Abstract
Background
Resistance training (RT) and adequate protein intake are recommended as strategies to preserve fat-free mass (FFM) and resting metabolic demand after bariatric surgery. However, the effect of both interventions combined in the late postoperative period is unclear. This study investigated the effects of RT, isolated and combined with protein supplementation, on body composition and resting energy expenditure (REE) in the late postoperative period of Roux-en-Y gastric bypass (RYGB).
Methods
This controlled trial involved patients who were 2–7 years postRYGB. Participants were partially matched on body mass index (BMI), age, sex, and years after surgery, and divided into four groups, placebo maltodextrin (control [CON]; n = 17), whey protein supplementation (PRO; n = 18), RT combined with placebo (RTP; n = 13), and RT combined with whey protein supplementation (RTP + PRO; n = 15)—considering the participants who completed the protocol. REE was measured by indirect calorimetry and body composition by multifrequency electrical bioimpedance.
Results
Participant characteristics (40.3 ± 8.3 years old; average BMI 29.7 ± 5.3 kg/m2; 88.9% females) were similar among groups. The RTP+PRO group showed an increase of 1.46 ± 1.02 kg in FFM and 0.91 ± 0.64 kg in skeletal muscle mass (SMM), which was greater than the equivalent values in the CON group (− 0.24 ± 1.64 kg, p = 0.006 and − 0.08 ± 0.96 kg, p = 0.008, respectively). There was no significant time-by-group interaction for absolute or relative REE.
Conclusion
Combined RT and adequate protein intake via supplementation can increase FFM and SMM in the late postoperative period without changing REE. These associated strategies were effective in improving muscle-related parameters and potentially in improving the patients’ physical function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Roux-en-Y gastric bypass (RYGB) frequently helps in achieving satisfactory weight loss, improved quality of life, and better control of comorbidities in patients with severe obesity [1,2,3,4]. However, in the second year after RYGB, many patients have difficulty in maintaining a low weight [5], which is associated with reduced adherence to systematic clinical monitoring [6,7,8]. Adoption of healthy lifestyle habits is paramount to long-term satisfactory excess weight loss after bariatric surgery [9, 10].
In individuals undergoing bariatric surgery, exercise has consistently been shown to provide positive effects, focusing on body weight, physical fitness, and cardiovascular health [11,12,13,14]. Meanwhile, training effects on resting energy expenditure (REE) remain poorly understood. REE is believed to be positively related to body mass, particularly fat-free mass (FFM) [15], to reduce abruptly in the first 6–12 months after bariatric surgery [16], and to remain decreased two years after that [17]. Therefore, in addition to bodyweight monitoring, information on alterations to body composition after bariatric surgery could provide insights for improved long-term management of patients’ postRYGB [18].
Resistance training (RT) improves body composition and increases FFM in diverse clinical populations, including those with obesity [19, 20]. The effects of RT, either isolated [21, 22] or combined with other types of exercise [23,24,25], have been studied during the first 2 years after bariatric surgery [21,22,23,24,25], but they remain unclear [14].
In addition to RT, adequate protein intake is essential for maintaining FFM and for avoiding a positive energy balance in patients undergoing RYGB because FFM is one of the most metabolically active compartments in the body. Relevant studies indicate that a protein intake ≥ 60 g/day is positively associated with better FFM preservation [26, 27]. However, protein intake is usually lower than recommended in patients who underwent bariatric surgery [28,29,30] owing to the reduction of the overall food consumption or because of intolerance to the protein sources present in food [29, 30]. Thus, high-quality protein supplements have been used to help achieve minimum intake recommendations. Whey protein is an example of a high-quality supplement that is easy to digest, quick to absorb [31, 32], and rich in all essential amino acids. Whey protein enhances muscle protein synthesis and can contribute to training-induced muscular hypertrophy [32,33,34]; hence, this is considered the first choice of protein supplementation in clinical practice.
To our knowledge, the only clinical trial that evaluated the effect of RT combined with protein supplementation found no changes in the body composition of women in the first 6 months after bariatric surgery [35]. However, the effect of these interventions combined with long-term postsurgery is yet to be investigated. Given that it is important to investigate effective and feasible strategies for the improvement of muscle-related parameters in patients who are at risk of weight regain and have low adherence to systematic clinical monitoring over time, this study aimed to investigate the effects of RT with and without whey protein supplementation on body composition and REE in the late postoperative period of RYGB.
Materials and Methods
Study Design
This study was part of the Nutrition and Resistance Exercise in Obesity (NERO) project, which was a controlled clinical trial with parallel groups. This study was registered (RBR-9k2s42) with the Brazilian Clinical Trials Registration Platform (ReBEC). The study was approved by the local Research Ethics Committee.
Participants
Adult individuals of both sexes who were at 2–7 years postRYGB were invited to participate through posters and social media announcements.
Patients with diabetes mellitus, heart disease, hormones or appetite regulator use, severe psychiatric disorders, recent elective surgery, and current pregnancy or breastfeeding were excluded. Individuals who used protein supplementation regularly and those who had been engaging in physical exercise since at least 2 months before the study were also excluded. To calculate the desired sample size, an effect size of 0.8 (a large effect size, according to Cohen [36]) was considered as indicative of a significant difference between the two groups. This calculation considered a level of significance of 5% and power of 80%, resulting in a minimum sample of 13 individuals in each group, with a total of 52 participants required for the study. Considering the high dropout rate found in this type of study, wide announcement of the study was made, and finally 119 participants were assessed after the exclusion criteria were applied.
Study Protocol
Allocation
Participants were paired according to body mass index (BMI), age, sex, and years after surgery and allocated to two separate groups that would or would not perform RT considering their ability to reach the place where the study was being conducted. Briefly, matched sets were created, bringing together four participants with similar characteristics. As the groups of participants were formed over time, the sets were filled in whole or in part, based on the characteristics of each set. Thus, the gaps observed in some matching sets were filled as new groups of volunteers were formed whenever possible, but it was not possible to guarantee the same number of participants in all groups.
Allocation to receive a protein supplement or placebo followed a randomized, double-blind procedure performed by an external researcher using the Research Randomizer® online software, version 4.0 (http://www.randomizer.org/). The protocol comprised two combined or isolated interventions, each lasting 12 weeks.
All assessments were performed before and after 12 weeks of the intervention, except for food intake, which was also evaluated at 6 weeks.
Sociodemographic and Clinical Characteristics
Sociodemographic and clinical data were collected with a questionnaire that included closed and open questions regarding sex, date of birth, education level (in years of study), and surgery date (in months and years).
Resistance Training Intervention
Volunteers allocated to either RTP or RTP+PRO took part in a 12-week RT program, performed thrice per week on nonconsecutive days. Before the training period, participants received three familiarization sessions to ensure that they had properly understood the technique. The training targeted all major muscle groups and involved the following exercises: chest press, knee extension, hamstring curl, leg press, hip abduction, latissimus pulldown, shoulder abduction, and plantar flexion (Rotech® Fitness Equipment, Brazil). Training loads were monitored and carefully adjusted using the OMNIResistance Exercise Scale (OMINI-RES) [37]. During the first 4 weeks of the training period, loads were carefully adjusted to correspond to 6 points on the OMINI-RES scale (“somewhat hard”), to 7 points (from “somewhat hard” to “hard”) during the next 4 weeks, and to 8 points (“hard”) in the last 4 weeks; repetitions were decreasing from 12 to 10 and 8, respectively, throughout the training period. Each exercise was performed in three sets with approximately 1-min breaks between sets. Each session lasted approximately 60 min and was preceded by a 10-min warm-up and followed by a 10-min cooldown. In order to assess the effectiveness of the RT program, knee extension isokinetic peak torque at 60°/s was measured before and after the intervention period using an isokinetic dynamometer (Biodex System 3®, Biodex Medical Systems, Inc., Shirley, NY, USA). All training sessions were closely supervised by qualified professionals. During the intervention period, participants were instructed to retain their usual physical activities and to refrain from joining any other exercise programs. Attendance at the training sessions was recorded; attendance < 70% was considered loss of follow-up.
Nutritional Intervention
The nutritional intervention consisted of whey protein supplementation or placebo (Maltodextrin). A concentrated 30-g portion of whey protein powder included the following: 120 kcal, 1.80 g of carbohydrates, 1.38 g of total fats, 23.10 g of proteins (5.61 g of branched-chain amino acids [BCAA] and, of these, 2.70 g of leucine), while a 30-g portion of Maltodextrin included 112 kcal and 28 g of carbohydrates.
Whey protein supplements or placebo portions were provided every 15 days, delivered in opaque packaging in the form of sachets, containing the amount corresponding to each individual daily dose. Participants were instructed to consume the entire dose at once with the last meal of the day. Empty packaging and sachets not used were returned to the researchers during scheduled consultations and duly registered.
Whey protein supplementation was prescribed at a dose of 0.5 g/kg of ideal body weight/day to the PRO and RTP+PRO groups. Maltodextrin was offered as a placebo to the CON and RTP groups at the same dose. A supplement or placebo intake < 70% was considered a loss to follow-up. All participants received general training on healthy eating.
Anthropometry and Body Composition Assessment
Anthropometric and body composition assessments were performed in the morning with the participant standing barefoot and wearing light clothing. Height measurement was performed using a portable stadiometer (Sanny®, American Medical of Brazil). Bodyweight and body composition were determined using a multifrequency bioelectrical impedance analysis (BIA) (InBody720®, Biospace, Korea). Participants were requested to refrain from physical activity and caffeine consumption in the 24 h before the exam; participants were also asked to fast for at least 8 h beforehand, present at a time outside a menstrual period, as relevant, and have an empty bladder. The variables obtained were body weight (BW; kg), FFM (kg), skeletal muscle mass (SMM; kg), fat mass (FM; kg), and the percentage of body fat (BF; %). Body weight and height were used to calculate BMI (kg/m2). Excess weight loss (EWL) and total weight loss (TWL) were considered satisfactory when greater than 50% and 20% [38], respectively. Weight regain was defined as weight gain greater than 10% of the lowest weight recorded during the postoperative period, and the ideal body weight was calculated using BMI equivalent to 25 kg/m2.
Resting Energy Expenditure Measurement
REE was analyzed by an open-circuit metabolic system IC (Vmax 29®, Sensor Medic Corp, Yorba Linda, CA, USA), as described elsewhere [39, 40]. In the morning before the test, participants remained at rest for 10 min in a supine position under thermoneutral conditions (22–24 °C) in a quiet and dimly lit room. They were instructed to remain awake, keep quiet, avoid hyperventilation and sudden movements, and breathe for 30 min through a transparent plastic ventilated canopy placed over the head. Oxygen and carbon dioxide sensors were calibrated using a reference mix of gases of known composition before each test. The average of measurements acquired in the last 20 min was used to determine the REE based on the Weir formula [41]; REE was expressed as kcal/day. A “steady state” was defined as a period when the average consumption of O2 and production of CO2 varied by less than 10% [42]. The variables thus obtained were absolute REE (kcal/day), relative REE by BW (REE/BW; kcal/kg), relative REE by FFM (REE/FFM; kcal/kg), and respiratory quotient (RQ).
Blood Parameters
Participants were instructed to undergo blood tests in the same week as the other tests. Blood samples were collected in the morning by qualified professionals, after an overnight fast of 8–14 h and measured by standard procedures. Levels of the following parameters were analyzed: serum albumin (colorimetric bromocresol green method), blood glucose (hexokinase method), basal insulin (chemiluminescence method), creatinine (amidinohydrolase/oxidase method), and lipid profile, including levels of total cholesterol (esterase/oxidase method), triglycerides (oxidase/peroxidase method), HDL-c (direct method), and LDL-c (calculated by Martin’s formula [43]). Homeostatic model assessment of insulin resistance and beta-cell function (HOMA-IR and HOMA-β) were calculated by standard formulas [44].
Dietary Intake
Energy and protein intake were analyzed using multiple 24-h dietary recalls (24hR) at baseline and after 6 and 12 weeks of intervention. At each evaluation point, two 24hR were made on non-consecutive days according to the 5-step multiple-pass method [45]. Data were analyzed using the Nutrition Data System for Research (NDSR®) software, version 2018 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, USA). Participants’ usual food consumption, with correction for intrapersonal variance in the complete sample [46], was performed at each of the follow-up assessments with PC-SIDE® software, version 1.0 (Iowa State University, Ames, IA, USA). Energy and protein intake were reported as absolute values and adjusted for the current and/or ideal BW.
To assess total protein intake during the follow-up period, the evaluation point at 6 weeks was chosen to analyze both dietary protein intake and protein supplementation. In addition, mean protein supplementation intake during the entire intervention was described in absolute values/day.
Data Analysis
Categorical variables were presented as frequencies and compared between groups with the chi-square test. Continuous variables were expressed as mean and standard deviation. To verify data distribution normality assumption, the Kolmogorov–Smirnov test was applied. A comparison between participants who completed the protocol and those who left the study was performed using the Student t test or the Mann–Whitney U test, as appropriate. Baseline among-group comparisons were performed with one-way ANOVA with Tukey’s post hoc test or the Kruskal–Wallis H test followed by Müller–Dunn post-hoc test, as appropriate. The effects of isolated and combined interventions were analyzed by testing the interaction effect in a two-way mixed ANOVA test with repeated measures, considering time as an intraindividual factor and group as an interindividual factor. A p value < 0.05 was indicative of statistical significance. All analyses were performed using the SPSS software, version 24.0 (IBM Corp., Armonk, NY, USA).
Results
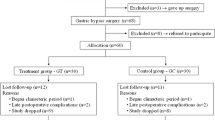
Among the 119 participants included in the study, a total of 63 completed the entire study protocol (Fig. 1). There was no difference in demographic or clinical characteristics between participants who completed (n = 63) and those who left the study (n = 56). The baseline characteristics of participants were similar among groups. Almost 90% of the participants were female, with a mean age of approximately 40 years old and BMI close to the upper limit of overweight classification. Participants exhibited adequate weight loss; however, approximately half of them showed weight regain, on average, 4 years after surgery (Table 1).
Flowchart capturing participant allocation process, sample randomization, and drop-out rates at each study stage. Abbreviations: RYGB: Roux-en-Y gastric bypass; BMI: Body mass index; CON: Control; PRO: Whey protein supplementation; RTP: Resistance training program. External illness/accident: car accident, musculoskeletal problems, diseases in the family, gout. Total sample loss n = 56
Mean adherence to the RT program was above 80%, with no difference between groups with this intervention. The adherence rates of protein supplementation and placebo were similar among the groups and above 90%.
Usual energy and protein intake were similar among the groups, except for a higher absolute protein intake of the PRO group than that of the CON group at 12 weeks (p = 0.031). The groups that received protein supplement had a usual protein intake approximately 30 g higher than placebo groups (p < 0.001), including after adjustment for the current and ideal weight (Table 2). Average levels of blood parameters were within normal reference ranges for all groups, without among-group differences (Table 3).
For anthropometric, body composition, and strength parameters, ANOVA demonstrated significant time-by-group interactions. Post hoc analyses revealed significant increases in the RTP+PRO group, relative to the CON group, observed for BW (p = 0.012), BMI (p = 0.012), FFM (p = 0.006), and SMM (p = 0.008). A significant post hoc analysis was also noted for knee extension isokinetic peak torque. These results were driven by significant improvements in the groups subjected to the RT program, and these results were not seen in the nonexercise groups (RTP: p = 0.001 and p < 0.001; RTP+PRO: p = 0.011 and p = 0.005; when compared to CON and PRO, respectively). There was no significant time-by-group interaction for absolute or relative REE, nor RQ (Table 4).
Discussion
To our knowledge, this is the first clinical trial that evaluated the effects of RT, separate or combined with protein supplementation, on body composition, relative REE, and blood parameters during the late postoperative period of RYGB. In patients in the “late” postRYGB period, RT combined with whey protein supplementation was associated with increased FFM. Although patients in the RTP group also exhibited an absolute mean increase in FFM, this change was not statistically significant.
Typically, 2 years postsurgery, patients achieve weight stabilization, and weight regain is often observed [47, 48]. In the present study, despite meeting the success criteria after surgery, as noted by EWL and TWL, weight regain was observed in about half of the sample. These findings might be due to participants not using protein supplementation and practicing physical exercise for at least two months, which were the inclusion criteria; they might also be associated with the end of adequate postoperative follow-up. Thus, at the beginning of the study, participants were not receiving any specific treatment, regardless of time after surgery.
The benefits of protein supplementation are illustrated by studies of patients undergoing bariatric surgery who do not reach the minimum recommended protein intake at any point during the postoperative period [28,29,30, 49]. Insufficient protein intake might be due to restricted overall food consumption imposed by the surgical procedure itself and characterized by intolerance or difficulty in consuming dairy products, fish, and red meat, even years after surgery [29, 30, 50]. In our study, energy and protein intake were similar for all groups, except for absolute protein intake of the PRO group, which was higher than that of the CON group after 12 weeks of intervention; this finding can be explained by an increased tolerance to protein, resulting from the intake of whey protein.
A protein intake of 60 g/day or 1.0–1.1 g/kg of ideal weight/day was associated with greater weight loss, lower BF, and better preservation of FFM after bariatric surgery in previous reports [26,27,28]. When associated with low energy intake, the consumption of approximately 1.5 g of protein/kg of ideal body weight can attenuate FFM loss, according to clinical practice guidelines [9]. Gomes et al. [51] evaluated the effect of whey protein supplementation at a dose similar to that used in the current investigation combined with a low-calorie diet on the weight loss and body composition of women with weight regain in the late postoperative period of RYGB. The authors observed that the intervention group demonstrated greater weight and FM losses than the control group, thus indicating the preservation of FFM. Therefore, optimal protein intake in the postoperative period of bariatric surgery until the moment defined by the consumption of approximately 1.5 g of protein/kg of ideal body weight should be encouraged because it can improve the evolution of patients’ body composition.
There is no currently established physical training protocol for the different follow-up stages after bariatric surgery. Training protocols differ in the type, volume, and duration of intervention, thus resulting in the heterogeneity of evidence included in systematic reviews [11, 14, 52]. The efficacy of our RT protocol was indicated by the improvements in isokinetic muscle strength observed in the exercise groups. Daniels et al. [21] and Huck et al. [22] applied RT protocols similar to those used in the present study; however, they did not find differences in FFM because they evaluated patients during the first postoperative year, which is a period marked by rapid and massive weight loss. Nevertheless, they found improvements in muscle strength and quality, thus demonstrating that RT has positive effects that go beyond body composition.
A metaanalysis of the effect of whey protein supplementation alone or combined with RT on body composition of adult individuals who had not undergone surgery has been reported [53]. Despite between-study heterogeneity, the authors concluded that the use of supplements for improving body composition is evidence-based and that the impact of supplements is greater when their use is combined with RT [53]. This finding might explain why the best FFM results were observed in the RTP+PRO group in the present study. Morton et al. [54] conducted a metaanalysis on the effect of protein supplementation on RT-induced gains in muscle parameters. Although the sample included healthy rather than energy-restricted individuals, the authors demonstrated RT combined with protein supplementation was associated with an additional increase in FFM, compared with isolated RT. Nevertheless, protein intake beyond approximately 1.6 g/kg/day did not provide additional benefits.
RT promotes muscle mass gain [54] and has a potent effect on the mammalian target of rapamycin (mTOR) signaling [55]. This pathway is responsible for stimulating muscle protein synthesis and downregulation of catabolic processes through phosphorylation and activation of target proteins [33]. In addition, skeletal muscle adaptation can increase when RT is combined with protein supplementation [56], since its rapid digestibility promotes hyperaminoacidemia and high plasma bioavailability of essential amino acids, in particular, leucine in muscle tissue [31,32,33], inducing muscle hypertrophy.
To date, only one clinical trial has evaluated the effect of RT combined with protein supplementation on body composition and physical fitness in women after RYGB [35]. In this trial, RT started 6 weeks after surgery, lasted 18 weeks, and the dose of whey protein used was 48 g/day for all patients. The authors noted no between-group differences regarding FFM, but the group that received combined interventions showed an increase in the relative muscular strength of the lower limbs compared to the remaining groups.
Studies that have evaluated the effect of physical exercise, protein supplementation, or both on energy expenditure after bariatric surgery are scarce, and results are controversial. In the present clinical trial, no significant changes were observed in REE among the groups, which might be due to patients undergoing RYGB maintaining a greater mass of trunk organs with a high metabolic rate, such as heart, kidneys, and liver, even in the late postoperative period [18]. Although FM influences REE, FFM corresponds to the most metabolically active body component for organs with a high metabolic rate [57, 58] and, to a lesser extent, for SMM [59, 60]. As such, the increase in FFM, mostly due to the increase in SMM, observed in the RTP+PRO group was probably not enough to cause changes in absolute or relative REE. An intervention of a longer duration comparing different types and intensities of training might be required to investigate the impact of body composition changes on REE in this population. RQ values outside the range of 0.67–1.30 suggest flaws in the indirect calorimetry test, such as air leakage in the respiratory circuit, agitation, or severe pain [61, 62]. The obtained RQ values demonstrated that the test was adequate for determining REE.
Blood parameters did not change significantly in any of the groups over 12 weeks, although the RTP group showed a marginally significant improvement to insulin levels and HOMA-IR. Physical exercise improves insulin sensitivity and could provide additional improvements to the effects of RYGB [63,64,65] by increasing mitochondrial oxidative capacity and reducing the levels of lipid types that impair adequate insulin signaling [63]. It should be noted that present participant biochemical and metabolic profile at baseline showed values within the reference range, which may explain why the observed effects were small.
This study contributes to the development of feasible interventions related to clinical care practice in the late postoperative period of bariatric surgery, a period characterized by frequent discontinuity of follow-up.
Strengths and Limitations
This study has several strengths and limitations. RT was closely supervised, and the whey protein supplement was administered at individualized doses. The evidence presented here extends the knowledge regarding exercise and nutritional interventions in individuals undergoing long-term bariatric surgery, a population for which not enough data exist. However, whether the participants retained their baseline physical activity levels were not evaluated in the nonexercising groups, and this could affect our findings. Owing to logistic and scheduling reasons, participants in both RT groups were not allocated in a randomized manner. The relatively large loss to follow-up observed in this study, which arose from intervention dropouts and noncompliance with the postblood tests and other phases of the protocol, should be noted. Sample losses reduce the statistical power of the tests and may have prevented the detection of the other effects of the intervention. These losses in clinical trials emerge from multiple transportation requirements for the assessment and training visits, along with the daily difficulties experienced by the volunteers. However, despite the sample loss, the methodological attention employed and the statistical treatment applied for the remaining sample support the validity of our results.
Conclusion
RT combined with whey protein supplementation for 12 weeks increase FFM and SMM in patients 2–7 years postRYGB. Although RT alone elicited mean absolute improvements, these results were not statistically significant. Concurrently, RT with or without whey protein supplementation did not promote alterations in REE and blood parameters. These findings support the use of RT combined with protein supplementation in the long-term outpatient care of patients after bariatric surgery as an effective strategy for improving SMM, which is known to decline as a result of RYGB. Of note, the development of muscle-related phenotypes potentially leads to augmented functional performance and enhances patients’ ability to perform activities of daily living.
References
Golzarand M, Toolabi K, Farid R. The bariatric surgery and weight losing: a meta-analysis in the long- and very long-term effects of laparoscopic adjustable gastric banding, laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy on weight loss in adults. Surg Endosc. 2017;31:4331–45.
Hayoz C, Hermann T, Raptis DA, et al. Comparison of metabolic outcomes in patients undergoing laparoscopic Roux-en-Y gastric bypass versus sleeve gastrectomy - a systematic review and meta-analysis of randomised controlled trials. Swiss Med Wkly. 2018;148:w14633.
Ahmed B, King WC, Gourash W, et al. Long-term weight change and health outcomes for sleeve gastrectomy (SG) and matched Roux-en-Y gastric bypass (RYGB) participants in the longitudinal assessment of bariatric surgery (LABS) study. Surgery. 2018;164:774–83.
Duvoisin C, Favre L, Allemann P, et al. Roux-en-Y gastric bypass: ten-year results in a cohort of 658 patients. Ann Surg. 2018;268:1019–25.
Silva LB, Oliveira BMPM, Correia F. Evolution of body composition of obese patients undergoing bariatric surgery. Clin Nutr ESPEN. 2019;31:95–9.
Larjani S, Spivak I, Hao Guo M, et al. Preoperative predictors of adherence to multidisciplinary follow-up care postbariatric surgery. Surg Obes Relat Dis. 2016;12:350–6.
Higa K, Ho T, Tercero F, et al. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011;7:516–25.
Khorgami Z, Zhang C, Messiah SE, et al. Predictors of postoperative aftercare attrition among gastric bypass patients. Bariatr Surg Pract Patient Care. 2016;10:79–83.
Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures – 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16:175–247.
Mingrone G, Bornstein S, Le Roux CW. Optimisation of follow-up after metabolic surgery. Lancet Diabetes Endocrinol. 2018;6:487–99.
Ren ZQ, Lu GD, Zhang TZ, et al. Effect of physical exercise on weight loss and physical function following bariatric surgery: a meta-analysis of randomised controlled trials. BMJ Open. 2018;8:e023208.
Mundbjerg LH, Stolberg CR, Cecere S, et al. Supervised physical training improves weight loss after Roux-en-Y gastric bypass surgery: a randomized controlled trial. Obesity. 2018;26:828–37.
da Silva ALG, Sardeli AV, André LD, et al. Exercise training does improve cardiorespiratory fitness in post-bariatric surgery patients. Obes Surg. 2019;29:1416–9.
Bellicha A, Ciangura C, Poitou C, et al. Effectiveness of exercise training after bariatric surgery—a systematic literature review and meta-analysis. Obes Rev. 2018;19:1544–56.
Westerterp KR. Control of energy expenditure in humans. Eur J Clin Nutr. 2017;71:340–4.
Lamarca F, Melendez-Araújo MS, Porto de Toledo I, et al. Relative energy expenditure decreases during the first year after bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2019;29:2648–59.
Wolfe BM, Schoeller DA, McCrady-Spitzer SK, et al. Resting metabolic rate, total daily energy expenditure, and metabolic adaptation 6 months and 24 months after bariatric surgery. Obesity. 2018;26:862–8.
Heshka S, Lemos T, Astbury NM, et al. Resting energy expenditure and organ-tissue body composition 5 years after bariatric surgery. Obes Surg. 2020;30:587–94.
Benito PJ, Cupeiro R, Ramos-Campo DJ, et al. A systematic review with meta-analysis of the effect of resistance training on whole-body muscle growth in healthy adult males. Int J Environ Res Public Health. 2020;17:1–27.
Zouhal H, Abderrahman AB, Khodamoradi A, et al. Effects of physical training on anthropometrics, physical and physiological capacities in individuals with obesity: a systematic review. Obes Rev. 2020:1–34.
Daniels P, Burns RD, Brusseau TA, et al. Effect of a randomised 12-week resistance training programme on muscular strength, cross-sectional area and muscle quality in women having undergone Roux-en-Y gastric bypass. J Sports Sci Routledge. 2018;36:529–35.
Huck CJ. Effects of supervised resistance training on fitness and functional strength in patients succeeding bariatric surgery. J Strength Cond Res. 2015;29:589–95.
Herring LY, Stevinson C, Carter P, et al. The effects of supervised exercise training 12–24 months after bariatric surgery on physical function and body composition: a randomised controlled trial. Int J Obes. 2017;41:909–16.
Campanha-Versiani L, Pereira DAG, Ribeiro-Samora GA, et al. The effect of a muscle weight-bearing and aerobic exercise program on the body composition, muscular strength, biochemical markers, and bone mass of obese patients who have undergone gastric bypass surgery. Obes Surg. 2017;27:2129–37.
Hassannejad A, Khalaj A, Mansournia MA, et al. The effect of aerobic or aerobic-strength exercise on body composition and functional capacity in patients with BMI ≥35 after bariatric surgery: a randomized control trial. Obes Surg. 2017;27:2792–801.
Ito MK, Gonçalves VSS, Faria SLCM, et al. Effect of protein intake on the protein status and lean mass of post-bariatric surgery patients : a systematic review. Obes Surg. 2017;27:502–12.
Raftopoulos I, Bernstein B, O’Hara K, et al. Protein intake compliance of morbidly obese patients undergoing bariatric surgery and its effect on weight loss and biochemical parameters. Surg Obes Relat Dis. 2011;7:733–42.
Moizé V, Andreu A, Rodríguez L, et al. Protein intake and lean tissue mass retention following bariatric surgery. Clin Nutr. 2013;32:550–5.
Nicoletti CF, De Oliveira BAP, Barbin R, et al. Red meat intolerance in patients submitted to gastric bypass: a 4-year follow-up study. Surg Obes Relat Dis. 2015;11:842–6.
Giusti V, Theytaz F, Di Vetta V, et al. Energy and macronutrient intake after gastric bypass for morbid obesity: a 3-y observational study focused on protein consumption. Am J Clin Nutr. 2016;103:18–24.
Dangin M, Boirie Y, Guillet C, et al. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr. 2002;132:3228S–33S.
Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. 2015;80:A8–15.
Dreyer HC, Drummond MJ, Pennings B, et al. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–400.
Phillips SM, Tang JE, Moore DR. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr. 2009;28:343–54.
Oppert JM, Bellicha A, Roda C, et al. Resistance training and protein supplementation increase strength after bariatric surgery: a randomized controlled trial. Obesity. 2018;26:1709–20.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Mahwah: Lawrence Erlbaum Associates; 1988.
Robertson RJ, Goss FL, Rutkowski J, et al. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc. 2003;35:333–41.
Berti LV, Campos J, Ramos A, et al. Posição da SBCBM - nomenclatura e definições para os resultados em cirurgia bariátrica e metabólica. Arq Bras Cir Dig. 2015;28:2–2.
Compher C, Frankenfield D, Keim N, et al. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881–903.
Fullmer S, Benson-Davies S, Earthman CP, et al. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non-critically ill individuals. J Acad Nutr Diet. 2015;115:1417–1446.e2.
Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9.
Matarese LE. Indirect calorimetry: technical aspects. J Am Diet Assoc. 1997;97:S154–60.
Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–8.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Conway JM, Ingwersen LA, Vinyard BT, et al. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–8.
Dodd KW, Guenther PM, Freedman LS, et al. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc. 2006;106:1640–50.
da Silva FBL, Gomes DL, de Carvalho KMB. Poor diet quality and postoperative time are independent risk factors for weight regain after Roux-en-Y gastric bypass. Nutrition. 2016;32:1250–3.
Hawkins RB, Mehaffey JH, McMurry TL, et al. Clinical significance of failure to lose weight 10 years after Roux-en-y gastric bypass. Surg Obes Relat Dis. 2017;13:1710–6.
Golzarand M, Toolabi K, Djafarian K. Changes in body composition, dietary intake, and substrate oxidation in patients underwent laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve Gastrectomy: a comparative prospective study. Obes Surg. 2019;29:406–13.
Aron-Wisnewsky J, Verger EO, Bounaix C, et al. Nutritional and protein deficiencies in the short term following both gastric bypass and gastric banding. PLoS One. 2016;11:e0149588.
Lopes Gomes D, Moehlecke M, Lopes da Silva FB, et al. Whey protein supplementation enhances body fat and weight loss in women long after bariatric surgery: a randomized controlled trial. Obes Surg. 2017;27:424–31.
Carretero-Ruiz A, del Carmen Olvera-Porcel M, Cavero-Redondo I, et al. Effects of exercise training on weight loss in patients who have undergone bariatric surgery: a systematic review and meta-analysis of controlled trials. Obes Surg. 2019;29:3371–84.
Miller PE, Alexander DD, Perez V. Effects of whey protein and resistance exercise on body composition: a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2014;33:163–75.
Morton RW, Murphy KT, Mckellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52:376–84.
Dreyer HC, Fujita S, Cadenas JG, et al. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–24.
Morton RW, McGlory C, Phillips SM. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front Physiol. 2015;6:245.
Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8.
Weigle DS, Sande KJ, Iverius PH, et al. Weight loss leads to a marked decrease in nonresting energy expenditure in ambulatory human subjects. Metabolism. 1988;37:930–6.
Bosy-Westphal A, Kossel E, Goele K, et al. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am J Clin Nutr. 2009;90:993–1001.
Wang Z, Heshka S, Gallagher D, et al. Resting energy expenditure-fat-free mass relationship: new insights provided by body composition modeling. Am J Physiol Metab. 2000;279:E539–45.
Oshima T, Berger MM, De Waele E, et al. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr. 2017;36:651–62.
McClave SA, Lowen CC, Kleber MJ, et al. Clinical use of the respiratory quotient obtained from indirect calorimetry. JPEN J Parenter Enteral Nutr. 2003;27:21–6.
Coen PM, Menshikova EV, Distefano G, et al. Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes. 2015;64:3737–50.
Coen PM, Tanner CJ, Helbling NL, et al. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. J Clin Invest. 2015;125:248–57.
Dantas WS, Roschel H, Murai IH, et al. Exercise-induced increases in insulin sensitivity after bariatric surgery are mediated by muscle extracellular matrix remodeling. Diabetes. 2020:db191180.
Acknowledgments
We are particularly thankful to Isabela Rios and Gabriela Sousa de Oliveira for participating in data collection, Gustavo Neves de Souza Gomes for conducting the supervised resistance training program applied to the participants of this study, and the Nucleus of Support on Research from Sabin Institute and Sabin Diagnostic medicine for partial funding and support for blood tests.
Funding
This study was funded by Foundation for Research Support of the Federal District (FAPDF; grant number 0193.001.462/2016), Brazilian National Technological and Scientific Development Council and Ministry of Health (CNPq/MS; grant number 408340/2017-7).
Author information
Authors and Affiliations
Contributions
The study was coordinated by Kênia Mara Baiocchi de Carvalho. Kênia Mara Baiocchi de Carvalho, Eliane Said Dutra, Nathalia Pizato, Teresa Helena Macedo da Costa, Ricardo Moreno Lima, and Fernando Lamarca wrote the study protocol and designed the study. Fernando Lamarca and Flávio Teixeira Vieira realized literature searches, data collection, and performed manuscript preparation. Eduardo Yoshio Nakano and Fernando Lamarca performed data analysis. All authors contributed to data analysis, figures, and tables conception, reviewed the manuscript, and approved the version to be submitted.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lamarca, F., Vieira, F.T., Lima, R.M. et al. Effects of Resistance Training With or Without Protein Supplementation on Body Composition and Resting Energy Expenditure in Patients 2–7 Years PostRoux-en-Y Gastric Bypass: a Controlled Clinical Trial. OBES SURG 31, 1635–1646 (2021). https://doi.org/10.1007/s11695-020-05172-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-05172-1