Abstract
In recent years, plenty of researches have reported in obese individuals with abnormal brain processes implicated in homeostatic regulation, reward, emotion, memory, attention, and executive function in eating behaviors. Thus, treating obesity cannot remain “brainless.” Behavioral and psychological interventions activate the food reward, attention, and motivation system, leading to minimal weight loss and high relapse rates. Pharmacotherapy is an effective weight loss method and regulate brain activity but with concerns about its brain function safety problems. Obesity surgery, the most effective therapy currently available for obesity, shows pronounced effects on brain activity, such as deactivation of reward and attention system, and activation of inhibition control toward food cues. In this review, we present an overview of alterations in the brain after the three common weight loss methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the average BMI increases globally, strategies to fight obesity have emerged one after another [1, 2]. Available treatments currently include behavioral modification (diet and exercise) [1, 3], psychological conditioning [4, 5], pharmacotherapy [6], as well as obesity surgery [7], gut microbiota therapy [8], and even Chinese traditional medicine or acupuncture therapy [9], all of which may be able to contribute to the success of battling the obesity epidemic. However, the effects of weight loss treatments vary significantly between individuals. In recent years, a growing amount of literature has highlighted brain processes involved in eating behavior, proposing that obese individuals exhibit brain functional abnormalities implicated in reward, attention, emotion, memory, homeostatic regulation of food intake, and executive function including inhibitory control of feeding behavior, appealing that treating obesity cannot remain “brainless” [10].
Behavioral and psychological interventions, the most common weight loss methods, often yield suboptimal results in long-term follow-up, resulting in minimal weight loss or high relapse rates [1], which could be partly attributable to the changes in brain activity [11]. Pharmacotherapies, most of which initially acts on or influence the brain [12], are also commonly used and have resulted in encouraging weight loss. However, concerns about brain function side effects have limited widespread use. Obesity surgery, the most effective treatment currently available for morbid obesity and diabetes, typically leads to 23%, 17%, 16%, and 18% changes in body weight for 2, 10, 15, and 20 years after surgery, respectively [7], with altered brain activity [13, 14]. In this narrative review, we present a brief overview of the brain regulation of eating, and then, we describe the crosstalk between brain and the three most commonly practiced weight loss treatments.
Central Nervous System Regulation of Eating
The Hypothalamus Regulation of Energy Homeostasis
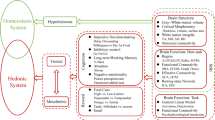
Brain regulation of eating is complicated, and almost all of the neural systems are involved (Fig. 1). The hypothalamus is the primary region responsible for energy homeostasis. The regulation of this process depends on the precise orchestration of complex physiological responses such as food intake and energy expenditure by the hypothalamus. The key pathway to this regulation is the melanocortin system, which consists of two functionally antagonistic neuronal populations: one subset expresses the orexigenic neuropeptides agouti-related peptide (AgRP) and neuropeptide Y (NPY), while the other subset expresses the anorexigenic peptides proopiomelanocortin (POMC) and cocaine and amphetamine-regulated transcript (CART) [15]. External factors activated or deactivated different groups of neurons in the hypothalamus, influencing eating behaviors and energy balance. For example, circulating levels of insulin and leptin, proportionate to nutritional status and adipose tissue stores, inhibit AgRP neurons and activate POMC neurons, leading to decreased energy intake and increased energy expenditure [16,17,18]. Other key peripheral molecules impacting the hypothalamus and energy homeostasis include ghrelin [19], GLP-1 [20,21,22], adiponectin [23], irisin [24], and inflammatory factors (discussed in the later). In addition to the crosstalk between groups of neurons inside the hypothalamus, it also receives external signals and communicates directly with other brain areas such as reward, emotion, memory systems, as well as the cognitive control and other cortical areas. Thus, the dysregulation of the hypothalamus may also be subject to control/influence by higher systems in humans.
Brain regulation of eating. Brain regulation of eating involves almost all of the neural systems and is influenced by inflammation in the brain, hormones, and blood-brain barrier (BBB) functions. Strikes from the environmental or gene mutations disrupt the normal regulation of energy balance in the brain, and result in obesity. AgRP agouti-related peptide, CART cocaine and amphetamine-regulated transcript, D2 receptors dopamine 2 receptors, DLPFC dorsolateral prefrontal cortex, GLP-1 glucagon-like peptide-1, NPY neuropeptide Y, OFC orbitofrontal cortex, POMC proopiomelanocortin, pre-SMA pre-supplementary motor area, SN substantia nigra, VTA ventral tegmental area
Higher Central Nerve System Regulation of Eating Behavior
The higher central nerve system in modification of energy balance is much more complicated than that in the hypothalamus. The regulation of eating behavior by activation or deactivation of certain regions in the brain is controversial in addition to their sophisticated cross-talks with each other. Conflicting results are reported by different research groups. Thus, we mainly discuss the mainstream ideas in the article.
The reward system, which consists of dopaminergic neurons originating in the ventral tegmental area (VTA) and substantia nigra (SN) in the midbrain project throughout the brain especially to key areas including the nucleus accumbens, striatum (especially the caudate), orbitofrontal cortex (OFC), and insula, has been extensively researched in relation to obesity in recent years. Numerous studies have indicated a respond of these areas to food cues during fMRI, especially in obese [25,26,27,28]. Some researchers have hypothesized that exposure to highly rewarding foods results in hyper-responsivity of the reward system to food cues, which leads individuals to seek foods more frequently and in greater quantity. This hyper-response theory is supported by fMRI studies in obese individuals with findings of increased activation in the nucleus accumbens, midbrain, and OFC to visual food cues [29, 30]. Other researchers are proposing a hypo-response theory that prolonged exposure to high-reward food results in lower availability of dopamine 2 (D2) receptors in the reward systems (especial the striatum), which are consistently reported in their PET studies [31, 32]. The decreased D2 receptors in reward system lead individuals to seek and consume more high-calorie foods to maintain reward and exacerbate obesity. These two seemingly paradoxical theories, the hyper-response and hypo-response theory, could in fact help to explain the occurrence and development of obesity, respectively.
The cognitive control system, sophisticated regulating of functions such as inhibitory control, food motivation, internal awareness, emotional processing, and impulse control, influences eating behavior in a very complicated and controversial way [33]. The prefrontal cortex comprises much of the cognitive control network, particularly the cingulate cortex, inferior frontal cortex, pre-supplementary motor area (pre-SMA), and dorsolateral prefrontal cortex (DLPFC). Several studies have demonstrated impaired inhibitory control in patients with obesity and a link between impaired control and future weight gain in normal-weight individuals [34,35,36]. Also, increased impulsivity has been found to be related to overweight and failing in attempts to lose weight [37].
The attention system, regulated by the parietal and visual cortices as well as some areas of the frontal cortex (e.g., anterior cingulate), has been repeatedly implicated in obesity. Obese individuals attend more to food cues under satiety and normal-weight individuals who pay more attention to food cues display patterns of overeating and weight gain [38].
The emotion system, which is primarily located at the amygdala, is well known to be a potent modulator of appetite. Joy and anger both increase appetite and create poorer dietary choices as compared to fear and sadness [39], and the effect is more pronounced in women than in men [39]. Indeed, food cues activate the amygdala [40], and activation of the amygdala could also predict the consumption of high fat or high-calorie foods [41].
Memory, primarily regulated by the hippocampus and parahippocampal formation, may also play a role in dysfunctional eating behaviors. The hippocampus and parahippocampal gyrus receive inputs regarding food cues from many other areas including the insula, orbitofrontal cortex, and arcuate nucleus of the hypothalamus [42]. It has been hypothesized that decreased functioning of the hippocampus leads to increased food intake and poorer dietary quality in turn leading to obesity [43,44,45] .
Generally, the increased activation of reward, attention, emotion, and impulsivity and motivation systems toward food, or decreased activation of the inhibitory control and impaired memory systems may play a vital role in the occurrence or exacerbation of obesity. However, no brain region acts in isolation in the regulation of eating, and there is also crosstalk with other contributors (e.g., hormones and inflammatory mediators), with intricate orchestration of complex signals through the body for regulation of eating behaviors.
Obesity Initiated by Inflammation in the Central Nerve System
Obesity commonly co-occurs with inflammation. Inflammation in the central nerve system happens earlier than in peripheral tissue and occurs preferentially in the hypothalamus since parts of it are unprotected by the blood-brain barrier (BBB) [46, 47]. It can induce hypothalamic dysfunction and lead to obesity in a two-phase process. During the early phase of inflammation, such as short exposure to a high-fat diet (HFD), the large amount of fat absorbed by the intestine causes activation of cytokines and inflammatory pathways in the hypothalamus [48]. Markers of hypothalamic inflammation increase significantly during the early days of HFD feeding, with reactive gliosis and neuronal injury manifesting during the first week, even before weight gain [46]. In parallel with the early occurrence of inflammation, 3 days of HFD feeding is sufficient to reduce hypothalamic insulin sensitivity significantly [49]. Importantly, these processes precede inflammatory events in peripheral tissues, such as the liver [47]. During the secondary inflammatory phase, prolonged inflammatory cascades lead to the activation of cellular stress mechanisms and inflammatory mediators released from non-neuronal cell types giving rise to long-lasting impaired metabolic control of the hypothalamus [50]. Proinflammatory cytokines, such as TNF-α, promote the onset of insulin and leptin resistance in the brain [51], and IKKβ/NF-κB, disrupts adult hypothalamic neural stem cells and mediates neurodegeneration [52]. Furthermore, long-term HFD feeding also alters synaptic plasticity, a change in synaptic strength in response to stimuli, in key hypothalamic neuronal systems [53].
In addition to the hypothalamus, the hippocampus is also vulnerable to inflammation despite full protection of the BBB [54, 55]. In a state of obesity, microglial action has been observed to impair hippocampal function by driving inflammation in the brain and resulting in impaired spatial recognition memory, depression and anxiety [54, 56]. Consequently, immune-mediated obesity may start with inflammation in the brain.
The BBB Dysfunction and Obesity
The BBB was first described for its ability to prevent the unregulated exchange of substances between the blood and central nerve system, including inflammatory factors described above. Over time, it has been characterized as an interface that enables regulated exchanges between the brain and peripheral cytokine and hormones. Hormones that regulate feeding are altered in obesity including insulin, leptin, adiponectin, and ghrelin that can cross the vascular BBB via specialized transport systems [57]. BBB transport is necessary for these proteins to exert their functions in the brain. Moreover, the BBB is also a secretory tissue which can secret some factors related to obesity either into the blood or the interstitial fluid of the brain [58].
Pathological changes to the BBB occur during obesity that may ultimately exacerbate disease and can lead to additional changes in the brain such as neuroinflammation and cognitive and memory impairment as described above. In addition to that, impaired BBB could result in the impaired transport of hormones [59] or the expression of other proteins at BBB [60], which leads to hormone resistance and altered cellular energy metabolism. However, the reversal of obesity can restore normal BBB functions. It has been reported that the reversal of obesity reduced FFA transport into the human brain by 17% [61]. Consequently, BBB, the mediator of peripheral communication to the brain, should not be neglected in the treatment for obesity.
The Changes of Behavioral and Psychological Interventions on Brain
Behavioral and Psychological Interventions: Conventional Weight Loss Therapy with Suboptimal Results
Behavioral and psychological interventions, the most commonly used weight loss method which modifies diet [1, 62, 63] and physical activity [1, 3, 64, 65], with or without the usage of psychosocial treatment (e.g., mindful eating, cognitive restructuring) for weight loss [4, 5, 66], also changes the brain activity. In an fMRI study of 19 postmenopausal obese women undergoing caloric restriction, Prehn et al. found improved memory score after 12-week negative energy balance for weight loss, paralleled by increased gray matter volume in the hippocampus (memory) [67].
While these interventions are simple and with low risk and economic burden, the short-term effects (weight loss) and longer-term effects (weight maintenance) of behavioral and psychological interventions are trivial, yielding minimal weight loss and high relapse rate [1, 68]. In the same study by Prehn et al., they also found that the brain restoring effects were transient only during the 12-week negative energy balance for bodyweight loss, and could not be detected after subsequent 4-week weight maintenance [67]. Moreover, subjects with more impulsivity and lower inhibition control have even worse outcomes, showing not only minimal weight loss and high relapse rates in the moment [37], but also greater risk for onset of binge eating, bulimic symptoms, and bulimia nervosa in the future [69, 70].
The Effects of Behavioral and Psychological Interventions on the Brain: an Explanation for Minimal Weight Loss
The suboptimal results of behavioral and psychological interventions could be attributable to the compensation response to the energy deficits during the interventions [71]. It resulted in the increased brain activation including regions implicated in motivation, attention, and reward valuation in response to food cues in subjects undergoing caloric restriction [72, 73], and biases brain reward systems toward high-calorie foods [74] (Fig. 2 and Table 1). By studying the fMRI of 196 dieting adolescents, Stice et al. found that fasting for hours correlated positively with activation in regions implicated in attention (anterior cingulate cortex), reward (putamen, OFC), and motivation (precentral gyrus) in response to food cues. Likewise, negative energy balance for 2 weeks (weight loss ≥ 1 kg) resulted in increased activation in attention (anterior cingulate cortex, ventral medial prefrontal cortex, superior visual cortex), and reward (caudate) regions in response to food, in addition to the restored memory system (hippocampus) [11].
The changes of behavioral and psychological interventions on brain. Behavioral and psychological interventions activates the food reward, attention, memory and motivation system, and always leads to minimal weight loss and high relapse rates. AgRP agouti-related peptide, CART cocaine and amphetamine-regulated transcript, D2 receptors dopamine 2 receptors, DLPFC dorsolateral prefrontal cortex, NPY neuropeptide Y, OFC orbitofrontal cortex, POMC proopiomelanocortin, pre-SMA pre-supplementary motor area, SN substantia nigra, VTA ventral tegmental area
Moreover, brain activation toward food cues could also predict weight loss. By studying 25 obese individuals before and after a 12-week psychosocial weight-loss treatment and at 9-month follow up, Murdaugh et al. found that those obese individuals who were least successful in losing weight during the treatment showed greater pretreatment activation in response to high-calorie food vs. control pictures in brain regions implicated in the reward-system (nucleus accumbens and insula) and attention processes (anterior cingulate, superior occipital cortex, inferior and superior parietal lobule, and prefrontal cortex). Furthermore, less successful weight maintenance at 9-month follow-up was predicted by greater post-treatment activation in brain regions implicated in reward (insula, VTA, putamen), and attention (fusiform gyrus) [4].
However, in the Look AHEAD study with 10-year follow-up, by studying 232 patients with type 2 diabetes and overweight or obesity, McDermott et al. found a better way for weight loss, a specially designed long-term intensive lifestyle intervention program (participants were assigned calorie, fat gram, and physical activity goals designed to produce 10% weight loss). Compared with participants in regular diabetes support and education programs, patients in their lifestyle intervention program showed less activated reward system (left caudate) by high-calorie food cues, though with greater activated attention system (left angular and occipital cortex) which might diminish the weight loss effect. Still, those patients in their lifestyle intervention program showed a much more effective weight loss result than patients in regular diabetes support and education programs since the first year of follow-up. Yet, unfortunately, the differences between the two groups diminished year by year and lasted up to the 9-year follow-up [75]. Therefore, behavioral and psychological interventions programs must consider their influence on brain activity to achieve optimal results.
The Influence of Weight Loss Medicine on Brain
Pharmacotherapies: a Double-Edged Sword for Obesity
Pharmacotherapies, most of which initially act or have an influence on the brain, are also effective in weight loss options [12, 76]. By analyzing 50 publications comprising 43,443 obese individuals undergoing pharmacotherapy in a system review, Dong et al. found that the maximal mean weight loss relative to placebo for orlistat (120 mg), lorcaserin, naltrexone-bupropion, phentermine-topiramate (7.5/46 mg), and liraglutide was − 2.94, − 3.06, − 6.15, − 7.45, and − 5.5 kg, at weeks 60, 54, 67, 59, and 65, with mean rates of regain of 0.51 kg, 0.48 kg, 0.91 kg, 1.27 kg, and 0.43 kg per year, respectively [76]. The brain target of pharmacotherapies is dopamine, norepinephrine, or serotonin, the increased activation of which in the brain can stimulate hypophagia, weight loss, and in some cases, energy expenditure (Fig. 3 and Table 1). However, brain function safety concerns exist, and side effects such as suicidal ideation and depression can occur due to the broad range of targets in the brain of these medications. In the same meta-analysis, Dong et al. also described the 1-year dropout rates for orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide were as high as 29.0, 40.9, 49.1, 34.9, and 24.3%, respectively, mainly due to adverse effects [76].
The influence of weight loss medicine on the brain. Pharmacotherapy is an effective weight loss method and regulates brain activity. Nevertheless, concerns about its brain function safety problems limit its extensive use. AgRP agouti-related peptide, CART cocaine and amphetamine-regulated transcript, D2 receptors dopamine 2 receptors, DLPFC dorsolateral prefrontal cortex, GLP-1 glucagon-like peptide-1, NPY neuropeptide Y, OFC orbitofrontal cortex, POMC proopiomelanocortin, pre-SMA pre-supplementary motor area, SN substantia nigra, VTA ventral tegmental area
Liraglutide
Liraglutide is an agonist of GLP-1 receptor, which presents in the human hypothalamus, medulla, and parietal cortex in addition to peripheral organs [77]. GLP-1 acts on the mesolimbic reward system in addition to the hypothalamus regulating homeostatic feeding. GLP-1 crosses the BBB at the area postrema and directly stimulates hypothalamic anorexigenic neurons (e.g., POMC) and deactivates the reward system involving the VTA and the nucleus accumbens [78]. Liraglutide has been reported to result in a weight loss of 4.7–6.1% during 56-week follow-up [79, 80]. By studying fMRI from 21 individuals with type 2 diabetes, Farr et al. found that liraglutide decreased the activation in the reward system (insula and putamen) as well as attention network (parietal cortex) in response to highly vs. less desirable food images. Meanwhile, participants taking liraglutide rated themselves as being fuller while fasting and trended toward feeling less pleasant to eat [77]. Nausea is the most common adverse effect, which is also related to the changes in activation in brain regions [77].
Naltrexone-Bupropion
Naltrexone-bupropion, approved for the treatment of obesity since 2014, stimulates dopamine and POMC neurons and blocks inhibitory feedback to mu-opioid receptors on POMC neurons, which results in decreased appetite and increased energy expenditure. Naltrexone-bupropion has been reported to result in 5.0–9.3% weight loss during 28–56 weeks observation [81,82,83,84]. As expected, fMRI study data from 40 obese women indicated that 4 weeks of naltrexone-bupropion treatment attenuated activation in the hypothalamus in response to food cues, as well as restored the activation of the memory system (hippocampal). Yet, researchers also found that the medicine influence the other higher central nerve systems, such as enhancing the activation of attention (anterior cingulate, superior parietal), reward (insula), and internal awareness systems (superior frontal) [85]. The wide range influence on the higher central nerve systems could enhance or diminish its effect on weight loss and cause side effects, which still need to be further investigated. Nausea is the most common adverse effect, and the FDA still carries the black box warning regarding suicidal ideation and actions.
Lorcaserin
Lorcaserin, a selective 5-hydroxytryptamine 2C receptor agonist, was approved in 2012. Evidence from plenty of studies suggests that lorcaserin has multiple psychological effects that contribute to weight loss, including elevation of satiety, reduction in craving, and impulsivity [86]. Clinical trials have reported resulting in a weight loss effect of 4.5–7.0% [87,88,89]. By studying 48 obese participants, Farr et al. found that lorcaserin exerts its weight-reducing effects by decreasing the attention (parietal and visual cortices), emotion (amygdala), and reward (insula) activity to food cues. Meanwhile, total caloric intake decreased about one quarter over 4 weeks in participants on lorcaserin. Moreover, baseline activation of the amygdala is associated with increased efficacy, suggesting that lorcaserin would be of particular benefit to emotional eaters [90]. Headache and dizziness are the most common adverse effects.
Currently, there are still plenty of centrally acting anti-obesity drugs, such as topiramate-phentermine that modulate norepinephrine release, GABA activity, voltage-gated ion channel modulation, inhibition of AMPA/kainite excitatory glutamate receptors, and inhibition of carbonic anhydrase; and phentermine, diethylpropion, benzphetamine, and phendimetrazine which are appetite-suppressant which stimulate norepinephrine release with minor dopamine release [12]. The wide range of targets in the brain, such as the energy homeostasis, reward, attention, and cognitive systems, allow pharmacotherapies with a fairly robust weight loss effect. However, the side effects, such as increased blood pressure and heart rate, insomnia, paresthesia, dry mouth, depression, anxiety, and constipation, which come from the unexpected targets of the medicines, limit their widespread use. In fact, concern about the safety of weight loss drugs has long been raised, with many weight loss drugs (such as sibutramine, rimonabant, caffeine, ephedra, and phenylpropanolamine among others) having been removed from the market [6, 12, 91].
Brain Regulation by Obesity Surgery
Obesity Surgery: the Most Effective Treatment for Obesity
Obesity surgery is currently the most effective long-term treatment for morbid obesity. The most commonly performed procedures are Roux-en-Y gastric bypass (RYGB), vertical sleeve gastrectomy (VSG), and adjustable gastric band (BAND). The mean changes in body weight for 2, 10, 15, and 20 years after surgery are 23%, 17%, 16%, and 18%, respectively [7, 14, 92]. In addition to the weight loss, our group and others have reported its ability to regulate glucolipid metabolism and insulin resistance, hormones balances (e.g., testosterone), inflammation, as well as decreasing the incidences of diabetes, myocardial infarction, stroke, cancer, and most importantly, overall mortality [7, 93,94,95,96,97]. Interestingly, patients also typically reported decreased hunger and lower caloric intake after surgery, in addition to decreased desire for core tastes and a shift in food preferences from high- to low-energy foods [98, 99]. This change in the motivation of caloric intake after obesity surgery is considered to be due to its effect on the brain, with plenty of fMRI studies showing decreased activation in the reward, attention, and motivation network and increase in the inhibition control system toward high vs. low energy food after bariatric surgeries [98, 100,101,102] (Fig. 4 and Table 1).
Brain regulation by bariatric surgery. Bariatric surgery is the most effective treatment currently available for morbid obesity and diabetes. It shows pronounced effects on brain activity, such as deactivation of regions implicated food reward, attention, motivation, memory, and emotion, and activation of regions implicated inhibition control toward food cues. AgRP agouti-related peptide, BAND adjustable gastric band, CART cocaine and amphetamine-regulated transcript, D2 receptors dopamine 2 receptors, DLPFC dorsolateral prefrontal cortex, NPY neuropeptide Y, OFC orbitofrontal cortex, POMC proopiomelanocortin, pre-SMA pre-supplementary motor area, RYGB Roux-en-Y gastric bypass, SN substantia nigra, VSG vertical sleeve gastrectomy, VTA ventral tegmental area
Regulation of Brain Activity by Obesity Surgery
Gastric Bypass
Gastric bypass is the most efficacious obesity surgery, resulting in a weight loss of 32 ± 8% at 1–2 years and 27 ± 12% at 15 years after the surgery [7]. This excellent result on weight loss could not be achieved without its effect on the brain, resulting in decreased activation of reward and attention systems and increased activation of inhibitory control. By studying the fMRI of 16 patients 4 months after RYGB, Baboumian et al. observed decreased activation in attention systems (fusiform gyrus, inferior temporal gyrus, and right middle occipital gyrus) and increased activation in inhibition control (DLPFC, right medial prefrontal gyrus, and paracingulate) in response to high vs. low energy food cues, together with patients’ BMI decrease of 9.1 kg/m2 [13]. In addition, the authors also observed decreased activation in parahippocampal [13], region implicating in memory or reward-processing, which is still controversial. Likewise, postsurgical reduction in brain responsiveness within the attention network (superior parietal and precuneus) toward high vs. low-energy foods has also been observed by Zoon et al. in the fMRI of 19 RYGB patients, together with their reports of a shift in food preferences from high-energy foods to low-energy foods [103]. By studying the fMRI of 10 female patients 1 month post-RYGB, Ochner et al. also found significant reductions in brain activation within the reward pathway (ventral striatum, VTA, putamen, lentiform nucleus) toward high vs. low-calorie food cues, in accordance with patients’ changes in desire to eat from high-energy food to low-energy food [102]. However, the author also reported reduced activity in inhibitory control (DLPFC) and other prefrontal cognitive systems (ventrolateral prefrontal cortex and dorsomedial prefrontal cortex) toward high vs. low-calorie food cues in these patients [102], which was contradictory to the results in other studies [13]. The authors believe that the activity changes in the prefrontal cognitive system could imply reduced reward-processing which was also a vital function of the cognitive system [102]. Thus, further studies are needed to advance our understanding of the relationship between the prefrontal cortex and obesity surgery. Still, there were plenty of similar findings reported in other studies [104]. In fact, Frank et al. reported an fMRI study that even showed a restoration by RYGB in patterns of brain activation to food stimuli similar to those observed in normal-weight individuals after an average of 3.4 ± 0.8 years following RYGB, though patients’ self-rated disinhibition and hunger were only partly restored [105].
VSG
VSG is currently considered as the optimal obesity surgery for most patients with morbid obese. Although the weight loss (25 ± 9% in 1–2 years and 18 ± 11% in 15 years post-surgery) percentage is a little lower than RYGB [7], the non-weight loss outcomes are controversial between the two procedures [106, 107], and serious complications are least in VSG [107]. By studying the fMRI of 9 patients 4 months after VSG, Baboumian et al. found that similar to RYGB though less robustly, VSG also exert its weight loss effect by increased inhibition (DLPFC) and decreased attention (fusiform gyrus) and reward or memory (parahippocampus) activation in response to high- vs. low-energy food cues [13]. Moreover, by studying 22 patients 1 month after VSG, Li et al. reported that VSG also significantly decreased brain activation in the food motivation (right DLPFC) in response to high-caloric vs. low-caloric food cues. Accordingly, the patients also rated a significant reduction of craving for high-calorie food. The decrease in right DLPFC activation in response to high-caloric vs. low-caloric food cues after surgery was positively correlated with the reduction in craving for high-caloric vs. low-caloric food cues [108].
BAND
BAND has been reported with a weight loss of 20 ± 10% in 1–2 years and 13 ± 14% in 15 years after surgery. Changes in brain activation toward food cues have also been found in cognitive and reward systems. Bruce et al. found significantly less self-reported hunger, disinhibition, and increased cognitive restraint rated by ten patients 12 weeks after BAND surgery, accompanied by decreased brain activation to food vs. nonfood pictures in regions implicated in food motivation (medial prefrontal cortex, inferior frontal gyrus) and reward and memory (insula, parahippocampus), and increased activation in cognitive control and inhibition (anterior prefrontal cortex). Moreover, correlation analysis indicated that less activation to food vs. non-food cues at post-meal in the right inferior frontal gyrus was associated with self-rated increased cognitive restraint and reduced hunger from before to after surgery, and less activation to food vs. non-food cues at post-meal in the right middle frontal gyrus was associated with larger reduction in self-rated disinhibited eating from before to after surgery [109].
Different Procedures with Varied Effects on Brain Activity
Although all kinds of bariatric surgeries exert weight loss and affect the brain, brain activity changes vary depending on the different procedures. Scholtz et al. compared the two most common bariatric surgical procedures, RYGB and BAND, and found that RYGB caused a greater reduction in the activation of brain reward areas to high-energy food pictures compared with BAND. Accordingly, high-calorie foods were rated as less appealing and percentage energy intake derived from fat was lower in patients after RYGB than after BAND. Also, patients after RYGB has shown healthier eating behavior and less eating disorder psychopathology compared with the BAND [104]. By comparing fMRI data from patients after RYGB and VSG, Baboumian et al. reported more pronounced changes in brain activity in regions implicated inhibitory control (DLPFC) after RYGB [13]. Thus, RYGB appears to be more effective than the other two procedures in modulating brain activity such as reward and inhibitory control systems.
In addition to variation among the different procedures, different individuals also respond differently with varying effects on the brain and eating behavior. Compare with individuals without weight regain, those with weight regain post-surgery have shown a 2.2-fold higher rate of eating psychopathology [110]. Consistently, patients who are less successful at losing weight after surgery have shown to have a lower increase in activation of the areas involved in inhibition but no significant change in the reward areas compared with their more successful weight loss counterparts [111]. Thus, brain activity is a pivotal factor in the prediction of weight loss.
Bariatric Regulation of Resting-State Functional Connectivity of Brain Regions
Aside from its effects on the brain activation, obesity surgery also modifies resting-state functional connectivity of different brain regions [112, 113]. By studying resting-state fMRI of 17 patients after VSG, Li et al. found that the obesity surgery could recover the dysfunction of some brain regions, e.g., a decreased resting-state activities and increased functional connectivity in reward processing and cognitive control regions (orbitofrontal cortex, middle frontal gyrus, superior frontal gyrus, and gyrus rectus), which were associated with BMI in the correlation analysis [112]. Also, using the functional connectivity density mapping in 22 obese participants 1 month after VSG, Li et al. found that significantly reduced functional connection in cortical regions implicated in self-referential processing and interoceptive awareness along with strengthening of connectivity of these regions with cortical (DLPFC) and striatal (caudate) regions implicated in executive control/self-regulation in them [113].
The Potential Mechanism by Which Obesity Surgery Affects the Brain Activity
Correlation Between Changes of Hormone and Brain Activity After Obesity Surgery
There is still controversy regarding the changes in hormones after surgery among different procedures, which could be related to changes in brain activity. Li et al. reported that decreased brain activation after VSG was positively correlated with a reduction in ghrelin levels but not insulin and leptin levels [108]. However, Zoon et al. found no change in ghrelin levels after RYGB though an alteration in brain activation was also observed [103]. RYGB has been reported to have a stronger correlation with postprandial plasma PYY and GLP-1 [104, 114,115,116]. Hormone changes after BAND are less clear than the other two surgeries [104]. Therefore, more studies are needed to explore the regulatory mechanisms of obesity surgery on brain activity, especially its connection with hormone levels.
Obesity Surgery Modifies Inflammation in the Brain
Obesity surgery also modifies inflammation in the brain in patients with obesity, which partially accounts for its outstanding weight loss effect. Van de Sande-Lee et al. have studied 13 patients after RYGB surgery and found that the obesity surgery increased cerebrospinal fluid (CSF) concentrations of interleukin (IL)-10 and IL-6 levels, accompanied by a partial reversal of hypothalamic dysfunction on fMRI, massive loss of body mass, and dramatically decreased caloric and saturated fats intake [117]. Consistently, by studying the changes of myo-inositol concentration (a putative marker of neuroinflammation) in 23 patients with morbidly obese and intra-gastric balloon surgery during the 3-month follow-up, Gazdzinski et al. found that the obesity surgery suppressed brain inflammatory responses consistent with weight loss, preceding the remission of metabolic abnormalities [118]. Thus, regulation of inflammation seems to be another contributor to the restoration of brain activity after the obesity surgery.
Obesity Surgery Changes the Expression of Receptors in the Brain
Additionally, obesity surgery changes the expression of brain receptors. By analyzing the PET images of 5 women before and 7 weeks after RYGB or VSG, Dunn et al. found dopamine type 2 (DA D2) receptor availability decreased after obesity surgery. Regional decreases were caudate 10 ± 3%, putamen 9 ± 4%, ventral striatum 8 ± 4%, substantia nigra 10 ± 2% (all four regions from reward systems), amygdala 9 ± 3% (emotion system), hypothalamus 9 ± 3% (energy homeostasis), and medial thalamus 8 ± 2%, together with patients’ decreased rate of depression and binge eating [119]. The above changes in the brain receptors could also explain the changes of brain activity after the obesity surgery to some extent.
Brain Regulation Comparing Obesity Surgery and Behavioral and Psychological Interventions
From the discussion above, it is clear that the changes in the activation of brain regions after obesity surgery are completely opposite compared with that after behavioral and psychological interventions. Obesity surgery always leads to the deactivation of food motivation, reward and attention networks with patients’ reporting of decreased craving for food and shift preference from high to low energy food. Conversely, these brain regions are usually activated after behavioral and psychological interventions, and patients are at greater risk for future binge eating. Baboumian et al. have compared the fMRI of 25 patients 3–4 months after obesity surgery and 14 patients 3–4 months after weight loss behavior intervention and found totally opposite changes in brain activity between these two groups of patients. Patients after surgery showed increased activity in inhibitory control (DLPFC) and decreased activity in memory and attention (left parahippocampal gyrus and fusiform) in response to high-energy diet vs. low-energy diet. While patients after behavior intervention showed decreased activity in inhibitory control and increased activity in memory and attention [13]. Another study by Bruce et al. compared the fMRI data from 16 behavioral dieters and 15 patients after obesity surgery with similar weight loss of about 10% and found that the behavioral dieters showed increased responses to food cues in the medial prefrontal cortex (attention system) when compared to patients with obesity surgery [120]. The differences in brain activation may help to explain why weight loss diets typically do not produce pronounced and sustainable weight loss as obesity surgery.
Conclusion
In conclusion, brain regulation of eating is a complicated process that involves brain regions implicated in homeostatic regulation, reward, emotion, memory, attention, and executive function including inhibitory control of feeding behavior and food motivation. The effects and safety of weight loss methods are greatly dependent on brain regulation. Although behavioral and psychological interventions are pervasive and easy to implement, they have shown paltry weight loss effect both in short- and long-term observation. The activation of the food reward, attention, and motivation systems may be responsible for the minimal weight loss and high relapse rate. Pharmacological therapy, especially drug acting on the brain, is also an effective weight loss method. However, its safety concerns related to the brain, such as depression, suicide, and nausea, have limited its widespread implementation. Obesity surgery, which shows an excellent weight loss effect, maybe efficacious due to its effect on brain regulation and deactivation in reward, attention, and motivation as well as activation in the inhibitory control network.
Nevertheless, it still would be worth highlighting that conclusions based on fMRI studies are limited by the small sample size of studies (most of which included only dozens of patients) in addition to some controversial results in these studies. Moreover, correlations between weight loss and changes in fMRI do not necessarily prove that these methods lead to changes in brain activity that lead to weight loss. More larger clinical studies and mechanism researches are needed to further prove the relation between brain regulation and weight loss. Still, targeting the brain to improve efficacy and avoid side effect is probably a vital way forward toward achieving sustainable weight loss.
References
Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755–67.
Wolfenden L, Ezzati M, Larijani B, et al. The challenge for global health systems in preventing and managing obesity. Obes Rev. 2019;
Reilly JJ, Hughes AR, Gillespie J, et al. Physical activity interventions in early life aimed at reducing later risk of obesity and related non-communicable diseases: a rapid review of systematic reviews. Obes Rev. 2019;20(Suppl 1):61–73.
Murdaugh DL, Cox JE, Cook 3rd EW, et al. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage. 2012;59(3):2709–21.
Fuentes Artiles R, Staub K, Aldakak L, et al. Mindful eating and common diet programs lower body weight similarly: systematic review and meta-analysis. Obes Rev. 2019;1
Krentz AJ, Fujioka K, Hompesch M. Evolution of pharmacological obesity treatments: focus on adverse side-effect profiles. Diabetes Obes Metab. 2016 Jun;18(6):558–70.
Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34.
Mikkelsen KH, Allin KH, Knop FK. Effect of antibiotics on gut microbiota, glucose metabolism and body weight regulation: a review of the literature. Diabetes Obes Metab. 2016;18(5):444–53. Epub 2016/01/29
Yao J, He Z, Chen Y, et al. Acupuncture and weight loss in Asians: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2019;98(33):e16815.
Farr OM, Li CS, Mantzoros CS. Central nervous system regulation of eating: insights from human brain imaging. Metabolism. 2016;65(5):699–713.
Stice E, Burger K, Yokum S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. NeuroImage. 2013;67:322–30.
Coulter AA, Rebello CJ, Greenway FL. Centrally acting agents for obesity: past, present, and future. Drugs. 2018;78(11):1113–32.
Baboumian S, Pantazatos SP, Kothari S, et al. Functional magnetic resonance imaging (fMRI) neural responses to visual and auditory food stimuli pre and post Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). Neuroscience. 2019;12
O'Brien PE, McPhail T, Chaston TB, et al. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032–40.
Schwartz MW, Porte Jr D. Diabetes, obesity, and the brain. Science. 2005;307(5708):375–9.
Benoit SC, Air EL, Coolen LM, et al. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22(20):9048–52.
Choudhury AI, Heffron H, Smith MA, et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115(4):940–50.
Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–4.
Zigman JM, Bouret SG, Andrews ZB. Obesity impairs the action of the neuroendocrine ghrelin system. Trends Endocrinol Metab. 2016;27(1):54–63.
Maniscalco JW, Zheng H, Gordon PJ, et al. Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J Neurosci. 2015;35(30):10701–14.
van Bloemendaal L, Veltman DJ, ten Kulve JS, et al. Emotional eating is associated with increased brain responses to food-cues and reduced sensitivity to GLP-1 receptor activation. Obesity (Silver Spring). 2015;23(10):2075–82.
Skow MA, Bergmann NC, Knop FK. Diabetes and obesity treatment based on dual incretin receptor activation: ‘twincretins’. Diabetes Obes Metab. 2016;18(9):847–54.
Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–26.
Bostrom PA, Fernandez-Real JM, Mantzoros C. Irisin in humans: recent advances and questions for future research. Metabolism. 2014;63(2):178–80.
Garcia-Garcia I, Horstmann A, Jurado MA, et al. Reward processing in obesity, substance addiction and non-substance addiction. Obes Rev. 2014;15(11):853–69.
DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15(10):1330–5.
Ziauddeen H, Alonso-Alonso M, Hill JO, et al. Obesity and the neurocognitive basis of food reward and the control of intake. Adv Nutr. 2015;6(4):474–86.
Volkow ND, Wang GJ, Tomasi D, et al. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14(1):2–18.
Stice E, Spoor S, Bohon C, et al. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–35.
Stice E, Yokum S, Bohon C, et al. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010;50(4):1618–25.
Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7.
Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage. 2008;42(4):1537–43.
Gluck ME, Viswanath P, Stinson EJ. Obesity, appetite, and the prefrontal cortex. Curr Obes Rep. 2017;6(4):380–8.
Kullmann S, Heni M, Veit R, et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care. 2015;38(6):1044–50.
Reyes S, Peirano P, Peigneux P, et al. Inhibitory control in otherwise healthy overweight 10-year-old children. Int J Obes. 2015;39(8):1230–5.
Levitan RD, Rivera J, Silveira PP, et al. Gender differences in the association between stop-signal reaction times, body mass indices and/or spontaneous food intake in pre-school children: an early model of compromised inhibitory control and obesity. Int J Obes. 2015;39(4):614–9.
Nederkoorn C, Jansen E, Mulkens S, et al. Impulsivity predicts treatment outcome in obese children. Behav Res Ther. 2007;45(5):1071–5.
Doolan KJ, Breslin G, Hanna D, et al. Attentional bias to food-related visual cues: is there a role in obesity? Proc Nutr Soc. 2015;74(1):37–45.
Macht M. Characteristics of eating in anger, fear, sadness and joy. Appetite. 1999;33(1):129–39.
O'Doherty JP, Deichmann R, Critchley HD, et al. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–26.
Mehta S, Melhorn SJ, Smeraglio A, et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96(5):989–99.
Wang GJ, Yang J, Volkow ND, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci U S A. 2006;103(42):15641–5.
Martin AA, Davidson TL. Human cognitive function and the obesogenic environment. Physiol Behav. 2014;136:185–93.
Parent MB, Darling JN, Henderson YO. Remembering to eat: hippocampal regulation of meal onset. Am J Physiol Regul Integr Comp Physiol. 2014;306(10):R701–13.
Loprinzi PD, Frith E. Obesity and episodic memory function. J Physiol Sci. 2018;68(4):321–31.
Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–62.
Prada PO, Zecchin HG, Gasparetti AL, et al. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology. 2005;146(3):1576–87.
De Souza CT, Araujo EP, Bordin S, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–9.
Clegg DJ, Gotoh K, Kemp C, et al. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103(1):10–6.
Jais A, Bruning JC. Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest. 2017;127(1):24–32.
Romanatto T, Cesquini M, Amaral ME, et al. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient--effects on leptin and insulin signaling pathways. Peptides. 2007;28(5):1050–8.
Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14(10):999–1012.
Chun SK, Jo YH. Loss of leptin receptors on hypothalamic POMC neurons alters synaptic inhibition. J Neurophysiol. 2010;104(5):2321–8.
Andre C, Dinel AL, Ferreira G, et al. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun. 2014;41:10–21.
Sobesky JL, Barrientos RM, De May HS, et al. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immun. 2014;42:22–32.
Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011 Sep 9;333(6048):1456–8.
Banks WA. Peptides and the blood-brain barrier. Peptides. 2015;72:16–9.
Banks WA. The blood-brain barrier as an endocrine tissue. Nat Rev Endocrinol. 2019;15(8):444–55.
Rhea EM, Salameh TS, Logsdon AF, et al. Blood-brain barriers in obesity. AAPS J. 2017;19(4):921–30.
Ouyang S, Hsuchou H, Kastin AJ, et al. Diet-induced obesity suppresses expression of many proteins at the blood-brain barrier. J Cereb Blood Flow Metab. 2014;34(1):43–51.
Karmi A, Iozzo P, Viljanen A, et al. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59(9):2171–7.
Andela S, Burrows TL, Baur LA, et al. Efficacy of very low-energy diet programs for weight loss: a systematic review with meta-analysis of intervention studies in children and adolescents with obesity. Obes Rev. 2019;20(6):871–82.
Astbury NM, Piernas C, Hartmann-Boyce J, et al. A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss. Obes Rev. 2019;20(4):569–87.
Yen HY, Chiu HL. The effectiveness of wearable technologies as physical activity interventions in weight control: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2019;20(10):1485–93.
Seo YG, Noh HM, Kim SY. Weight loss effects of circuit training interventions: a systematic review and meta-analysis. Obes Rev. 2019;19
Yang Y, Shields GS, Wu Q, et al. Cognitive training on eating behaviour and weight loss: a meta-analysis and systematic review. Obes Rev. 2019;28
Prehn K, Jumpertz von Schwartzenberg R, Mai K, et al. Caloric restriction in older adults-differential effects of weight loss and reduced weight on brain structure and function. Cereb Cortex. 2017;27(3):1765–78.
Michel S, Raab R, Drabsch T, et al. Do lifestyle interventions during pregnancy have the potential to reduce long-term postpartum weight retention? A systematic review and meta-analysis. Obes Rev. 2019;20(4):527–42.
Neumark-Sztainer D, Wall M, Guo J, et al. Obesity, disordered eating, and eating disorders in a longitudinal study of adolescents: how do dieters fare 5 years later? J Am Diet Assoc. 2006;106(4):559–68.
Stice E, Davis K, Miller NP, et al. Fasting increases risk for onset of binge eating and bulimic pathology: a 5-year prospective study. J Abnorm Psychol. 2008;117(4):941–6.
Doucet E, McInis K, Mahmoodianfard S. Compensation in response to energy deficits induced by exercise or diet. Obes Rev. 2018;19(Suppl 1):36–46.
Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring). 2008;16(5):945–50.
Leidy HJ, Lepping RJ, Savage CR, et al. Neural responses to visual food stimuli after a normal vs. higher protein breakfast in breakfast-skipping teens: a pilot fMRI study. Obesity (Silver Spring). 2011;19(10):2019–25.
Goldstone AP, Prechtl de Hernandez CG, Beaver JD, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30(8):1625–35.
McDermott KD, Williams SE, Espeland MA, et al. Impact of intensive lifestyle intervention on neural food cue reactivity: action for health in diabetes brain ancillary study. Obesity (Silver Spring). 2019;27(7):1076–84.
Dong Z, Xu L, Liu H, et al. Comparative efficacy of five long-term weight loss drugs: quantitative information for medication guidelines. Obes Rev. 2017;18(12):1377–85.
Farr OM, Sofopoulos M, Tsoukas MA, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;59(5):954–65.
van Bloemendaal L, Ten Kulve JS, la Fleur SE, et al. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221(1):T1–16.
Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes. 2013;37(11):1443–51.
Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–99.
Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605.
Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21(5):935–43.
Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19(1):110–20.
Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–9.
Wang GJ, Tomasi D, Volkow ND, et al. Effect of combined naltrexone and bupropion therapy on the brain's reactivity to food cues. Int J Obes. 2014;38(5):682–8.
Roth BL, Willins DL, Kristiansen K, et al. 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): where structure meets function. Pharmacol Ther. 1998;79(3):231–57.
Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–56.
Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011 Oct;96(10):3067–77.
O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20(7):1426–36.
Farr OM, Upadhyay J, Gavrieli A, et al. Lorcaserin administration decreases activation of brain centers in response to food cues and these emotion- and salience-related changes correlate with weight loss effects: a 4-week-long randomized, placebo-controlled, double-blind clinical trial. Diabetes. 2016;65(10):2943–53.
Colman E. Food and Drug Administration's obesity drug guidance document: a short history. Circulation. 2012;125(17):2156–64.
Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65.
Zhu C, Mei F, Gao J, et al. Changes in inflammatory markers correlated with increased testosterone after laparoscopic sleeve gastrectomy in obese Chinese men with acanthosis nigricans. J Dermatol. 2019;46(4):338–42.
Zhang Y, Zhu C, Wen X, et al. Laparoscopic sleeve gastrectomy improves body composition and alleviates insulin resistance in obesity related acanthosis nigricans. Lipids Health Dis. 2017;16(1):209.
Gao J, Zhang M, Zhu C, et al. The change in the percent of android and gynoid fat mass correlated with increased testosterone after laparoscopic sleeve gastrectomy in Chinese obese men: a 6-month follow-up. Obes Surg. 2018;28(7):1960–5.
Cardoso L, Rodrigues D, Gomes L, et al. Short- and long-term mortality after bariatric surgery: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(9):1223–32.
Yeo C, Kaushal S, Lim B, et al. Impact of bariatric surgery on serum uric acid levels and the incidence of gout-A meta-analysis. Obes Rev. 2019;
Hansen TT, Jakobsen TA, Nielsen MS, et al. Hedonic changes in food choices following Roux-en-Y gastric bypass. Obes Surg. 2016;26(8):1946–55.
Gero D, Dib F, Ribeiro-Parenti L, et al. Desire for Core tastes decreases after sleeve Gastrectomy: a single-center longitudinal observational study with 6-month follow-up. Obes Surg. 2017;27(11):2919–26.
Alosco ML, Galioto R, Spitznagel MB, et al. Cognitive function after bariatric surgery: evidence for improvement 3 years after surgery. Am J Surg. 2014;207(6):870–6.
Behary P, Miras AD. Food preferences and underlying mechanisms after bariatric surgery. Proc Nutr Soc. 2015;74(4):419–25.
Ochner CN, Kwok Y, Conceicao E, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253(3):502–7.
Zoon HFA, de Bruijn SEM, Smeets PAM, et al. Altered neural responsivity to food cues in relation to food preferences, but not appetite-related hormone concentrations after RYGB-surgery. Behav Brain Res. 2018;353:194–202.
Scholtz S, Miras AD, Chhina N, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63(6):891–902.
Frank S, Wilms B, Veit R, et al. Altered brain activity in severely obese women may recover after Roux-en Y gastric bypass surgery. Int J Obes. 2014;38(3):341–8.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Panagiotou OA, Markozannes G, Adam GP, et al. Comparative effectiveness and safety of bariatric procedures in Medicare-eligible patients: a systematic review. JAMA Surg. 2018;153(11):e183326.
Li G, Ji G, Hu Y, et al. Reduced plasma ghrelin concentrations are associated with decreased brain reactivity to food cues after laparoscopic sleeve gastrectomy. Psychoneuroendocrinology. 2019;100:229–36.
Bruce JM, Hancock L, Bruce A, et al. Changes in brain activation to food pictures after adjustable gastric banding. Surg Obes Relat Dis. 2012;8(5):602–8.
Mauro M, Papelbaum M, Brasil MAA, et al. Is weight regain after bariatric surgery associated with psychiatric comorbidity? A systematic review and meta-analysis. Obes Rev. 2019;20(10):1413–25.
Goldman RL, Canterberry M, Borckardt JJ, et al. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obesity (Silver Spring). 2013;21(11):2189–96.
Li P, Shan H, Liang S, et al. Sleeve gastrectomy recovering disordered brain function in subjects with obesity: a longitudinal fMRI study. Obes Surg. 2018;28(8):2421–8.
Li G, Ji G, Hu Y, et al. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum Brain Mapp. 2018;39(12):4755–65.
le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243(1):108–14.
Borg CM, le Roux CW, Ghatei MA, et al. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93(2):210–5.
Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Rev Obes Surg. 2016;26(2):410–21.
van de Sande-Lee S, Pereira FR, Cintra DE, et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes. 2011;60(6):1699–704.
Gazdzinski SP, Gazdzinska AP, Orzel J, et al. Intragastric balloon therapy leads to normalization of brain magnetic resonance spectroscopic markers of diabetes in morbidly obese patients. NMR Biomed. 2018;31(9):e3957.
Dunn JP, Cowan RL, Volkow ND, et al. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res. 2010;1350:123–30.
Bruce AS, Bruce JM, Ness AR, et al. A comparison of functional brain changes associated with surgical versus behavioral weight loss. Obesity (Silver Spring). 2014;22(2):337–43.
Acknowledgments
We would like to thank Dr. Aaron M Gusdon (University of Texas Health Science Center at Houston) for discussion and critical reading of the manuscript.
Funding
The work was supported by the National Key R&D Program of China (No. 2018YFC1314100), National Natural Science Foundation of China (81970677), and National Natural Science Foundation of China for Youth (81500687).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Does not apply.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, Z., Qu, S. Legend of Weight Loss: a Crosstalk Between the Bariatric Surgery and the Brain. OBES SURG 30, 1988–2002 (2020). https://doi.org/10.1007/s11695-020-04474-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04474-8