Abstract
Introduction
In bariatric surgery, new surgical techniques are continually being developed. The one anastomosis gastric bypass (OAGB) has become increasingly common since 2001. However, some patients experience bile reflux or excessive weight loss. This study aimed to assess a new bariatric procedure designed to avoid some of the drawbacks of conventional OAGB.
Material and Methods
To lower the complication rate and pathophysiological impact after OAGB, we performed an omega loop gastroileal bypass (OLGIBP/SAGI) with a 300-cm common limb. We compared this technique with OAGB.
Results
Seventeen patients underwent OLGIBP and 23 underwent OAGB. Mean operative time was 108 min for OLGIBP vs 103 min for OAGB. The mean hospital length of stay was 3 days (1 to 7). No complications related to the gastroenterostomy occurred. At 3 years, among OAGB patients, there were 5 (21.7%) cases of bile reflux including 2 (8.7%) requiring a revision to Roux-en-Y gastric bypass. Among OLGIBP patients, there were 3 (17.6%) cases of bile reflux 1 (5.9%) requiring a revision to Roux-en-Y gastric bypass. There was no albumin deficiency. At 3 years, % of total weight loss (TWL) was 43.6 + − 6.2 in the OAGB group vs 48.2 + − 7.4 in the OLGIBP group.
Conclusions
The bariatric and metabolic outcomes of OLGIBP are expected to be similar to those of OAGB. The OLGIBP technique should reduce the risks of malnutrition and bile reflux. The two techniques can be safely performed and offer alternatives in bariatric surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
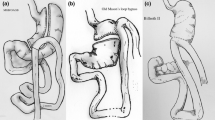
The current laparoscopic one anastomosis gastric bypass (OAGB) is a mixed restrictive and malabsorptive procedure that consists of a long, narrow, vertical gastric tube along the lesser curvature of the stomach that is connected to a loop of small bowel approximately 200 cm from the ligament of Treitz in an antecolic fashion [1] (Fig. 1).
Due to its simplicity, OAGB is now the third most performed primary bariatric procedure in the world (4.8%), after sleeve gastrectomy (SG) (53.6%) and Roux-en-Y gastric bypass (RYGB) (30.1%), according to the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) [2].
Although OAGB is internationally considered mainstream, it is not officially endorsed to several national societies including France [3], due to malnutrition and gastroesophageal reflux disease (GERD). Unlike RYGB, the malabsorptive aspects of OAGB are still unknown, since its physiopathology has never been clearly studied so far in the literature to our knowledge [4]. It could result from 2 mechanisms: from one hand, the delayed exposure of the food bolus with an already partially digested bile, less rich in bile salts, and from the other hand, less absorption of nutrients by the shorter common limb (CL) [5].
The total small bowel length (TSBL) is inconsistent in humans, with a median length of 6.9 m, which may vary from 3.50 to 10.49 m, according to Tacchino et al. [6]. TSBL correlates with sex (men have longer TSBL than women) and height, but does not correlate with weight [6]. As a result, malnutrition after OAGB is favored by the fixed measurement of the biliopancreatic limb (BPL) at 200 cm, without measurement of the remaining CL. In patients whose TSBL is less than 4 m, there is a high risk of type I short-bowel syndrome [7]. Bile reflux is another major concern of OAGB that remains controversial [8]. Its single-loop anastomosis could lead to GERD, with the risk of gastritis, esophagitis, anastomostic ulcer [9], and potentially long-term esogastric cancer [10].
To avoid these complications, we developed in 2013 an omega loop gastroileal bypass (OLGIBP) (Fig. 2). This new technique combines the simplicity of the OAGB procedure, with a single gastroileal anastomosis, and the safety of a 300 cm CL that ensures the absence of malnutrition, by analogy to the modified single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) technique [11] and the stomach intestinal pylorus-sparing (SIPS) surgery [12].
The bile acid physiology is complex. Upon reaching the ileum, primary bile acids are deconjugated by the intestinal bacteria into secondary bile acids, taken up by specific transport proteins [13], thanks to protein ileal bile acid transporter (IBAT, SLC10A2), which move bile acids from the gut lumen into the ileocyte. Then, bile acids uptake, intracellular transport, and secretion into the portal vein require the apical sodium-dependent bile acid transporter (ASBT), the cellular intestinal bile acids binding protein (I-BABP), and the basolateral heterodimeric organic solute transporter (OSTα/β) [14]. Bile acids are efficiently reabsorbed before the end of the terminal ileum (> 95%) performing the biliary cycle described by Schiff [15]. Therefore, the more distal localization of the gastroileal anastomosis lets both passive bile acids reabsorption in the jejunum and active bile acids reabsorption in the proximal ileum of the BPL [16]. This may reduce the quantity and thus the aggressiveness of the already digested bile salts at the level of the anastomosis, in case of a possible bile reflux.
A similar technique was first described in 2016 by De Luca et al. as a single anastomosis gastroileal bypass (SAGI) [17]. Since the principles of the 2 techniques are identical, we will use the acronym SAGI, firstly used in the scientific literature, to describe our own procedure.
The aim of this study is to describe our SAGI technique; assess the feasibility, safety, and efficacy of SAGI; and compare medium-term outcomes with our series of OAGB, in agreement with the French recommendations [3].
Material and Methods
This was a prospective observational study. Between January 2013 and December 2015, 40 morbidly obese patients underwent a one anastomosis gastric bypass (OAGB) (Table 1). During the same period, 226 sleeve gastrectomies, 214 Roux-en-Y gastric bypasses, 38 gastric bandings, and 83 gastric band removals were performed. All patients were allowed to select their surgical procedure and then, following this choice, enroll in the study. Explicit written informed consent for operation and data recording was obtained from all patients. Collected data included length of hospital stay, preoperative BMI, presence of medical comorbidities, intra- and postoperative complications, total operative time, common channel length, and weight loss. All data were entered prospectively into a custom-designed database.
All patients were ≥ 18 years of age. The indication for surgery was morbid obesity, defined as BMI > 40 kg/m2, or BMI > 35 kg/m2 with associated comorbidities, based on French guidelines. Duration of obesity had to be at least 5 years. All patients were evaluated preoperatively by a multidisciplinary team including an endocrinologist, psychologist, dietician, bariatric nurse, and surgeon. All patients underwent preoperative screening, including physical examination (comorbidities, use of medication, body mass index (BMI)), nutritional status (laboratory tests), psychological examination, screening for obstructive sleep apnea, preoperative upper endoscopic evaluation, preoperative abdominal tomography, and a preoperative upper gastrointestinal study. The patients went through the standard seminar and educational program available as part of the Center of Excellence since 2014. The exclusion criteria were the presence of severe esophagitis, Barrett’s esophagus, hiatal hernia, as well as the presence of alcoholism, drug addiction, or psychological disorders. One month postoperatively, all patients started the supplementation. Follow-up was conducted by the surgeon and endocrinologist at 1, 3, 6, 9, and 12 months postoperatively in the first year and then every 6 months. At each consultation, BMI was calculated, and patients were screened for signs and symptoms of deficiencies and use of supplements. Blood samples to evaluate nutritional status were obtained once or at various intervals within the first year after surgery and at least once a year after the first year.
Surgical Technique
The patient is placed in supine position, the left arm along the body. We use 5 (OAGB) or 6 (OLGIBP) bladeless trocars (Fig. 3). To perform OLGIBP, the ileocecal junction is identified. The length of the small bowel is measured from the ligament of Treitz or from the ileocecal junction to the angle of Treitz. We use a 200-cm biliopancreatic limb to perform OAGB and a 300-cm common channel/alimentary limb to perform OLGIBP. The selected loop is brought up to the stomach and fixed to the antrum. A vertical gastric pouch approximately 120–150 ml in volume is created first by horizontal section of the proximal stomach on the angle of the lesser curvature side using a 60-mm linear stapler, following by vertical ascending transection toward the angle of Hiss using a 60-mm linear stapler that is fired three or four times under the guidance of a 37 Fr gastric tube (MID-sond, Medical Innovation Developpement, Dardilly, France). The omega loop is anastomosed in an isoperistaltic fashion. We perform a posterior or anterior vertical side-to-side mechanical anastomosis using a 45-mm linear stapler. The enterotomies are closed with a resorbable V-loc (Covidien Healthcare, Mansfield, MA). The anastomosis is tested for leaks with methylene blue instillation through the nasogastric tube. The last step of the operation is the creation of an anti-reflux mechanism: the first 5 cm of the afferent loop is sutured to the pouch vertically, reinforcing the vertical staple line and reducing the tension of the anastomosis, thereby providing a preferential route for food and liquid to travel toward the alimentary efferent limb. No nasogastric tube and no drain are left in place.
Revisional operations
Abdominal adhesions between the liver and the stomach are divided. In the presence of severe adherences, the dissection should be kept close to the liver. In case of a difficult dissection or a suspicion of a breach to the gastric pouch, we recommend a methylene blue test or an intraoperative endoscopy. In case of an history of VBG. The band is approached first. Then it should be removed before gastric division. If there is some compromise of the lumen to the extent that creation of a blind limb or pouch is a concern, then we may either resect this portion of the stomach or incise the ring to open it up. In case of a history of RYGB, the intervention starts by restoring the normal anatomy of the small bowel, with the resection of the gastrojejunal anastomosis. The gastric pouch was not modified because it was already long and narrow.
Results
Forty patients (37 women and 3 men) were included in this study (Table 1). Table 1 summarizes patients characteristics. Twenty-three patients were treated with OAGB and 17 with OLGIBP. The average BMI was 44 kg/m2 (32–54) in the OAGB group vs 45 in the OLGIBP group. Ten patients had a history of gastric bypass surgery, and 2 patients had a history of GVC (gastroplastie verticale calibrée/intervention of Mason). Mean operative time was 103 min (Table 2) in the OAGB group vs 108 in the OALGIB group. The mean hospital stay was 3 days [1,2,3,4,5,6,7]. In the OAGB group, there were five intraoperative incidents (bowel perforation) and two postoperative complications (bowel perforation after OAGB with peritonitis). In spite of a prompt reoperation (1 POD), one patient died from multiorgan failure. At 3 years, among OAGB patients, there were 5 (21.7%) cases of bile reflux including 2 (8.7%) requiring a revision to Roux-en-Y gastric bypass; the other patients refused the revision surgery. Among OLGIBP patients, there were 3 (17.6%) cases of bile reflux 1 (5.9%) requiring a revision to Roux-en-Y gastric bypass. There was no albumin deficiency (Table 3). At 3 years, % of total weight loss (TWL) was 43.6 + − 6.2 in the OAGB group vs 48.2 + − 7.4 (Table 3).
Discussion
This study describes the SAGI technique, as a variation of the OAGB, with a single-loop anastomosis and a fixed 300-cm CL length. Our results confirm the feasibility of this procedure, which could be carried out without further difficulty in all the 17 cases. The operating times were comparable in both SAGI and OAGB groups (103 vs 108 min). The SAGI group tends to have fewer per operative complications than the OAGB group: there were no leaks or bleeding during the 17 procedures. These encouraging results can be explained by the ease of unrolling the distal ileum, which remains superficial, starting from the ileocecal valve. The absence of per operative leak is favored by the solidity of the OAGB-like tension-free gastroileal anastomosis, thanks to the long gastric tube and the length of the ileum.
On the other hand, the OAGB BPL measurement, starting from the angle of Treitz deep under the transverse colon and the omentum, carries a risk of bowel perforation. In our study, 5 of the 23 patients of the OAGB group needed a per procedure bowel suture (n = 5 vs n = 0 in the SAGI group). An extra perforation went unnoticed during surgery. This led to secondary stercoral peritonitis, and the patient’s death. On the contrary, there was no death in the SAGI group. Considering the large number of intestinal wounds during this study in the OAGB group, we decided to change the grasper after the study was completed. The use of a disposable Epix® grasper (Applied Medical, Rancho Santa Margarita, CA) allowed us to decrease this risk. In the immediate postoperative period, no complications occurred among the 17 patients who benefited from the SAGI procedure. These results are consistent with the preliminary results of the De Luca et al. study [17]. Unlike the 3 to 6 months follow-up of the latter, the strength of the current study is the 3 years follow-up, which allows us to have a good assessment of the SAGI benefits in the medium term, especially on malnutrition and bile reflux.
OAGB enables excellent weight loss with a low complication rate, but in rare cases, weight loss is too large. Thereby, operative revision after OAGB is often due to malnutrition [18, 19]. To avoid this problem, numerous limb-length combinations have been tested. In OAGB, the gastro-jejunostomy is originally formed at 150–200 cm [20]. Focusing on the BPL optimal length, some authors suggest an increase in BPL length by 10 cm for every BMI point above 40 kg/m2 [21]. Others, like Ahuja et al. [22], propose 3 different BPL lengths, depending on the weight: a standard 150-cm BPL with very minimal nutritional complications and good results, a 180-cm BPL for super obese, and a 250-cm BPL for extreme weights.
However, in these studies, the CL is unknown, leading to a risk of short bowel with excessive weight loss and malnutrition. A more rigorous approach is to establish the length of both BPL and CL by measuring the TSBL. Komaei et al. [23] describe less nutritional deficiencies compared to OAGB, when tailoring BPL length relatively to TSBL, by bypassing 40% of the TSBL. More recently, Tovar et al. [4] use the formula BPL = 50% TSBL + 50 cm, with a CL length of at least 180 cm. According to this study, the CL/TSBL ratio and the CL length are the most accurate parameters to predict a 5-year postoperative BMI ≤ 25 kg/m2. They conclude with an ideal range between 0.40 and 0.43 for the CL/TSBL ratio, and 200 to 220 cm for the CL length, which is unexpected to our concern, since numerous cases of malnutritions are described in the literature with this length.
Although the TSBL measurement seems more precise, it makes the operation more difficult, tedious, and time-consuming while increasing the risk of bowel perforations or lacerations in the serosa. Furthermore, per operative ratio calculations can also lead to a poor BPL and CL measurements, by typing or mental calculation errors. Last but not least, considering the variations of the BPL and the CL among patients, and the fact that TSBL measurement is not always achievable [24], this leads to a lack of standardization of the technique.
Soong et al. had initially tailored limb according to preoperative body mass index but modified their technique due to malnutrition, eventually measuring the TSBL to keep the CL at least 400–600 cm long [25]. The remaining CL is considered responsible for more nutritional deficiencies, when its length is smaller than 50% of the TSBL [26]. Thus, the third approach to single anastomosis surgeries is to only measure the CL, starting from the ileocecal valve. SADI-S popularized this method firstly described in 1979 in the Scopinaro’s biliopancreatic bypass technique, with a 50 cm CL [27]. In an OAGB-like procedure, for maximum safety, the CL must never be under 200 cm.
Sanchez-Pernaute et al. extensively reviewed limb length variations when initially describing the SADI-S operation, ultimately deciding to form a CL of 250 cm [11]. The 300-cm CL SIPS procedure tends to decrease malabsorption [28], with an EWL equivalent to SADI-S [29]. As the SIPS operation has proven to be safe and effective with no relevant malabsorption in mid-term follow-up, the anastomosis position for SAGI should be similar.
In 2019, Nabil et al. presented a randomized control trial which compared OAGB vs OAGB with a relatively fixed 400 to 600-cm CL (named “distal group”) [24]. One year after surgery, the distal group presented more nutritional deficiencies: the levels of hemoglobin, serum cholesterol, triglycerides, iron, total proteins, and albumin were significantly lower but without hypoalbuminemia. The levels of SGOT and parathormone were significantly higher. The gastrointestinal quality of life index (GIQLI) score was also lower in the distal group, without specifying whether the patient’s discomfort was due to bile reflux or intestinal disturbances. It is also unfortunate that 10 patients with a short TSBL (< 6 m) were excluded from the study, because it is precisely these who would have been most likely to benefit from a fixed CL.
These results are not consistent with the results of our study: when we performed the SAGI technique, no patient experienced malnutritions symptoms after 3 years. Major bariatric surgeries combine a restrictive gastric component with a rearrangement of the small intestinal passage. When reconnecting the stomach pouch to the intestine, the pylorus can either be preserved or excluded.
The gold standard RYGB was developed for two reasons: to prevent tension in a distant anastomosis and to protect the gastric mucosa against pancreaticobiliary secretions. More recently, the duodenal switch (DS) and the SADI-S techniques preserve the pylorus when bypassing the duodenum, avoiding dumping syndrome and marginal ulcers [30]. Disregarding these principles, more and more surgeons use nowadays a single-loop reconstruction without rerouting biliopancreatic fluids, when performing an OAGB. The absence of a mechanical pylorus barrier and Y-shaped loop can favor GERD, which in rare cases may require surgical revision. Interestingly, marginal ulcers and bile reflux also occur after conventional RYGB [31, 32], and with a preservation of the pylorus [30].
SAGI has the same type of surgical arrangement as OAGB, but thanks to a more distal anastomosis, the bile is theoretically largely absorbed before the gastroileal anastomosis, which reduces the risk of biliary reflux. Despite the creation of an anti-reflux mechanism, three OAGB patients had bile reflux versus none after SAGI surgery, and no patient experienced clinical signs of GERD. However, the absence of systematic endoscopic control and pH monitoring over the long term prevents us from drawing any conclusions about the benefits of the technique on bile reflux and its consequences.
This is the 2nd clinical study on SAGI in the literature, with the exception of a case report from 2019 [33], and it is the first with mid-term results. In De Luca’s study, the first clinical results of SAGI are comparable to OAGB, not only in terms of weight loss but also in terms of remission of dyslipidemia and OSAS comorbidities [17]. In our study, excess weight loss was also comparable in the 2 techniques at 1 year, as well as at 3 years.
This study has several limitations. Firstly, it has a prospective monocentric design, conducted on a small number of patients, with retrospective analysis of the data. Moreover, a significant number of patients were lost to follow-up, making the results difficult to interpret. We focused more on the feasibility and effectiveness on excess weight loss of this new technique, leaving aside the effects on comorbidities and the quality of life after surgery. During our procedure, we did not investigate TSBL. When performing a malabsorptive procedure, the ideal is to measure the length of the CL according to TSBL. This is why we cannot interpret the weight loss outcomes in this study. That said, we can extrapolate the length of the remaining BPL from other studies. When measuring a 400 to 600-cm CL, Nabil et al. had a mean remaining BPL length of 200 cm (301 ± 104) [24]. For Komaei et al., mean TSBL was 625.6 ± 110.5 cm [23]. Therefore, we think that the BPL of the SAGI is on average greater than 250 cm. Nevertheless, given the lack of certainty, we have decided to prospectively study the SAGI procedure with measurement of the TSBL.
Conclusion
This study suggests that SAGI is a simple, feasible, and safe malabsorptive bariatric procedure, with a good efficacy on excess of weight loss. The mid-term results of this small series most importantly show no mortality and no complications related to the SAGI. The difference between OAGB and SAGI is that in the latter, the alimentary anastomosis is placed more distally in the ileum with a 300-cm CL. This avoids the risk of malnutrition and reduces the risk of bile reflux. This new technique will have to be evaluated in randomized control trials.
References
Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg juin. 2001;11(3):276–80.
Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–94.
Lafarge J-C. Traitement chirurgical de l’obésité sévère et massive par court-circuit (bypass) gastrojéjunal avec anse en oméga. Haute Autorité de Santé. 2019;71
Ruiz-Tovar J, Carbajo MA, Jimenez JM, et al. Are there ideal small bowel limb lengths for one-anastomosis gastric bypass (OAGB) to obtain optimal weight loss and remission of comorbidities with minimal nutritional deficiencies? World J Surg mars. 2020;44(3):855–62.
Caiazzo R, Torres F, Sterkers A, et al. Complications du bypass gastrique. In: Complications de la chirurgie bariatrique. Montrouge: John Libbey Eurotext; 2016. p. 97. (Monographies de l’association française de chirurgie)
Tacchino RM. Bowel length: measurement, predictors, and impact on bariatric and metabolic surgery. Surg Obes Relat Dis avr. 2015;11(2):328–34.
Scolapio JS. Current update of short-bowel syndrome. Curr Opin Gastroenterol. 2004;20(2):143–5.
Mahawar KK, Himpens J, Shikora SA, et al. The first consensus statement on one anastomosis/mini gastric bypass (OAGB/MGB) using a modified Delphi approach. Obes Surg. 2018;28(2):303–12.
Fisher BL, Buchwald H, Clark W, et al. Mini-gastric bypass controversy. Obes Surg. 2001;11(6):773.
Bruzzi M, Duboc H, Gronnier C, et al. Long-term evaluation of biliary reflux after experimental one-anastomosis gastric bypass in rats. Obes Surg. 2017;27(4):1119–22.
Sánchez-Pernaute A, Herrera MAR, Pérez-Aguirre ME, et al. Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S). One to three-year follow-up. Obes Surg déc. 2010;20(12):1720–6.
Mitzman B, Cottam D, Goriparthi R, et al. Stomach intestinal pylorus sparing (SIPS) surgery for morbid obesity: retrospective analyses of our preliminary experience. Obes Surg. 2016;26(9):2098–104.
Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50 Suppl:S120–125.
Di Ciaula A, Garruti G, Lunardi Baccetto R, et al. Bile acid physiology. Ann Hepatol. 2017;16(Suppl. 1: s3–105):s4–14.
Reuben A. The biliary cycle of Moritz Schiff. Hepatology. 2005;42(2):500–5.
Dawson PA. Role of the intestinal bile acid transporters in bile acid and drug disposition. Drug Transporters. 2011:169–203.
De Luca M, Himpens J, Angrisani L, et al. A new concept in bariatric surgery. Single anastomosis gastro-ileal (SAGI): technical details and preliminary results. Obes Surg. 2017;27(1):143–7.
Bolckmans R, Arman G, Himpens J. Efficiency and risks of laparoscopic conversion of omega anastomosis gastric bypass to Roux-en-Y gastric bypass. Surg Endosc. 2019;33(8):2572–82.
Robert M, Espalieu P, Pelascini E, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open-label, non-inferiority trial. Lancet. 2019;393(10178):1299–309.
Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg. 2005;15(9):1304–8.
Noun R, Skaff J, Riachi E, et al. One thousand consecutive mini-gastric bypass: short- and long-term outcome. Obes Surg. 2012;22(5):697–703.
Ahuja A, Tantia O, Goyal G, et al. MGB-OAGB: effect of biliopancreatic limb length on nutritional deficiency, weight loss, and comorbidity resolution. Obes Surg. 2018;28(11):3439–45.
Komaei I, Sarra F, Lazzara C, et al. One anastomosis gastric bypass–mini gastric bypass with tailored biliopancreatic limb length formula relative to small bowel length: preliminary results. Obes Surg. 2019;29(9):3062–70.
Nabil TM, Khalil AH, Mikhail S, et al. Conventional versus distal laparoscopic one-anastomosis gastric bypass: a randomized controlled trial with 1-year follow-up. Obes Surg. 2019;29(10):3103–10.
Soong T-C, Almalki OM, Lee W-J, et al. Measuring the small bowel length may decrease the incidence of malnutrition after laparoscopic one-anastomosis gastric bypass with tailored bypass limb. Surg Obes Relat Dis. 2019;15(10):1712–8.
Abellan I, Luján J, Frutos MD, et al. The influence of the percentage of the common limb in weight loss and nutritional alterations after laparoscopic gastric bypass. Surg Obes Relat Dis. 2014;10(5):829–33.
Scopinaro N, Gianetta E, Civalleri D, et al. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg. 1979;66(9):618–20.
Neichoy BT, Schniederjan B, Cottam DR, et al. Stomach intestinal pylorus-sparing surgery for morbid obesity. JSLS. 2018;22(1)
Brown WA, Ooi G, Higa K, et al. IFSO-appointed task force reviewing the literature on SADI-S/OADS. Single anastomosis duodenal-Ileal bypass with sleeve gastrectomy/one anastomosis duodenal switch (SADI-S/OADS) IFSO position statement. Obes Surg. 2018;28(5):1207–16.
Grueneberger JM, Karcz-Socha I, Marjanovic G, et al. Pylorus preserving loop duodeno-enterostomy with sleeve gastrectomy - preliminary results. BMC Surg. 2014;14:20.
Obeid A, Long J, Kakade M, et al. Laparoscopic Roux-en-Y gastric bypass: long term clinical outcomes. Surg Endosc. 2012;26(12):3515–20.
Swartz DE, Mobley E, Felix EL. Bile reflux after Roux-en-Y gastric bypass: an unrecognized cause of postoperative pain. Surgery for Obesity and Related Diseases. 2009;5(1):27–30.
Debs T, Petrucciani N, Ben Amor I, et al. Laparoscopic conversion of failed sleeve gastrectomy to single anastomosis gastro ileal bypass (SAGI) (with video). J Visceral Surg. 2019;156(4):358–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Informed Consent
“Informed consent was obtained from all individual participants included in the study.”
Statement of Human and Animal Rights
“Informed consent was obtained from all individual participants included in the study.”
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kassir, R., Giudicelli, X., Lointier, P. et al. Omega Loop Gastroileal Bypass (OLGIBP/SAGI) Versus One Anastomosis Gastric Bypass (OAGB): Medium-Term Results. OBES SURG 31, 1597–1602 (2021). https://doi.org/10.1007/s11695-020-05165-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-05165-0