Abstract
Background
To evaluate the differences in 24-h urine profiles, radiographic imaging, and stone events post-Roux-en-Y gastric bypass versus sleeve gastrectomy in patients with a history of nephrolithiasis.
Methods
A retrospective review was conducted on 102 patients with a history of nephrolithiasis who then underwent bariatric surgery at our tertiary academic center. Computed tomography imaging and 24-h urine profile values were performed pre-operatively and at 1-year follow-up.
Results
A total of 60 patients underwent Roux-en-Y gastric bypass and 42 had sleeve gastrectomy. The Roux-en-Y gastric bypass group had significant increases in oxalate and decreases in citrate (p = 0.009 and 0.003, respectively), while the sleeve gastrectomy group had decreases in oxalate and stable citrate (p = 0.013 and 0.906, respectively). Roux-en-Y gastric bypass was the only significant predictor of post-operative hyperoxaluria (OR 7.1 [95% CI 2.3–21.3], p = 0.001). Radiographically, 38.3% of the Roux-en-Y gastric bypass group and 26.2% of the sleeve gastrectomy group had an increase in stone burden, and post-operative stone procedure rate was 10.0% and 7.1%, respectively.
Conclusions
At 1-year post-bariatric surgery, patients who underwent Roux-en-Y gastric bypass had exacerbated lithogenic urinary profiles, while those in sleeve gastrectomy patients improved. Although not statistically significant, stone burden increase and stone procedure rate were higher post-Roux-en-Y gastric bypass and will likely worsen at a longer follow-up due to the group’s lithogenic 24-h urine profiles. These findings support pre-bariatric counseling and urinary monitoring in patients with a history of kidney stones who undergo RYGB, with a multi-disciplinary approach between urologists and general surgeons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is the most effective treatment for patients with morbid obesity (BMI > 30 kg/m2) [1]. Rates of bariatric procedures have increased over the past several years due to their efficacy in sustained weight loss and reduction of obesity-related comorbidities [2, 3]. The two most common types of bariatric surgery are Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) [3].
The propensity for developing nephrolithiasis post-bariatric surgery is an important pre-operative clinical consideration. Studies have shown that the malabsorptive properties of RYGB lead to the development of lithogenic urinary profiles [4,5,6,7,8,9,10], as well as de novo kidney stone formation [11,12,13]. This is in contrast to the SG, in which studies show that patients may have improved urinary profiles and a decreased risk of stone formation when compared to RYGB and obese controls [14,15,16].
These previous studies have focused on populations that are kidney stone naïve; and, to our knowledge, there are no data on stone formation or urinary changes in patients with a history of nephrolithiasis who then undergo bariatric surgery. It is vital to understand changes within this patient subpopulation that are at a high risk of post-operative stone formation. The purpose of this study was to evaluate, at 1-year follow-up, any differences in post-operative 24-h urine (24HU) values, radiographic imaging, and stone events in patients with a documented history of kidney stones undergoing RYGB versus SG.
Materials and Methods
Data Extraction
Following approval from our institutional research ethics board, clinical data were retrospectively queried for patients who had undergone either laparoscopic RYGB or SG at our academic center. Patients were included if they had a history of nephrolithiasis and completed the pre- and post-operative urological investigations detailed below. A history of nephrolithiasis was defined as spontaneous passage of a kidney stone, presence of kidney stones on imaging, and/or a history of a stone procedure. Patients were excluded if they had known comorbidities that predisposed them to nephrolithiasis such as inflammatory bowel disease, metabolic bone disease, any type of malignancy, gout, recurrent pyelonephritis, and/or any previous bowel surgery that included removal of the terminal ileum, stomach, and/or small bowel. A total of 102 eligible patients were included in the final analysis.
Patients underwent urological assessment prior to surgery that included nephrolithiasis history, 24HU profiles, bloodwork, and imaging. Patients were weighed for BMI calculations (kg/m2). 24HU profiles included volume, pH, urate, sodium, phosphate, citrate, and calcium, while serum levels included creatinine, urate, and calcium. Baseline computed tomography imaging of the kidneys, ureters, and bladder (CT KUB) were conducted. At 1-year post-bariatric surgery follow-up, these assessments were repeated. These 24HU profiles are conducted routinely for recurrent stone formers at our center, and the use of CT imaging for stone surveillance is often necessary given the technical limitations of ultrasound and x-ray for the obese population.
Hyperoxaluria was defined as urinary excretion of oxalate ≥ 450 μmol/day, hypocitraturia as ≤ 1.6 mmol/day, and low urinary volume as ≤ 800 mL/day. Post-operative stone events were defined as renal colic confirmed by imaging, spontaneous or medical expulsion of a stone, and/or undergoing any stone procedure. Increase in stone burden was defined as a radiological development of a new stone and/or an increase in interval size of previously imaged stones.
Surgical Technique
RYGB and SG were performed by three bariatric surgeons using identical techniques. Indications for surgery were based on institutional guidelines that included a BMI ≥ 40 or a BMI ≥ 35 with at least one of the following: coronary artery disease, type II diabetes mellitus, hypertension, sleep apnea, and gastroesophageal reflux disease. SG was indicated for patients with anemia, gastric ulcers, inflammatory bowel disease, diverticular disease, and/or high BMI (BMI ≥ 40) [17]. RYGB consisted of the creation of a 15–20-mL gastric pouch, a 100-cm Roux limb, and a 50-cm biliopancreatic limb. The SG involved gastric volume reduction of 75–80% by resecting the stomach alongside a 30-French endoscope beginning 3 cm from the pylorus and ending at the angle of His.
Diet Recommendations
Per institutional protocol, patients were required to meet with a dietitian 4–5 times during the first post-operative year. Recommendations included daily consumption of at least 2 L of water, 400–800 IU of vitamin D, 1200 mg of calcium citrate, 2000–4000 IU of calcium, 1000 μg of vitamin B12, and avoidance of high oxalate foods [18].
Statistical Analysis
Descriptive data were compared between surgical groups. Student’s t tests (2-tailed, paired) were used to compare means between groups, and chi-square tests to compare proportions. Wilcoxon’s signed-rank sum test (2-tailed) was used for the comparison of pre- and post-operative 24HU values. Multivariate binary logistic regression determined predictors of post-operative hyperoxaluria and development of new stones. Covariates were chosen a priori based on other similar papers [12]. Chi-square tests compared abnormal post-operative urinary states, radiologically determined stone development, and stone event frequency between groups. The α-level was set at 0.05 for statistical significance. Analysis was performed using commercially available software (IBM SPSS Statistics version 25.0., Armonk, NY).
Results
Demographic and Baseline Data
A total of 102 patients with a documented history of nephrolithiasis underwent bariatric surgery at our center between October 2013 and June 2019 (60 RYGB and 42 SG). The SG group was slightly older (53.6 years and 49.2 years, respectively; p = 0.016) and had a higher baseline BMI (51.0 kg/m2 and 47.1 kg/m2, respectively; p = 0.012) (Table 1). Both groups had similar proportions of patients with a history of stone procedures and stones present during the bariatric surgery (p = 0.799 and p = 0.704, respectively). Baseline serum and 24HU profiles did not differ (p = 0.105–0.779) (Appendix Table 5). There were no differences in follow-up periods (RYGB 12.4 ± 1.9 months and SG 12.5 ± 1.8, p = 0.654).
Post-operative Serum Levels and 24HU Profiles
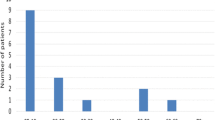
Both groups had significant decreases in serum urate and parathyroid hormone (PTH), as well as 24HU volume, urate, sodium, and phosphate (p = < 0.001–0.008) (Table 2). There were slight decreases in serum creatinine and 24HU calcium in the RYGB group (p = < 0.001 and 0.003, respectively). However, the RYGB group had worsened lithogenic urinary profiles when compared to their baseline 24HU. Specifically, the RYGB group had significant increases in oxalate and a decrease in citrate (respectively, p = 0.009 and 0.003). In contrast, the SG group had a significant decrease in oxalate and stable citrate (respectively, p = 0.013 and 0.906). There was a higher proportion of patients with post-operative hyperoxaluria in the RYGB group versus SG (23/47 (48.9%) and 2/37 (5.6%), respectively; p < 0.001) (Fig. 1). Additionally, there were more patients with hypocitraturia in the RYGB group versus SG (11/47 [23.4%] and 3/35 [8.6%], respectively; p = 0.034). There was no difference in the frequency of post-operative low urinary volume between groups (p = 0.633). On multivariate analysis, RYGB was the only significant predicator for post-operative hyperoxaluria (OR 7.1 [95% CI: 2.3–21.3]; p = 0.001) (Table 3).
Radiographic Findings and Post-operative Stone Events
In the RYGB group, 16/60 (26.7%) of patients developed de novo stones and 9/60 (15.0%) had interval growth of stones, with a total of 23/60 (38.3%) of patients having an increase in overall stone burden (Table 4). Regarding stone events, 3/60 (5.0%) had spontaneously passed a stone and 6/60 (10.0%) had a stone procedure. In the SG group, 10/42 (23.8%) of patients developed de novo stones and 5/42 (11.9%) had interval growth of stones previously present, with 11/42 (26.2%) demonstrating an increase in stone burden. With respect to stone events, 3/42 (7.1%) had a stone procedure. There were no significant differences in the rate of stone procedures between groups; although the RYGB group had a higher proportion of stone burden increase when compared to the SG group, this was not statistically significant (23/60 [38.3%] versus 11/42 [26.2%], respectively; p = 0.143). On multivariate analysis, the presence of stones at the time of bariatric surgery was the only significant predicator for post-operative formation of nephrolithiasis (OR 3.1 [95% CI: 1.1–8.4]; p = 0.027) (Table 3).
Weight Loss
Patients who underwent RYGB had higher rates of weight loss at 1-year post-bariatric surgery when compared to the SG group (measured via change from baseline BMI), respectively − 16.7 ± 4.7 kg/m2 and − 14.0 ± 5.2 kg/m2 (p < 0.015).
Discussion
Bariatric surgery is a well-established and effective treatment for morbid obesity [1]. Unfortunately, there is an association with post-operative nephrolithiasis development in kidney stone–naïve patients [8]. To our knowledge, this is the first study to investigate this association in patients with a pre-existing history of kidney stones prior to bariatric surgery. We compared 24HU changes, radiographic imaging, and frequency of stone events in patients with a history of kidney stones who underwent either RYGB or SG.
Our data showed, at 1-year post-bariatric surgery, that patients with a history of nephrolithiasis who underwent RYGB had exacerbated lithogenic 24HU profiles, while 24HU profiles in SG patients improved (Table 2). Post-operative hyperoxaluria was significantly higher in patients who had RYGB versus those who had SG, 48.9% and 5.6%, respectively (p = < 0.001). Both groups had similar rates of post-surgical stone procedures; however, we hypothesize that the RYGB patients will experience increased stone development at follow-up beyond 1 year due to the presence of post-operative hyperoxaluria. These findings support a need for urological assessment and dietary counseling, along with close dietary and urinary monitoring in patients with a history of kidney stones who undergo RYGB.
In our cohort, patients who underwent RYGB had increased 24HU oxalate and decreased 24HU citrate and volume (Table 2). These changes are likely due to the malabsorptive characteristics of RYGB, leading to an accumulation of intra-luminal free fatty acids that saponify calcium ions and decrease levels of unbound calcium. Intestinal oxalate, which is usually bound by calcium, is then more readily absorbed, which subsequently leads to hyperoxaluria [12]. Secondly, low urinary citrate develops due to intestinal bicarbonate loss from malabsorption, along with secondary acidosis [5, 19, 20]. Third, a decreased gastric reservoir and intestinal absorption subsequently decreases the amount of fluid absorption, leading to low urinary outputs [21]. These lithogenic urinary changes are accurately assessed with a 24HU metabolic evaluation, a commonly utilized test for excreted urine metabolites. In fact, this assessment is the first step in the medical management of nephrolithiasis in recurrent stone formers, and provides the physician insight on a patient’s diet, water intake, and gastrointestinal and renal absorption characteristics [22].
These findings of post-RYGB lithogenic urinary profiles are consistent with those of the literature on kidney stone–naïve patients [4,5,6,7, 9, 10, 23]. Park et al. performed a study on 45 kidney stone–naïve patients who underwent RYGB and found that at 1-year post-bariatric surgery, patients developed increased urine oxalate and decreased citrate and urinary volume [5]. Valezi et al. also reported hyperoxaluria, hypocitraturia, and low urine volume in their cohort of 151 RYGB patients. These studies’ reported post-operative 24HU oxalate levels are similar to those in our RYGB cohort (both resulted in a median oxalate excretion of 444 μmol/day) and suggest that even with dietary counseling, patients undergoing RYGB may still develop hyperoxaluria [5, 10]. Overall, our study provides evidence of this lithogenic relationship also occurring in patients with a pre-operative history of kidney stones who undergo RYGB.
Patients who underwent SG had improved urinary profiles from baseline, with decreases in urinary oxalate (Table 2). This procedure, although inducing similar alterations to gastrointestinal hormones as the RYGB, does not lead to intestinal oxalate hyperabsorption [21, 24]. Reported data on post-SG nephrolithiasis risk are lacking, and we present the largest cohort to our knowledge that examines urinary changes post-surgery. There is only one other small study, by Semins et al., that examined urinary changes of 18 kidney stone–naïve patients who underwent either gastric banding (n = 14, a pure restrictive procedure) or SG (n = 4). They found that at 1-year follow-up, urinary oxalate was significantly lower compared to RYGB controls [16]. These findings are further supported by Penniston et al. who also found higher proportions of hyperoxaluria in RYGB patients compared to gastric banding only [20]. Our larger cohort of patients (n = 40) had similar results, which suggests that urinary oxalate levels decrease after SG regardless of pre-operative stone history.
Although the RYGB cohort had exacerbated lithogenic 24HU profiles, there was no statistically significant difference in rates of stone events or stone procedures between groups (Table 4). At 1-year post-operative follow-up, the procedure rates for the RYGB and SG group were 10.0% and 7.1%, respectively. However, the frequency of procedures in both groups was higher than what has been previously reported in the literature. Matlaga et al. conducted a large retrospective study of 4639 patients who underwent RYGB and found that in 3 years, only 3.3% of their population underwent a urological procedure post-bariatric surgery [12]. Furthermore, Chen and colleagues found that post-SG patients had a low rate of stone formation, with a person-time stone incidence rate of 5.25, meaning that for every 1000 SGs, only 5.25% patients developed nephrolithiasis [15]. These aforementioned studies examined populations with the majority of the sample size being kidney stone naïve, and may explain our discordant findings. Our study’s higher procedural rates may suggest that the underlying history of kidney stones predisposes patients to increased rates of stone treatment in the immediate post-operative period. We hypothesize that the hyperoxaluria found in our RYGB cohort may compound these effects, and the difference in stone events may be more apparent at a longer follow-up (greater than 1 year).
There are some limitations to this study. First, utilization of one 24HU collection may have a risk of aberrant urine values not representative of a patient’s urinary profile. However, the difficulty of obtaining 24HU profiles is well established, and requesting multiple collections is challenging. Second, due to the retrospective nature of the study, there may be asymptomatic patients who were not motivated to seek follow-up appointments. Third, with this study design, patients are not randomized to treatment groups, which is associated with inherent selection bias and differences in baseline characteristics. Furthermore, although nutrition guidelines were consistent, the actual diet consumed is a variable that cannot be controlled for in this study design. Finally, stone analysis data of the patients that underwent operative procedures were not available for extraction.
Conclusions
In conclusion, this is the first study to evaluate urinary changes and nephrolithiasis risk in patients with a pre-existing history of kidney stones who undergo either RYGB or SG. We found that at 1-year follow-up, RYGB patients had exacerbated lithogenic urinary profiles, while urinary profiles in SG patients improved. Although not statistically significant, stone burden increase and stone procedure rate were higher post-RYGB and will likely worsen at a longer follow-up due to the group’s post-operative lithogenic 24HU. These findings support pre-bariatric counseling and close urinary monitoring in patients with a history of kidney stones who undergo RYGB, with a multi-disciplinary approach between urologists and general surgeons.
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62–70.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27:1–11.
Nelson WK, Houghton SG, Milliner DS, et al. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1(5):481–5.
Park AM, Storm DW, Fulmer BR, et al. A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J Urol. 2009;182(5):2334–9.
Duffey BG, Alanee S, Pedro RN, Hinck B, Kriedberg C, Ikramuddin S, et al. Hyperoxaluria is a long-term consequence of Roux-en-Y gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg. 2010; 1;211(1):8–15.
Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177(2):565–9.
Wu JN, Craig J, Chamie K, et al. Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg Obes Relat Dis. 2013;9(1):83–7.
Agrawal V, Liu XJ, Campfield T, et al. Calcium oxalate supersaturation increases early after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2014;10(1):88–94.
Valezi AC, Fuganti PE, Junior JM, et al. Urinary evaluation after RYGBP: a lithogenic profile with early postoperative increase in the incidence of urolithiasis. Obes Surg. 2013;23(10):1575–80.
Durrani O, Morrisroe S, Jackman S, et al. Analysis of stone disease in morbidly obese patients undergoing gastric bypass surgery. J Endourol. 2006;20(10):749–52.
Matlaga BR, Shore AD, Magnuson T, et al. Effect of gastric bypass surgery on kidney stone disease. J Urol. 2009;181(6):2573–7.
Lieske JC, Mehta RA, Milliner DS, et al. Kidney stones are common after bariatric surgery. Kidney Int. 2015;87(4):839–45.
Thongprayoon C, Cheungpasitporn W, Vijayvargiya P, et al. The risk of kidney stones following bariatric surgery: a systematic review and meta-analysis. Ren Fail. 2016;38(3):424–30.
Chen T, Godebu E, Horgan S, et al. The effect of restrictive bariatric surgery on urolithiasis. J Endourol. 2013;27(2):242–4.
Semins MJ, Asplin JR, Steele K, et al. The effect of restrictive bariatric surgery on urinary stone risk factors. Urology. 2010;76(4):826–9.
Ontario Bariatric Network. Surgical program description [Internet]. OBN. [cited 2019 Aug 11]. Available from: https://www.ontariobariatricnetwork.ca/our-programs/surgical-program. Accessed 11 Aug 2019.
St. Joseph’s Healthcare Hamilton. Bariatric surgery – gastric bypass and vertical sleeve gastrectomy [Internet]. [cited 2019 Aug 11]. Available from: https://www.stjoes.ca/patients-visitors/patient-education/patient-education-a-e/pd-6000-bariatric-surgery-gastric-bypass-2017-june.pdf. Accessed 11 Aug 2019.
Maalouf NM, Tondapu P, Guth ES, et al. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol. 2010;183(3):1026–30.
Penniston KL, Kaplon DM, Gould JC, et al. Gastric band placement for obesity is not associated with increased urinary risk of urolithiasis compared to bypass. J Urol. 2009;182(5):2340–6.
Sakhaee K, Griffith C, Pak CYC. Biochemical control of bone loss and stone-forming propensity by potassium-calcium citrate after bariatric surgery. Surg Obes Relat Dis. 2012;8(1):67–72.
Dion M, Ankawi G, Chew B, et al. CUA guideline on the evaluation and medical management of the kidney stone patient–2016 update. Can Urol Assoc J. 2016;10(11–12):E347.
Park S, Kim YJ, Choi CY, et al. Bariatric surgery can reduce albuminuria in patients with severe obesity and normal kidney function by reducing systemic inflammation. Obes Surg. 2018;28(3):831–7.
Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg. Endosc. 2012; 1;26(8):2231–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study (retrospective), formal consent is not required. Formal ethical approval by the Hamilton Integrated Research Ethic Board was obtained prior to study initiation (HIREB: 1058).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Uy, M., Di Lena, R., Hoogenes, J. et al. Bariatric Surgery in Patients with a History of Nephrolithiasis: 24-h Urine Profiles and Radiographic Changes After Roux-en-Y Gastric Bypass Versus Sleeve Gastrectomy. OBES SURG 31, 1673–1679 (2021). https://doi.org/10.1007/s11695-020-05178-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-05178-9