Abstract
Background
There are currently few pre-operative predictors of initial and long-term weight loss following bariatric surgery.
Objectives
We evaluated the role of pre-operative patient characteristics and baseline gut and adipose-derived hormones in predicting maximal total body weight loss (WLmax) and risk of weight regain (WR) after Roux-en-Y gastric bypass (RYGB) surgery.
Methods
One hundred five adult patients undergoing primary RYGB were prospectively recruited. Baseline demographics were recorded and fasting plasma glucose, glycosylated hemoglobin (A1C), insulin, glucagon, leptin, active ghrelin, glucagon-like peptide 1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP) levels were measured on day of surgery.
Results
Our cohort had a mean age of 44.4 ± 13.0 years, and initial BMI (body mass index) of 45.1 ± 6.7 kg/m2 with mean post-operative follow-up of 40 months. Eighty patients were female and 26 had type 2 diabetes mellitus (T2D). Average WLmax was 35.3 ± 7.4%. On univariate analysis, higher baseline fasting ghrelin, lower age, lower CRP (C-reactive protein), lower A1C, and negative T2D status were associated with greater WLmax (p < 0.05). Controlling for these variables using stepwise multivariate regression, only higher fasting ghrelin and younger age were associated significantly with greater WLmax (p < 0.05). In subgroup multivariate regression analysis of T2D patients, higher ghrelin and glucagon were significantly associated with greater WLmax. Following stepwise multivariate regression, lower initial BMI and lower glucagon were associated with greater WR (p < 0.05).
Conclusions
Incorporation of baseline biological and hormonal markers may help in developing more accurate predictive models for weight loss following bariatric surgery that help inform patient counseling and decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a global epidemic and a risk factor for diabetes, cardiovascular diseases, cancer, and overall mortality. Bariatric surgery is the most effective therapy for obesity [1]. Laparoscopic Roux-en-Y gastric bypass (RYGB) is a highly effective treatment for morbid obesity, leading to weight loss, remission of obesity-associated comorbidities including type 2 diabetes (T2D), and reduction in long-term mortality [2].
Long-term follow-up studies of bariatric surgery patients suggest that most patients achieve durable weight loss, with a small subgroup that experiences inadequate weight loss after primary operation (non-responders) and a larger group that experience substantial weight regain after achieving adequate initial weight loss (weight regainers) [1, 3]. Factors predisposing patients to significant weight regain are unknown, but important to recognize as concern about weight regain is a significantly deterrent for surgery in patients considering bariatric surgery. This weight recidivism has important health consequences including recurrence of obesity-related comorbidities, as well as economic repercussions with recurrent costs associated with managing ongoing obesity and the associated conditions [4]. The ability to identify patients at risk of inadequate weight loss or weight regain remains therefore an important clinical priority.
The potential predictive factors for long-term outcomes include patient demographics and comorbidities, behavioral and socioeconomic parameters, and biological factors. In a large prospective cohort study looking at pre-operative patient characteristics and post-operative behavior, only a few baseline variables such as age, race, and diabetes status were associated with post-operative weight change, and the effect size of these predictive variables was small [5]. Biological factors have been understudied, with only a few reports involving small cohorts and poor follow-up [6].
Collectively, there is good scientific data indicating that hormonal changes are responsible for weigh outcomes and metabolic effects of RYGB, but their clinical predictive ability has not been studied well. We were interested in studying correlation of pre-operative serum biomarkers, specifically hormones involved in weight and glucose regulation, to weight loss (WL) and weight regain (WR).
Considering cost and risks of bariatric surgery, it is important to identify the individuals who will benefit the most from intervention. Moreover, improved pre-operative predictors of surgical outcomes will allow for improved counseling of patients regarding weight regain or inadequate weight loss. This prospective human cohort study evaluates the role of pre-operative clinical, biochemical, and metabolic hormones in predicting weight loss and weight regain following laparoscopic RYGB.
Methods
Patients
The study protocol was reviewed and approved by our Institutional Review Board. Consecutive patients undergoing a primary laparoscopic RYGB by the senior author were offered to participate in the study as part of which fasting pre-operative blood values were collected. Inclusion criteria for this study included patients who successfully underwent a RYGB. One hundred and eight adult patients were initially included in the study. Three patients were excluded from the analysis, including 1 for the incidental intra-operative finding of a gastric GIST (gastrointestinal stromal tumor), 1 for intra-operative finding of cirrhosis, and 1 patient who had type 1 diabetes. The patients had a standard pre-operative evaluation including meetings with dieticians, psychologist, and the surgeon before being cleared for surgery. They underwent a standard laparoscopic RYGB surgery, with 10-cm Roux and 40-cm BP (biliopancreatic) limbs. The surgical technique remained unchanged for all patients. All patients were asked to go on a modified 2-week pre-operative diet to help with weight loss.
Demographic data including age, gender, and race were collected through questionnaire and patients’ electronic records. Clinical outcomes were then followed by chart review and clinic visits. Patients with glycosylated hemoglobin (A1C) of 6.5% or above, or those with history of diabetes and taking diabetic medications before surgery, were considered T2D. Parameters of interest were pre-operative weight loss, maximal post-operative weight loss, and weight regain. These were defined as below.
Pre-operative Weight Change
Pre-operative weight loss was calculated as the highest weight recorded in 1 year before surgery minus the patient’s weight at the last pre-operative visit, which was available for 103 (98.1%) patients.
Maximal Post-operative Weight Loss
Baseline weight and BMI (body mass index) were regarded as those recorded during the patients’ final pre-operative visit, generally within 2 weeks prior to surgery. Post-operative weights were measured during in-person follow-up visits by qualified staff and were recorded for the following time points: 1 month, 6 months, and then annually up to 5 years. For the patients not following up with bariatric clinic, data was collected from the patients’ electronic records.
In this study, weight changes were presented as percentage total body weight loss. Maximum %TBWL (WLmax) was calculated based on the recorded nadir weight within the first 2 years after surgery and their pre-operative weight.
For most of the patients, the nadir weight was reached between 1 and 2 years after surgery, so for the patients who missed both 1- and 2-year follow-ups (N = 12), WLmax was not calculated. These cases were removed from weight loss analysis. The baseline demographics of these patients, including BMI, age, and race, were similar to the overall cohort.
Weight Regain
For WR analysis, we included just the patients with at least a 3-year follow-up (n = 49). Weight regain was defined as percentage of WLmax that was regained at 3 years post-operatively. In other words, weight regain was calculated using the formula:
Maximal WR = (highest recorded weight after reaching nadir weight until 3 post-op years − nadir weight) × 100/(pre-op weight − nadir weight).
Biochemical and Hormonal Measurements
Blood samples were obtained pre-operatively by venipuncture after an overnight fast in the operating room, immediately after induction of anesthesia and before starting the surgical procedure. Blood samples were immediately sent to the hospital reference lab, Center for Clinical Investigation (CCI). Serum biochemical data including CRP (C-reactive protein), fasting glucose, A1C, insulin, leptin, glucagon, active ghrelin, glucagon-like peptide 1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP) levels were measured by routine clinical chemistry, immediately after extraction by the LabCorp and BRAC (Brigham Research Assay Core) labs. Active (acylated) ghrelin was measured by radioimmunoassay (RIA). Preservative enzymes and pretreatment including acidifying solution for ghrelin and DPP4 inhibitor for GLP-1 and GIP were added to the EDTA (ethylenediaminetetraacetic acid) collection tubes. Serum samples for these hormonal measurements were immediately frozen and stored at − 80 °C until assayed together later.
Statistical Analysis
Variables are presented as mean ± standard deviation (SD). The quantitative data in counterpart groups were compared by t test. The chi-squared test was used to compare qualitative values. Single correlations among variables were evaluated with the Pearson coefficient of correlation. Multivariate linear regression models were constructed separately for weight loss and weight regain to ascertain the statistical significance of independent baseline predictors. For this multivariate analysis, pre-operative variables with p < 0.2 in univariate regression analysis were included. Analyses were performed with IBM SPSS Statistics for Windows, version 22.0., Armonk, NY: IBM Corp. and GraphPad Prism Software, version 7.03, La Jolla, CA. All reported P values are 2-sided. P values less than 0.05 are considered significant.
Results
Pre-operative Anthropometrics and Metabolic Parameters
The study cohort of 105 patients had a mean age, weight, and BMI of 44.4 ± 13.0 years, 125.0 ± 24.7 kg, and 45.1 ± 6.7 kg/m2, respectively. Age ranged from 22 to 70 years and BMI from 35.0 to 77.3 kg/m2. Twenty-five (23.8%) were male and 80 (76.2%) were female. Age (45.8 ± 12.3 vs. 44.0 ± 13.3 years, p = NS) and BMI (46.6 ± 8.3 vs. 44.7 ± 6.3, p = NS) were similar between men and women. Mean follow-up was 39.9 ± 26.8 months.
Twenty-six (24.8%) patients had T2D with 2 diet-controlled, 13 on oral medications, and 11 on insulin. The T2D patients were significantly older than non-diabetic (non-DM) patients (53.3 ± 9.6 vs. 41.5 ± 12.7 years, p < 0.01). However, there was no significant difference in M to F ratio. Table 1 summarizes demographic and baseline blood values of the overall cohort and compares the T2D and non-DM subgroups.
Post-operative Weight Loss
The mean %TBWL after surgery was 33.9 ± 7.4% after 1 year, 32.0 ± 9.2% after 2 years, 29.1 ± 8.2% after 3 years, 27.0 ± 8.8% after 4 years, and 25.7 ± 9.9% after 5 years (Fig. 1). Figure 1 also depicts follow-up rates in different time points. As expected, there was variation in weight loss outcomes (Supp. Figure 1). WLmax in male and female patients was 33.9 ± 7.3% and 35.7 ± 7.5% (p = NS). WLmax in T2D patients was significantly lower than non-DM patients (31.4 ± 6.2 vs 36.5 ± 7.4%; p < 0.01), as well as average %TBWL 6 months, 1, 2, and 3 years after surgery; however, this difference became non-significant after 4 years, likely due to decreased number of the patients (Fig. 1).
In a univariate linear regression analysis of the whole cohort, correlation between WLmax and pre-operative demographics, degree of pre-operative weight loss, and baseline hormone levels was calculated. Lower A1C, lower CRP, negative T2D status, higher active ghrelin, and younger age at surgery were significantly correlated with higher WLmax (Supp. Table 1). WLmax in patients younger than 60 years of age was significantly higher compared with older patients (36.3 ± 7.2% vs. 30.7 ± 6.9%; p < 0.01). Degree of perioperative WL was not significantly correlated with WLmax (p = 0.07). In a multivariable linear regression, only initial age and fasting active ghrelin remained significantly associated with WLmax (p < 0.05, Table 2).
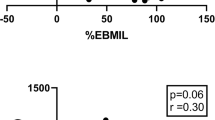
T2D is an important comorbidity in many patients undergoing RYGB, and since weight loss can impact post-operative T2D outcomes, we performed a similar analysis to identify predictors of WLmax in the T2D patient subgroup. Univariate regression analysis shows correlation between higher pre-operative ghrelin and glucagon levels with greater WLmax (Supp. Table 1). In a multivariable linear regression model, higher fasting active ghrelin and fasting glucagon were correlated with greater WLmax in T2D patient cohort (p < 0.01, Table 2 and Fig. 2).
Weight Regain
For the overall cohort, average WR was 20.7 ± 13.5% of the maximum weight loss achieved. There was variability in the degree of weight regain, with approximately a third of the patients maintaining their weight (Supp. Figure 2). WR was not different between male and female genders (23.8 ± 14.5 vs. 19.8 ± 13.3%; p = NS). WR was also similar between different race groups, with 21.0 ± 12.9%, 22.4 ± 22.1%, and 18.3 ± 8.3% in white, African-American, and Hispanic races, respectively. It was also similar in the T2D vs. non-DM groups (23.4 ± 11.5% vs. 19.8 ± 14.1%; p = NS).
On univariate linear regression analysis, initial BMI, glucagon, leptin, and glucose were correlated with higher WR with p value of less than 0.2 (Supp. Table 2). Correlation of WLmax with later WR was not significant (p = 0.10). In multivariate linear regression analysis, lower baseline fasting glucagon level and lower initial BMI were correlated with greater weight regain (p < 0.05, Table 3).
Discussion
Weight Loss
Weight loss following RYGB is an important outcome measure for both patients and surgeons. Significant post-operative WL is associated with resolution or remission of obesity-related comorbidities, improved quality of life, and reduced long-term mortality [7]. Variability in outcomes after bariatric surgery is well documented [1], and optimizing WL after surgery remains a challenge in management of bariatric patients. The reasons behind this variability remains poorly understood. Several attempts at correlating patient phenotypes with post-operative outcomes have failed to identify significant factors. Although patient dietary choices are often cited as an explanation for this variability, the main reason is likely multifactorial with at least some biological determinants [8]. Genetic association studies support that biology can drive success as WL outcomes correlate with genetic factors such as serotonin receptor [9] or UCP-2 genes [10], and several single-nucleotide polymorphisms (SNPs) [11,12,13,14]. Routine genetic assessment of potential bariatric patients is however challenging and expensive, and association of these factors with WL outcomes is weak and the effect size is small.
In this study, we set out to identify pre-operative serum biomarkers to help us assess outcomes following RYGB. We prospectively enrolled 105 patients undergoing RYGB and measured pre-operative hormone levels to see if we can identify pre-surgical markers that can help predict post-operative outcomes. Our patients experienced the majority of weight loss in the first 12 months after surgery with 75% of them reaching their nadir weight at 1-year time point.
Ghrelin, a peptide-secreted predominantly by the gastric enteroendocrine cells, stimulates food intake and growth hormone (GH) secretion. Ghrelin is found in the circulation in both acylated (active) and des-acylated forms. Only the acylated form (active ghrelin as measured in our study) acts at the GHS-R1a to affect appetite, GH release, and metabolism [15]. Our study showed a significant positive correlation between WLmax and baseline fasting active ghrelin levels. A previous rodent RYGB study had similarly demonstrated that pre-operative ghrelin levels are correlated to WL [16]. The importance of pre-operative ghrelin level on 1 year weight loss data was also demonstrated in a small study of 15 patients undergoing laparoscopic sleeve gastrectomy [17]. Our data is also consistent with Labayen et al. findings in a dietary weight loss study showing that lower baseline ghrelin levels were correlated with resistance to fat mass loss [18]. In contrast to our study, Pellitero et al. [19] found that pre-operative total ghrelin was lower in patients with higher WL 12 months after RYGB, and total ghrelin was not a predictor of patients who maintained their WL at 12 to 24 months after surgery. This difference in finding however may be explained by our measurement of active (acylated) ghrelin, which is not necessarily reflected in total ghrelin levels. Furthermore, the patients in this study underwent a modified version of the RYGB (ringed or a distal bypass), which likely affects hormonal changes and outcomes.
Our finding is biologically plausible, as high active ghrelin levels are associated with increased appetite, and therefore, those with higher levels of this hormone may benefit most from appetite suppressing/anorexic effects of bariatric surgery.
Fasting glucagon level also had a significant positive correlation with WLmax in T2D subgroup of patients. Glucagon is an amino acid peptide that is secreted from pancreatic α-cells in response to low levels of blood glucose. Non-glycemic effects of glucagon include modulation of food intake and satiety, lipid homeostasis, insulin secretion, and energy expenditure [20]. In non-surgical models, chronic administration of glucagon has been shown to substantially reduced body weight (up to 25%) in diet-induced obese (DIO) mice [21, 22]. Although glucagon studies in RYGB are limited, our results support the aforementioned studies indicating the role of glucagon in weight modification and suggest it might be used as a predictor of weight outcome after bariatric surgeries among T2D patients.
Looking at other serum biomarkers, there was no correlation between WLmax and pre-operative gut derived incretin hormones GLP-1 and GIP. Similarly, Werling et al. showed that GLP-1 (and PYY; peptide YY) did not correlate to WL after RYGB [23].
Based on multivariate analysis, we find that patient age also has a significant inverse correlation with WLmax. Most published studies concur with our findings, showing better WL in younger patients [24, 25]. Similarly, sex, baseline weight, and initial BMI were not found to correlate with WLmax, consistent with reports by Courcoulas et al. on 3-year weight outcomes of the Longitudinal Assessment of Bariatric Surgery (LABS) cohort [5]. We found that T2D is significantly correlated with less WL after RYGB in univariate analysis, but this was not confirmed in multivariate regression. There are several studies using univariate-like analysis suggesting that T2D leads to less favorable weight outcomes [5, 26, 27]. However, when we perform a multivariate analysis and control for differences in fasting ghrelin and the higher age of the diabetic cohort, the T2D effect washed out. Specifically, these analyses highlight the importance of ghrelin levels in influencing post-operative weight loss, a parameter not examined in prior studies. Because weight loss in T2D patients undergoing RYGB is particularly important and can impact T2D outcomes, we performed a separate analysis to identify predictors of WL in this subgroup and found that similar to the overall cohort, ghrelin is correlated with WLmax. Specific to the T2D subgroup, however, higher glucagon levels are also correlated to WLmax. Considering smaller number of patients in our T2D subgroup, more studies are needed to evaluate and validate this finding.
Our findings are novel in that we have identified hormonal factors that influence post-RYGB outcomes, suggesting that there are biological factors that influence post-operative outcomes. As we were primarily interested in identifying pre-operative markers of post-operative outcomes, we did not measure post-surgery levels of the same hormones, which can be the subject of future studies. Since we focused on pre-operative factors, we also did not include post-operative eating habits and activity levels which have been proposed as factors influencing outcomes, although previous studies have confirmed that their contribution may be limited [28]. We believe larger prospective studies are needed to validate this finding with the ultimate goal of assessing pre-operative hormone profile of patients to educate them on their likely maximal weight loss and help to improve patient education and consent.
Weight Regain
Although some degree of weight regain is commonly seen after patients reach their nadir weight, several patients experience significant weight regain and concern about this possibility lowers many patients’ enthusiasm for bariatric surgery. Identifying pre-operative factors which may predict risk of post-operative weight gain are therefore very important in helping providers council patients. There are various definitions for significant weight regain after bariatric surgeries. One of the most commonly used definitions is gaining > 20% of the maximal initial weight loss [29, 30]. In a national study of bariatric surgery patients with 7 years of follow-up, Courcoulas et al. defined 6 trajectories for weight outcome patterns after RYGB [31], with the trajectories being distinguishable at 3 years. Based on this data, we chose the 3-year time point to study weight regain in our cohort.
Our data identified lower baseline fasting glucagon level and lower initial BMI were correlated with greater WR. Some studies have highlighted the importance of initial weight loss on subsequent weight regain, including a study by Cooper et al. who showed that greater initial WL leads to more successful long-term weight outcomes (less WR) [32]. In our study, WLmax however was not a significant predictor of WR when controlling for biological hormonal factors. T2D status also did not influence risk of WR. This suggests that different mechanisms may be at play and influence these outcomes.
The role of leptin on post-operative weight loss and subsequent WR is unclear with mixed results in rodent studies [33, 34]. Although there was a trend towards this in our univariate analysis, we could not confirm this on our multivariate analysis. Age, sex, and race did not show a correlation with WR in our study, consistent with the mixed literature on this topic [35].
This study is unique and practical in that it tries to link fasting pre-operative hormone levels to long-term outcomes in a prospective fashion; this is an approach that can be replicable in a clinical setting. It does however have some limitations including high rate of female patients which is inherent to most bariatric studies, and low follow-up rates beyond 3 years. Although we believe a 3-year follow-up is long enough to establish weight regain patterns and has been used in other studies [1, 36], longer follow-ups are needed for confirmatory future studies. This study focused on RYGB, which at the time of initiation of the study remained a very popular surgical option. With current changes in bariatric surgical options and popularity of sleeve gastrectomy, we plan to perform similar studies looking at outcomes of sleeve gastrectomy patients too.
Conclusions
In this study, we have assessed the ability of pre-operative clinical and serum metabolic biomarkers to predict maximum WL and WR after LRYGB. We found that higher pre-operative ghrelin and younger age are associated with greater maximum weight loss (WLmax), and lower baseline BMI and glucagon levels were associated with greater WR. These findings could help guide larger future studies to confirm and ultimately be used to develop a care protocol that involves measurements of these hormones in the pre-operative phase and the data used to discuss individual level likelihood of weight regain and more accurate prediction of weight loss after RYGB. Such discussions will improve shared decision-making with the patient and pre-operative counseling.
References
Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013/11/06. 2013;310:2416–25. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=24189773
Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012/09/20. 2012;308:1122–31. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22990271
Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008/04/09. 2008;18:648–51. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18392907
Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013/09/03. 2013;23:1922–33. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23996349
Courcoulas AP, Christian NJ, O’Rourke RW, Dakin G, Patchen Dellinger E, Flum DR, et al. Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surg Obes Relat Dis. 2015/04/01. 2015;11:1109–18. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=25824474
Hatoum IJ, Greenawalt DM, Cotsapas C, Reitman ML, Daly MJ, Kaplan LM. Heritability of the weight loss response to gastric bypass surgery. J Clin Endocrinol Metab. 2011/08/13. 2011;96:E1630–3. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21832118
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004/10/14. 2004;292:1724–37. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15479938
Tavakkoli A. Comment on: revisional surgery after failed gastric banding: results of one-stage conversion to RYGB in 195 patients. Surg Obes Relat Dis. 2014/12/03. 2014;10:1083–4. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=25443065
Novais PF, Weber TK, Lemke N, Verlengia R, Crisp AH, Rasera-Junior I, et al. Gene polymorphisms as a predictor of body weight loss after Roux-en-Y gastric bypass surgery among obese women. Obes Res Clin Pr. 2016/08/25. 2016;10:724–7. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=27554131
Nicoletti CF, de Oliveira AP, Brochado MJ, Pinhel MA, de Oliveira BA, Marchini JS, et al. The Ala55Val and -866G>A polymorphisms of the UCP2 gene could be biomarkers for weight loss in patients who had Roux-en-Y gastric bypass. Nutrition. 2016/10/17. 2017;33:326–30. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=27743836
Sarzynski MA, Jacobson P, Rankinen T, Carlsson B, Sjostrom L, Bouchard C, et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int J Obes. 2010/08/25. 2011;35:676–83. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20733583
Rinella ES, Still C, Shao Y, Wood GC, Chu X, Salerno B, et al. Genome-wide association of single-nucleotide polymorphisms with weight loss outcomes after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2013/05/02. 2013;98:E1131–6. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23633212
Magi R, Manning S, Yousseif A, Pucci A, Santini F, Karra E, et al. Contribution of 32 GWAS-identified common variants to severe obesity in European adults referred for bariatric surgery. PLoS One. 2013/08/21. 2013;8:e70735. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23950990
Bandstein M, Voisin S, Nilsson EK, Schultes B, Ernst B, Thurnheer M, et al. A genetic risk score is associated with weight loss following Roux-en Y gastric bypass surgery. Obes Surg. 2016/02/03. 2016;26:2183–9. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=26832135
Prudom C, Liu J, Patrie J, Gaylinn BD, Foster-Schubert KE, Cummings DE, et al. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and interpretation of plasma ghrelin levels. J Clin Endocrinol Metab. 2010
Stylopoulos N, Davis P, Pettit JD, Rattner DW, Kaplan LM. Changes in serum ghrelin predict weight loss after Roux-en-Y gastric bypass in rats. Surg Endosc. 2005/05/28. 2005;19:942–6. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15920683
Kruljac I, Mirosevic G, Kirigin LS, Nikolic M, Ljubicic N, Budimir I, et al. Changes in metabolic hormones after bariatric surgery and their predictive impact on weight loss. Clin Endocrinol. 2016/07/21. 2016;85:852–60. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=27439154
Labayen I, Ortega FB, Ruiz JR, Lasa A, Simon E, Margareto J. Role of baseline leptin and ghrelin levels on body weight and fat mass changes after an energy-restricted diet intervention in obese women: effects on energy metabolism. J Clin Endocrinol Metab. 2011/04/08. 2011;96:E996–1000. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21470990
Pellitero S, Perez-Romero N, Martinez E, Granada ML, Moreno P, Balibrea JM, et al. Baseline circulating ghrelin does not predict weight regain neither maintenance of weight loss after gastric bypass at long term. Am J Surg. 2015/02/24. 2015;210:340–4. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=25701890
Kleinert M, Sachs S, Habegger KM, et al. Glucagon regulation of energy expenditure. Int J Mol Sci. 2019;20(21):5407. https://doi.org/10.3390/ijms20215407.
Habegger KM, Stemmer K, Cheng C, et al. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62:1453–63.
Kim T, Nason S, Holleman C, et al. Glucagon receptor signaling regulates energy metabolism via hepatic farnesoid X receptor and fibroblast growth factor 21. Diabetes. 2018;
Werling M, Fandriks L, Vincent RP, Cross GF, le Roux CW, Olbers T. Preoperative assessment of gut hormones does not correlate to weight loss after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2014/10/06. 2014;10:822–8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=25282191
Snyder B, Nguyen A, Scarbourough T, Yu S, Wilson E. Comparison of those who succeed in losing significant excessive weight after bariatric surgery and those who fail. Surg Endosc. 2009/02/03. 2009;23:2302–6. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19184204
Carlin AM, O’Connor EA, Genaw JA, Kawar S. Preoperative weight loss is not a predictor of postoperative weight loss after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007/12/11. 2008;4:481–5. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18065295
Ma Y, Pagoto SL, Olendzki BC, Hafner AR, Perugini RA, Mason R, et al. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. 2006/09/23. 2006;16:1227–31. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16989709
Campos GM, Rabl C, Mulligan K, Posselt A, Rogers SJ, Westphalen AC, et al. Factors associated with weight loss after gastric bypass. Arch Surg. 2008/09/17. 2008;143:877–83; discussion 884. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18794426
Welch G, Wesolowski C, Piepul B, Kuhn J, Romanelli J, Garb J. Physical activity predicts weight loss following gastric bypass surgery: findings from a support group survey. Obes Surg. 2008/03/28. 2008;18:517–24. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18365295
Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol. 2011;
Yanos BR, Saules KK, Schuh LM, et al. Predictors of lowest weight and long-term weight regain among Roux-en-Y gastric bypass patients. Obes Surg. 2015;
Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg. 2018;153:427.
Cooper TC, Simmons EB, Webb K, Burns JL, Kushner RF. Trends in weight regain following Roux-en-Y gastric bypass (RYGB) bariatric surgery. Obes Surg. 2015/01/18. 2015;25:1474–81. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=25595383
Hao Z, Munzberg H, Rezai-Zadeh K, Keenan M, Coulon D, Lu H, et al. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int J Obes. 2014/10/29. 2015;39:798–805. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=25349056
Mokadem M, Zechner JF, Uchida A, Aguirre V. Leptin is required for glucose homeostasis after Roux-en-Y gastric bypass in mice. PLoS One. 2015/10/09. 2015;10:e0139960. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=26445459
Shantavasinkul PC, Omotosho P, Corsino L, Portenier D, Torquati A. Predictors of weight regain in patients who underwent Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016/12/19. 2016;12:1640–5. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=27989521
Keith Jr. CJ, Gullick AA, Feng K, Richman J, Stahl R, Grams J. Predictive factors of weight regain following laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2017/10/27. 2017; Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=29067574
Funding
This study receiving funding from Harvard Catalyst (USA) (Award No: KL2 TR002542); Eric G. Sheu, MD, PhD, is the grant recipient.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ali Tavakkoli is cofounder and consultant for AltrixBio with an equity stake in the company. Eric Sheu is consultant for Medtronic. All the other authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Figure 1
Average total body weight loss (%) following Roux-en-Y gastric bypass in in top third WLmax (one third with highest WLmax) and bottom third WLmax patients. WLmax = maximal total body weight Loss (DOCX 650 kb)
Supplementary Figure 2
Average total body weight loss (%) following Roux-en-Y gastric bypass in top third WR (one third with highest weight regain) and bottom third WR patients. WR = weight regain (DOCX 646 kb)
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Aliakbarian, H., Bhutta, H.Y., Heshmati, K. et al. Pre-operative Predictors of Weight Loss and Weight Regain Following Roux-en-Y Gastric Bypass Surgery: a Prospective Human Study. OBES SURG 30, 4852–4859 (2020). https://doi.org/10.1007/s11695-020-04877-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04877-7