obesity, such as those coding for apolipoprotein receptors (APOB) [7]. These genetic loci may interact with obesity treatments and influence weight loss outcomes. Previous reviews have examined the interaction between genomic information and lifestyle interventions [8,9,10,11,12]. However, the literature on genetic factors in relation to weight loss after bariatric surgery has not been well synthesized.
Identifying genetic factors related to weight loss after bariatric surgery may help to guide weight management strategies pre- and post-surgery and to identify and develop novel interventions. The purpose of this systematic review was to provide a narrative synthesis of research on the association between genetic factors and weight loss heterogeneity in patients who received bariatric surgery.
Methods
Our study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [13]. Articles were identified using the databases PubMed, Embase, Web of Science, PsycInfo, and Cochrane Library. A research librarian was consulted to create the search strategy (Table S1). The reference lists of selected articles were manually searched. Randomized controlled trials, observational reports, and cohort studies were considered for this review. Eligible journal articles were peer reviewed and published in the English language. Studies that measured changes in gene expression, epigenetics, proteomics, or metabolomics were excluded. The literature search was conducted from inception to July 2, 2020.
Data Extraction
We piloted a data abstraction form. Data were extracted on country, sample size, age, BMI, race/ethnicity, sex, and association between the gene and weight loss outcomes.
Quality Analysis
The Q-Genie tool was used to assess study quality [14]. The tool was developed by collating published guidelines and recommendations for genetic association studies, such as STrengthening the REporting of Genetic Association Studies (STREGA) and Strengthening the Reporting of Genetic Risk Prediction Studies (GRIPS). The Q-Genie tool consists of 11 items including the rationale for the study; classification for the genetic variant; sources of bias and confounding factors; test of genetic assumptions (e.g., agreement with the Hardy Weinberg equilibrium); statistical methods and control; and inferences drawn from the results. Each item was scored on a Likert scale from 1 to 7, with 7 indicating the highest score in the category. Studies with control groups and composite scores >45 indicate good-quality studies, those with 36–45 were of moderate quality, and those with ≤35 indicate poor-quality studies.
Results
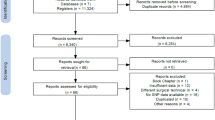
The literature search yielded a total of 2598 eligible articles and 3 additional studies were found by checking reference lists of relevant studies (Figure 1). After removing duplicates, 1836 articles remained for title and abstract screening. Of these, 1585 were excluded. The remaining 251 articles were screened at full-text level. After full-text review, 194 were excluded. In total, 57 articles met inclusion criteria. All studies were rated as good-quality studies based on the Q-Genie assessment tool.
Demographics
The sample size ranged from 4 to 1443 participants (Table 1). In most studies, the major inclusion criteria for surgery followed standard guidelines for bariatric surgery: BMI ≥40 or ≥35 kg/m2 with one or more obesity-related comorbidity. The range of average BMIs reported in studies was 35–55 kg/m2. The participants in the selected studies had a range of average age from 16 to 52 years old. The range of average percent female of a sample was 50–100%.
FTO
Ten studies explored FTO in relation to weight loss after bariatric surgery (Table 2). Of these studies, four studies showed an association between FTO and improved weight loss, two demonstrated a lesser weight loss, and the rest showed no significant association. Of the four articles that showed improved weight loss, three specifically studied the rs9939609 variant of FTO and found an association between 3 and 24 months. However, Rodrigues et al. found that participants with the rs9939609 polymorphism lost less weight at 24–60 months after RYGB [18]. Thus, there was mixed support that differences in weight change based on FTO gene variants were associated with bariatric surgery outcomes.
MC4R
Fourteen articles examined the melanocortin-4 receptor (MC4R) gene and weight loss after bariatric surgery, and results were mixed (Table 2). Nine of 14 studies found no association between MC4R and weight loss. Four studies concluded that variations in the MC4R gene were associated with a poorer weight loss outcome. These studies reported outcomes from 3 to 60 months and included patients who underwent various types of bariatric surgeries including SG, RYGB, and GB. BMI at the start of the study ranged from 43 to 51 kg/m2. On the other hand, Javanrouh et al. found that patients with the rs17773430 variant of MC4R experienced a greater reduction in percent excess weight loss (%EWL) up to 12 months after gastric bypass [21]. Mirshahi et al. also reported improved weight loss in patients with the rs5282087 variant in the MC4R promoter region [22]. However, this relationship was only significant up to 36 months post-RYGB and was not sustained thereafter at 48 months.
Leptin
Six articles examined the association of either leptin or the leptin receptor genes (LEP/LEPR) with weight loss after bariatric surgery (Table 2). Between 12 and 24 months after RYGB, Kops et al. noted that patients with the rs1137101 variant of LEP223 experienced a greater percent excess weight loss [23]. De Luis et al. also found patients had better weight loss outcomes with a rs1805094 mutation in LEPR after BPD [24]. However, the remaining studies did not find an association between genetic variants of leptin and weight loss post-surgery.
Uncoupling Protein
Eight studies explored different variants of the uncoupling protein (UCP) gene (Table 2). Four of six studies that examined the rs660339 variant of UCP2 found that this variant was associated with greater weight loss after bariatric surgery, while two studies found no association with this variant. Of the four articles that highlighted greater weight loss, significant findings occurred between 6 and 12 months after surgery. Liou et al., Chen et al., and Lee et al. measured weight loss after LAGB [16, 25, 26]. Six months after surgery, Liou et al. reported that participants with a risk genotype of “CT/TT” lost more weight than those without the genotype (−7.5 versus −6 kg/m2, respectively) [16]. Chen et al. similarly demonstrated a greater weight loss for patients with the risk genotype compared to those with the “CC” genotype, 12.2 versus 8.1 kg/m2 at 12 months post-surgery and 13.1 versus 9.3 kg/m2 at 24 months post-surgery, respectively [25]. Nicoletti et al. measured weight loss after RYGB and found improved weight loss with the rs660339 variant [27]. They also found the G866A variant in the UCP2 gene had a similar, positive association 12 months after RYGB. Finally, 6 months after LAGB, Sesti et al. found that patients with the A866A variant in the promoter region of UCP2 experienced greater weight loss [28]. The remaining studies did not find an association between variants of the uncoupling protein gene and bariatric surgery outcomes.
Peroxisome Proliferator–Activated Receptor
Five studies explored the association between genetic variations of the peroxisome proliferator–activated receptor (PPAR) gene and weight loss after bariatric surgery (Table 2). While Lee et al. found the rs4684846 of PPAR to be associated with improved weight loss outcomes 24 months after LAGB, the other four studies revealed there was no association between these two variables.
Other Genes
Several other genes were examined in five or fewer studies (Table 2).
Interleukin-6
Of the four studies reporting findings on interleukin-6 (IL-6) gene, two found significant short-term associations. Sesti et al. noted the G174G mutation in the promoter region was associated with greater percent BMI decrease 6 months after LAGB [28]. Di Renzo et al. found those with a G174C to have poorer weight loss outcomes 6 months after LAGB [29, 30].
Ghrelin
Three studies examined either the ghrelin gene or ghrelin receptor (GHRL/GHSR). Vitolo et al. found the rs696217 variant of GHRL was associated with greater percent total weight loss after RYGB at 6, 26, and 52 weeks post-surgery [31]. In a longer-term study, Matzko et al. found the rs490683 variant of GHSR to be associated with greater weight loss 30 months after RYGB [32].
Proopiomelanocortin
Three articles reported on variations in the proopiomelanocortin (POMC) gene. Velázquez-Fernández et al. found the rs1042571 variant to be associated with greater percent excess weight loss after RYGB at 6, 12, 18, and 24 months [33]. The other two studies did not find any association.
FK506-Binding Protein 5
The two studies reporting on the rs1360780 variation of the FK5506-binding protein 5 (FKBP5) gene both noted a lower percent excess weight loss after RYGB for individuals who carried the T allele up to 24 months after surgery. In Hartmann et al., the association was significant at the 12–14, 18–20, and 24–26 month time points, while the association in Peña et al. was significant at the 24-month mark [34, 35].
Estrogen Receptor 1
Significant short-term findings were reported for the rs712221 variant of the estrogen receptor-1 (ESR1) gene. Liou et al. found that patients undergoing LAGB or laparoscopic mini-gastric bypass experienced better weight loss 6 months after surgery [16]. Similarly, Velázquez-Fernández et al. found an association between rs712221 and percent excess body weight loss 6 and 12 months after RYGB, with a difference of 10% or more at both timepoints [33].
Genes with no Significant Relationships with Weight Loss
Four articles explored adiponectin (ADIPOQ), none of which found a significant association between this gene and weight loss (Table 2). These studies ranged in duration from 6 weeks to 6 years with no associations at any of the timepoints. The one article that explored the insulin receptor substrate 1 (IRS1) gene did not find an association with weight loss 6 months after bariatric surgery. Two studies explored polymorphisms in the tumor necrosis factor (TNF) gene, none of which found a significant association between weight loss and outcomes 1 year and 6 years following bariatric surgery. Three articles reported on variations of the guanine nucleotide–binding protein (GNBP) gene. None of the studies had significant findings 6, 12, 18, 24, and 72 months after surgery. Two articles explored the relationship between fatty acid–binding protein-2 (FABP2) and weight loss 1 and 2 years after bariatric surgery. Neither study reported a significant association.
Genes Identified in One Study Only
We found several genes that were only found in one study but had a significant association with weight loss after bariatric surgery. Serotonin receptor (5-HT2C), angiopoietin-like 4 (ANGPTL4), mitochondrial translational initiation factor 3 (MTIF3), lysophospholipase-like 1 (LYPLAL), fatty acid amid hydrolase (FAAH), ST8 sialyltransferase 2 (ST8SIA2), and interleukin-1 receptor–associated kinase-3 (IRAK3) were genes that had polymorphisms associated with them resulting in improved weight loss after bariatric surgery. The associations were significant 12 months, 2 years, 1–9.5 years, 2 years, 1 year, at time of weight nadir, and 3 months after bariatric surgery, respectively. Conversely, a variation in the CD40 ligand (CD40L) gene was associated with poorer weight loss outcomes 6, 26, and 52 weeks after RYGB.
Genetic Risk Score
Seven articles used genetic risk scores to predict the weight loss outcomes over a range of time periods, from 12 to 96 months after bariatric surgery (Table 3). Rinella et al. combined 17 genes into a predictive model that assessed the association with weight loss 24 months after RYGB [36]. After conducting a primary investigation of genes associated with weight loss after RYGB, these 17 single nucleotide polymorphisms (SNP) were identified as having the most potential clinical utility. Ciudin et al. similarly created a predictive model using 57 genes to ascertain the weight regain 60 months after RYGB [37]. The 57 SNPs were found to be associated with obesity, appetite regulation, and weight loss in response to bariatric surgery. The polygenic risk score created by de Toro-Martín et al. combined 186 SNPs and was also found to be helpful to predict weight loss up to 96 months after BPD [38]. BMI-associated SNPs were selected from the GWAS Catalog. Aasbrenn et al. incorporated 77 SNPs into a genetic risk score and found it to be associated with predicting percent excess BMI loss (%EBMIL) 24 months after RYGB but stated it may not be ready for clinical practice yet [39]. Notably, patients in the highest tertile of the genetic risk score lost 1.7kg more weight than those in the lowest tertile, EBMIL of 81.1% versus 73.9%, respectively. Katsareli et al. found that their models using 108 SNPs were significantly able to predict percent total weight loss (%TWL) 12 and 24 months after RYGB [40]. Specifically, one proposed model found a 4.62% decrease in %EWL at 12 months after surgery. The predisposition score composed of 7 SNPs, by Nicoletti et al., found that patients with a higher score had greater metabolic benefits 1 year after RYGB [41]. Patients with a higher predisposition score were found to have a greater reduction in glycemia, total cholesterol, and triglycerides. Finally, the genetic risk score created by Käkelä et al. using 33 SNPs was not able to predict weight changes 36 months after bariatric surgery [42].
Discussion
Fifty-seven articles were considered in this review to find a genetic association with weight loss after bariatric surgery. The UCP variant rs660339 demonstrated significant, positive correlations with excess weight loss after bariatric surgery. Specifically, four studies concluded the rs660339 variant of UCP2 was associated with greater weight loss after LAGB and RYGB. Both articles exploring FKBP5 noted lower weight loss for patients with the rs1360780 variant. A combination of various genes, measured by genetic risk scores, was also shown to have significant predictive value after surgery. Our review supports that weight loss is influenced by multiple genetic variants that have modest individual effects, yet together produce a larger aggregate effect. As such, polygenic risk scores may better capture the genetic architecture of weight loss with bariatric surgery.
Although numerous genes have an established association with obesity, our findings show a lack of consistently identifiable genetic factors that can reliably predict weight loss outcomes after bariatric surgery. While genetic risk score evaluations show promise in predicting weight loss, evidence of efficacy in predicting longer-term outcomes (3 years or longer) is needed before such measures can be used by clinicians. Studies are necessary to determine whether genetic risk score evaluations can provide patients with an accurate anticipated amount of weight loss post-surgery, which holds implications for greater satisfaction with the chosen treatment. However, the feasibility, cost, and added clinical value of these analyses need to be examined. In addition to the relationship of weight loss after surgery, genetic factors and risk scores may be useful in determining postoperative changes, such as response to drug therapies and alterations in dietary habits. Thus, future studies would be useful to further explore this relationship (reviewer #6, comment #2).
The FTO and MC4R genes were inconsistent in their relationship with the amount of weight loss after surgery, as some articles found a significant change in weight while others did not. This result was noteworthy as these genes have a well-known relationship to obesity [6, 7]. A few explanations may underlie these findings. Firstly, multiple genes explain the interindividual variability in weight loss; thus, it seems that FTO and MC4R are more useful in a genetic risk score than alone. Additionally, not enough studies have been conducted on the multitude of variants present for the FTO and MC4R genes. For example, the rs9930506 variant of FTO has been identified as a significant marker of BMI and obesity yet only one study explored this SNP [72]. Finally, it is possible that the genetic variants associated with the development of obesity are not involved in weight loss following treatment.
Several limitations are present within this study. First, the variability in sample size and participant characteristics may affect the generalizability to a larger patient population. Second, there was a large amount of heterogeneity in the type of surgery, method of sample collection, length of study, and gene identification. Third, case studies and articles not published in the English language were excluded, possibly missing relevant reports. Furthermore, LAGB, VBG, and BPD currently make up a low percentage of the total number of bariatric procedures performed annually in the USA and worldwide but were included in many of the reviewed studies [73]. Findings from these studies may not translate to more commonly used procedures of sleeve gastrectomy and RYGB.
Conclusions
This review suggests some evidence of the importance of UCP and FKBP variants and genetic risk scores in predicting the weight loss outcomes of bariatric surgery. Our study contributes to the numerous factors that clinicians may consider when discussing surgery with their patients. Nevertheless, more research is needed to explore the interaction between genetic, lifestyle, and environmental factors and their effect on weight loss after bariatric surgery. Given the rise in popularity of sleeve gastrectomy, future studies examining the relationship between genetic predictors and weight loss outcomes would add to the literature. Long-term studies evaluating genetic factors and weight regain beyond the “honeymoon” period of 2–3 years after bariatric surgery are needed.
References
Chang S-H, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–87.
Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg. 2018;153(5):427–34.
Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52.
Karlsson J, Taft C, Ryden A, et al. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes. 2007;31(8):1248–61.
Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27(4):325–51.
Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94.
Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206.
Heianza Y, Qi L. Gene-diet interaction and precision nutrition in obesity. Int J Mol Sci. 2017 Apr 7;18(4):787.
Bayer S, Winkler V, Hauner H, et al. Associations between genotype–diet interactions and weight loss—a systematic review. Nutrients. 2020;12(9):2891.
Xiang L, Wu H, Pan A, et al. FTO genotype and weight loss in diet and lifestyle interventions: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103(4):1162–70.
Martínez JA, Milagro FI. Genetics of weight loss: a basis for personalized obesity management. Trends Food Sci Technol. 2015;42(2):97–115.
Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6(3):223–36.
Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–12.
Sohani ZN, Meyre D, de Souza RJ, et al. Assessing the quality of published genetic association studies in meta-analyses: the quality of genetic studies (Q-Genie) tool. BMC Genet. 2015;16(1):1–8.
Bandstein M, Schultes B, Ernst B, et al. The role of FTO and vitamin D for the weight loss effect of Roux-en-Y gastric bypass surgery in obese patients. Obes Surg. 2015;25(11):2071–7.
Liou T-H, Chen H-H, Wang W, et al. ESR1, FTO, and UCP2 genes interact with bariatric surgery affecting weight loss and glycemic control in severely obese patients. Obes Surg. 2011;21(11):1758–65.
de Luis DA, Aller R, Conde R, et al. Effects of RS9939609 gene variant in FTO gene on weight loss and cardiovascular risk factors after biliopancreatic diversion surgery. J Gastrointest Surg. 2012;16(6):1194–8.
Rodrigues GK, Resende CM, Durso DF, et al. A single FTO gene variant rs9939609 is associated with body weight evolution in a multiethnic extremely obese population that underwent bariatric surgery. Nutrition. 2015;31(11-12):1344–50.
Sarzynski M, Jacobson P, Rankinen T, et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int J Obes. 2011;35(5):676–83.
Figueroa-Vega N, Jordán B, Pérez-Luque EL, et al. Effects of sleeve gastrectomy and rs9930506 FTO variants on angiopoietin/Tie-2 system in fat expansion and M1 macrophages recruitment in morbidly obese subjects. Endocrine. 2016;54(3):700–13.
Javanrouh N, Khalaj A, Guity K, et al. Presence of CC genotype for rs17773430 could affect the percentage of excess weight loss 1 year after bariatric surgery: Tehran Obesity Treatment Study (TOTS). Obes Surg. 2020;30(2):537–44.
Mirshahi UL, Still CD, Masker KK, et al. The MC4R (I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96(12):E2088–96.
Kops NL, Vivan MA, Horvath JD, et al. FABP2, LEPR223, LEP656, and FTO polymorphisms: effect on weight loss 2 years after bariatric surgery. Obes Surg. 2018;28(9):2705–11.
De Luis DA, Aller R, Sagrado MG, et al. Influence of Lys656asn polymorphism of leptin receptor gene on surgical results of biliopancreatic diversion. J Gastrointest Surg. 2010;14(5):899–903.
Chen H-H, Lee W-J, Wang W, et al. Ala55Val polymorphism on UCP2 gene predicts greater weight loss in morbidly obese patients undergoing gastric banding. Obes Surg. 2007;17(7):926–33.
Lee YC, Liew P-L, Lee W-J, et al. Prediction of successful weight reduction after laparoscopic adjustable gastric banding. Hepatogastroenterology. 2009;56(93):1222–6.
Nicoletti CF, de Oliveira APR, Brochado MJF, et al. The Ala55Val and-866G> A polymorphisms of the UCP2 gene could be biomarkers for weight loss in patients who had Roux-en-Y gastric bypass. Nutrition. 2017;33:326–30.
Sesti G, Perego L, Cardellini M, et al. Impact of common polymorphisms in candidate genes for insulin resistance and obesity on weight loss of morbidly obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. J Clin Endocrinol Metab. 2005;90(9):5064–9.
Di Renzo L, Carbonelli MG, Bianchi A, et al. Impact of the− 174 G> C IL-6 polymorphism on bioelectrical parameters in obese subjects after laparoscopic adjustable gastric banding. J Obes. 2012;2012:1–7.
Di Renzo L, Carbonelli M, Bianchi A, et al. Body composition changes after laparoscopic adjustable gastric banding: what is the role of− 174G> C interleukin-6 promoter gene polymorphism in the therapeutic strategy? Int J Obes. 2012;36(3):369–78.
Vitolo E, Santini E, Seghieri M, et al. Heterozygosity for the rs696217 SNP in the preproghrelin gene predicts weight loss after bariatric surgery in severely obese individuals. Obes Surg. 2017;27(4):961–7.
Matzko ME, Argyropoulos G, Wood GC, et al. Association of ghrelin receptor promoter polymorphisms with weight loss following Roux-en-Y gastric bypass surgery. Obes Surg. 2012;22(5):783–90.
Velázquez-Fernández D, Mercado-Celis G, Flores-Morales J, et al. Analysis of gene candidate SNP and ancestral origin associated to obesity and postoperative weight loss in a cohort of obese patients undergoing RYGB. Obes Surg. 2017;27(6):1481–92.
Hartmann IB, Fries GR, Bücker J, et al. The FKBP5 polymorphism rs1360780 is associated with lower weight loss after bariatric surgery: 26 months of follow-up. Surg Obes Relat Dis. 2016;12(8):1554–60.
Peña E, Caixàs A, Arenas C, et al. Role of the FKBP5 polymorphism rs1360780, age, sex, and type of surgery in weight loss after bariatric surgery: a follow-up study. Surg Obes Relat Dis. 2020;16(4):581–9.
Rinella ES, Still C, Shao Y, et al. Genome-wide association of single-nucleotide polymorphisms with weight loss outcomes after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2013;98(6):E1131–6.
Ciudin A, Fidilio E, Ortiz A, et al. Genetic testing to predict weight loss and diabetes remission and long-term sustainability after bariatric surgery: a pilot study. J Clin Med. 2019;8(7):964.
de Toro-Martin J, Guenard F, Tchernof A, et al. Polygenic risk score for predicting weight loss after bariatric surgery. JCI Insight. 2018;6:3(17).
Aasbrenn M, Schnurr TM, Have CT, et al. Genetic determinants of weight loss after bariatric surgery. Obes Surg. 2019;29(8):2554–61.
Katsareli E, Amerikanou C, Rouskas K, et al. A genetic risk score for the estimation of weight loss after bariatric surgery. Obes Surg. 2020;30(4):1482–90.
Nicoletti CF, Pinhel MAS, de Oliveira BAP, et al. The genetic predisposition score of seven obesity-related single nucleotide polymorphisms is associated with better metabolic outcomes after Roux-en-Y gastric bypass. J Nutrigenet Nutrigenomics. 2016;9(5-6):222–30.
Käkelä P, Jääskeläinen T, Torpström J, et al. Genetic risk score does not predict the outcome of obesity surgery. Obes Surg. 2014;24(1):128–33.
Scott F, Elahi S, Adebibe M, et al. Farnesoid X receptor-a molecular predictor of weight loss after vertical sleeve gastrectomy? Obes Sci Pract. 2019;5(3):273–80.
Balasar Ö, Çakır T, Erkal Ö, et al. The effect of rs9939609 FTO gene polymorphism on weight loss after laparoscopic sleeve gastrectomy. Surg Endosc. 2016;30(1):121–5.
Novais PFS, Weber TK, Lemke N, et al. Gene polymorphisms as a predictor of body weight loss after Roux-en-Y gastric bypass surgery among obese women. Obes Res Clin Pract. 2016;10(6):724–7.
Wang C-Y, Liu K-H, Tsai M-L, et al. FTO variants are associated with ANGPTL4 abundances and correlated with body weight reduction after bariatric surgery. Obes Res Clin Pract. 2020;14(3):257–63.
Cooiman M, Kleinendorst L, Aarts E, et al. Genetic obesity and bariatric surgery outcome in 1014 patients with morbid obesity. Obes Surg. 2020;30(2):470–7.
Potoczna N, Branson R, Kral JG, et al. Gene variants and binge eating as predictors of comorbidity and outcome of treatment in severe obesity. J Gastrointest Surg. 2004;8(8):971–82.
Resende CMM, Durso DF, Borges KBG, et al. The polymorphism rs17782313 near MC4R gene is related with anthropometric changes in women submitted to bariatric surgery over 60 months. Clin Nutr. 2018;37(4):1286–92.
Aslan IR, Campos GM, Calton MA, et al. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg. 2011;21(7):930–4.
Censani M, Conroy R, Deng L, et al. Weight loss after bariatric surgery in morbidly obese adolescents with MC4R mutations. Obesity (Silver Spring). 2014;22(1):225–31.
Hatoum IJ, Stylopoulos N, Vanhoose AM, et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2012;97(6):E1023–31.
Moore BS, Mirshahi UL, Yost EA, et al. Long-term weight-loss in gastric bypass patients carrying melanocortin 4 receptor variants. PLoS One. 2014;9(4):e93629.
Valette M, Poitou C, Le Beyec J, et al. Melanocortin-4 receptor mutations and polymorphisms do not affect weight loss after bariatric surgery. PLoS One. 2012;7(11):e48221.
Zechner JF, Mirshahi UL, Satapati S, et al. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144(3):580–590. e587.
De Luis D, Pacheco D, Aller R, et al. Influence of-55CT polymorphism of UCP3 gene on surgical results of biliopancreatic diversion. Obes Surg. 2010;20(7):895–9.
de Luis DA, Sagrado MG, Izaola O, et al. Influence of Ala54Thr polymorphism of fatty acid–binding protein-2 on clinical results of biliopancreatic diversion. Nutrition. 2008;24(4):300–4.
Hulsmans M, Geeraert B, De Keyzer D, et al. Interleukin-1 receptor-associated kinase-3 is a key inhibitor of inflammation in obesity and metabolic syndrome. PLoS One. 2012;7(1):e30414.
De Luis D, Pacheco D, Aller R, et al. Influence of G308A polymorphism of tumor necrosis factor alpha gene on surgical results of biliopancreatic diversion. Obes Surg. 2010;20(2):221–5.
Alexandrou A, Armeni E, Kaparos G, et al. Bsm1 vitamin D receptor polymorphism and calcium homeostasis following bariatric surgery. J Investig Surg. 2015;28(1):8–17.
Hatoum IJ, Greenawalt DM, Cotsapas C, et al. Weight loss after gastric bypass is associated with a variant at 15q26. 1. Am J Hum Genet. 2013;92(5):827–34.
Beisani M, Pappa S, Moreno P, et al. Laparoscopic sleeve gastrectomy induces molecular changes in peripheral white blood cells. Clin Nutr. 2020;39(2):592–8.
Poitou C, Lacorte J-M, Coupaye M, et al. Relationship between single nucleotide polymorphisms in leptin, IL6 and adiponectin genes and their circulating product in morbidly obese subjects before and after gastric banding surgery. Obes Surg. 2005;15(1):11–23.
Rasmussen-Torvik LJ, Baldridge AS, Pacheco JA, et al. rs4771122 predicts multiple measures of long-term weight loss after bariatric surgery. Obes Surg. 2015;25(11):2225–9.
Bandstein M, Mwinyi J, Ernst B, et al. A genetic variant in proximity to the gene LYPLAL1 is associated with lower hunger feelings and increased weight loss following Roux-en-Y gastric bypass surgery. Scand J Gastroenterol. 2016;51(9):1050–5.
De Luis D, Sagrado MG, Pacheco D, et al. Effects of C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase on weight loss and cardiovascular risk factors 1 year after biliopancreatic diversion surgery. Surg Obes Relat Dis. 2010;6(5):516–20.
Leyvraz C, Verdumo C, Suter M, et al. Changes in gene expression profile in human subcutaneous adipose tissue during significant weight loss. Obes Facts. 2012;5(3):440–51.
Ruiz-Lozano T, Vidal J, De Hollanda A, et al. Evening chronotype associates with obesity in severely obese subjects: interaction with CLOCK 3111T/C. Int J Obes. 2016;40(10):1550–7.
Potoczna N, Wertli M, Steffen R, et al. G protein polymorphisms do not predict weight loss and improvement of hypertension in severely obese patients. J Gastrointest Surg. 2004;8(7):862–8.
Goergen M, Manzoni D, De Blasi V, et al. Influence of obesity-susceptibility loci (MC4R and INSIG2) on the outcome of weight loss and amelioration of co-morbidity in obese patients treated by a gastric-bypass. Bull Soc Sci Med Grand Duche Luxemb. 2011;2:7–24.
de Luis D, Calvo SG, Primo D, et al. Polymorphism rs3123554 in the cannabinoid receptor type 2 (CB2R) gene is associated with metabolic changes after biliopancreatic diversion surgery. Endocrinol Diabetes Nutr (Engl Ed). 2019;66(3):157–63.
Scuteri A, Sanna S, Chen W-M, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7):e115.
Alalwan AA, Friedman J, Park H, et al. US national trends in bariatric surgery: a decade of study. Surgery. 2021;170:13–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Does not apply.
Conflict of Interest
Ariana M. Chao reports grants and consulting fees from WW International, Inc., outside the submitted work. Thomas A. Wadden discloses serving on advisory boards for Novo Nordisk and WW International, Inc. Robert I. Berkowitz reports receiving funding through grant support from Eisai Inc and Novo Nordisk and scientific consulting fees from WW International. The other authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• FTO and MC4R are inconsistently related to weight loss after bariatric surgery.
• Genetic risk scores may be useful to predict the amount of weight loss after surgery.
• UCP was associated with excess weight loss after surgery in multiple studies.
Supplementary Information
ESM 1
(DOC 121 kb)
Rights and permissions
About this article
Cite this article
Gupta, S.R., Zhou, Y., Wadden, T.A. et al. A Systematic Review of Genetic Correlates of Weight Loss After Bariatric Surgery. OBES SURG 31, 4612–4623 (2021). https://doi.org/10.1007/s11695-021-05585-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05585-6