Abstract
Background
A worrying increase of gastroesophageal reflux disease (GERD) and Barrett esophagus has been reported after sleeve gastrectomy (SG). Recent reports on combined fundoplication and SG seem to accomplish initial favorable results. However, no study included manometry or pH monitoring to evaluate the impact of fundoplication in SG on esophageal physiology.
Method
In this study, 32 consecutive bariatric patients with GERD and/or esophagitis had high-resolution impedance manometry (HRiM) and combined 24-h pH and multichannel intraluminal impedance (MII-pH) before and after laparoscopic sleeve gastrectomy associated to anterior fundoplication (D-SLEEVE). The following parameters were calculated at HRiM: lower esophageal sphincter pressure and relaxation, peristalsis, and mean total bolus transit time. The acid and non-acid GER episodes were assessed by MII-pH, symptom index association (SI), and symptom-association probability (SAP) were also analyzed.
Results
At a median follow-up of 14 months, HRiM showed an increased LES function, and MII-pH showed an excellent control of both acid exposure of the esophagus and number of reflux events. Bariatric outcomes (BMI and EWL%) were also comparable to regular SG (p = NS).
Conclusion
D-SLEEVE is an effective restrictive procedure, which recreates a functional LES pressure able to control and/or prevent mild GERD at 1-year follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As for any other surgical procedure able to change anatomy of stomach, sleeve gastrectomy (SG) has an important impact on either gastric or esophageal function [1, 2]. Given the gastric fundus removal, partial section of the muscular collar Helvetius’s fibers [3], reduced volume, and increased pressure of the tube, SG has potential risk of promoting “de novo” postoperative reflux. Recently, some authors have reported a worrying increase of gastroesophageal reflux disease (GERD) and Barrett esophagus after SG [4,5,6,7,8] eventually associated to biliary duodenogastric refluate [9].
We recently observed that SG with a regular tube, preserving antrum and LES anatomy, does not induce “de novo” GERD in patients without preoperative evidence of pathological reflux [10]. However, concerns may rise in patients with preoperative GERD symptoms or clinical evidence of LES incompetence at preoperative instrumental assessment.
In attempt to reduce postoperative risk of GERD symptoms and to expand our indication for SG in patients suffering from mild reflux or potentially at risk of developing postoperative GERD (i.e., symptoms, LES incompetence or L.A. grade A), we designed an original technique adding to SG an anterior fundoplication (D-SLEEVE).
This prospective longitudinal study aims to provide objective data of D-SLEEVE on the esophageal physiology by means of combined multichannel intraluminal impedance and pH (MII-pH) and high-resolution manometry with impedance (HRiM).
Materials and Methods
Study Design and Patient Selection
From a prospectively maintained database of 520 patients referred for bariatric procedure to the Division of General, Mini-Invasive and Obesity Surgery, University of Campania “Luigi Vanvitelli,” a consecutive series of 20 women and 12 men (median age, 38 years [27–49]) underwent between May 2016 and July 2017 HRiM and MII-pH before and after D-SLEEVE. Our institutional review board approved the study protocol. Each patient was informed about the investigational nature of the study and received detailed information about the study protocol. Before subjects entered the study, specific informed consent was obtained from each.
Patient inclusion criteria were as follows: age 18 years or older and younger than 60 years. Patients who for at least 5 years had morbid obesity (BMI > 40 or > 35 with co-morbidities) with transient or insufficient response to nutritional treatment were offered bariatric surgery according to the Italian society of bariatric and metabolic surgery (S.I.C.O.B.) recommendation [11]. Patients with history of GERD-related symptoms (i.e., heartburn, pyrosis, regurgitation), and/or endoscopic evidence of Los Angeles grade A and/or LES incontinence, were offered the opportunity of participating to the protocol. The surgical treatment options (i.e., laparoscopic antireflux procedure [12, 13], laparoscopic gastric bypass [14], or a combined bariatric-antireflux procedure D-SLEEVE) were offered after a multidisciplinary meeting once preoperative work-up was completed and definitively chosen with the patient.
Patient exclusion criteria were as follows: previous upper gastrointestinal surgery, paraesophageal (type 2), mixed (type 3) or sliding hiatal hernias of 3 cm or more, persistence of hiatal hernia at manometric swallows, presence of esophagitis > grade B sec. Los Angeles, and Barrett’s metaplasia at upper endoscopy [15]. Symptoms were assessed by submitting to patients, pre- and postoperatively, a standardized questionnaire (GERDQ score) dealing with the frequency and intensity of esophageal symptoms (such as heartburn, regurgitation, epigastric pain, bloating) [16].
Patients who underwent regular SG without fundoplication, with a similar technique by the same surgeons, were used as the control group [17].
High-Resolution Impedance Manometry
A solid-state combined manometry and impedance recording assembly incorporating 36 circular and unidirectional strain gauge pressure sensors spaced at 1-cm intervals and 9 impedance-recording rings (five impedance segments) spaced at 2-cm intervals spanning was used (Sandhill Scientific Inc., Highlands Ranch, CO, USA). Subjects were off medication (any antacid medication or prokinetic was stopped at least 2 weeks before testing) and fasted for at least 6 h before trans-nasal placement of the HRiM. Studies were conducted with the subjects in supine position, catheter was introduced trans-nasally and placed to record the entire esophageal length and at least 3–4 cm of proximal stomach, including LES and the fundoplication. The HRiM protocol included a 5-min period to assess EGJ pressure at resting followed by ten 5-mL swallows of 0.3% saline solution to evaluate esophageal body function [18].
MII Definitions
Data were analyzed using BioView analysis software (Sandhill Scientific Inc.), and each tracing was personally reviewed by one blinded investigator to upper endoscopy data [S.T.]. For each swallow, complete (effective) bolus transit occurred when the bolus entered the first pair of sensors and exited all the distal pair of sensors [19]. The study was considered abnormal if complete bolus transit occurred less than 80% of the time for liquid swallows. Bolus transit time was expressed as time in seconds from entrance of the bolus in the proximal channel (channel 1) to the exit in the most distal channel (channel 5) [20].
Manometry Definitions
After LES identification, its resting pressure and relaxation response to swallow (integrated relaxation pressure over 4 s) were recorded. Crural diaphragm (CD) was discernible as the axial point with the maximal inspiratory pressure augmentation. Patients were then classified to have normal EGJ (with LES and CD superimposed) or hiatal hernia (with a presence of axial separation, measured in centimeters, between LES and CD). EGJ morphology was classified based on the presence of axial cranial separation between lower LES and CD, measured in centimeters, as follows: type I, no separation between the LES and the CD; type II, minimal separation (> 1 and < 2 cm); type III (hiatal hernia), ≥2 cm of separation [21]. Proximal intragastric pressures (IGP), distal contractile integral (DCI), distal latency (DL), integrated relaxation pressure (IRP), and esophagogastric junction contractile integral (EGJ-CI) were automatically calculated. HRiM motility patterns were categorized according to the Chicago Classification V 3.0 [22]; thus, patients were graded to have “normal” or “abnormal” motility by means of calculation of the integrated relaxation pressure, distal contractile integral, and distal latency.
Combined 24-H ph-MII-pH
Twenty-four-hour ambulatory combined pH-multichannel intraluminal impedance studies were performed to document the presence of GERD. A dedicated catheter (Sandhill Scientific Inc., Highlands Ranch, CO, USA) with a pH sensor 5 cm above the LES/anterior fundoplication and six pairs of impedance sensors positioned in the esophagus 3, 5, 7, 9, 15, and 17 cm above the upper limit of the high-pressure zone (LES/fundoplication) were placed trans-nasally. Abnormal total, acid and non-acid exposure and reflux at impedance were defined as previously described [23]. Acid exposure time was defined as time percentage of ph < 4 in distal esophagus.
Patients were asked to record every meal, changes in body position (i.e., from upright to recumbent), and symptoms occurrence during the monitoring day. After excluding meal periods, MII-pH data were analyzed with the Bioview GERD Analysis Software (Sandhill Scientific). At MII-pH, the following features were evaluated: percentage of distal acid exposure time (AET%) with pH < 4; abnormal AET%, defined as > 6.0% for total time, > 4.2% for upright time, and > 1.2% for recumbent period; number of reflux episodes identified at MII and their quality (acid, weakly acid, and weakly alkaline; normal value < 80); symptom index association (SI) and symptom-association probability (SAP), as described elsewhere [23].
Surgical Technique
As in standard SG [17], procedure begins with dissection of greater omentum perpendicularly to incisura angularis alongside greater gastric curvature. Starting at this point, grater curvature is divided upward. Dissection is terminated once fundus is entirely detached, deciding the portion involved into anterior fundoplication. This can be easily achieved mimicking the antireflux plication to detect the upper limit of dissection. Usually, 2–3 cm of gastro-phrenic ligament, corresponding to 1–2 short-gastric vessel, was left intact.
Anterior fundoplication: lifting up the left liver lobe, the proximal portion of hepatogastric ligament was opened to expose the right crus. The gastroesophageal junction and the Laimer-Bertelli membrane were left intact. Within a 40 F bougie, a 180° wrap was fashioned with the anterior gastric wall with two single extracorporeal stitches incorporating the endoabdominal fascia at right crus. Finally, gastric section was started alongside bougie up-to the inferior edge of the wrap preserving majority of antrum. Attention was paid to obtain a regular shape and to not create an excessive narrowing of the gastric lumen at the incisura angularis while achieving a complete removal of posterior fundus. Intraoperative endoscopy double checked intraluminal bleeding and size, defective stapler line, position, and geometry of the fundoplication. The stapler line was routinely reinforced by over-sewing running suture (Fig. 1).
Sleeve gastrectomy with anterior fundoplication (D-SLEEVE). The gastroesophageal junction, the Laimer-Bertelli membrane, and 1–2 short-gastric vessel were left intact. A 180° wrap was fashioned with the anterior gastric wall with two single extracorporeal stitches incorporating the endoabdominal fascia at right crus. Gastric section was conducted until the inferior edge of the wrap preserving majority of antrum. The stapler line was routinely reinforced by over-sewing running suture
Statistical Analysis
Statistical analysis was performed using SPSS for Windows (version 22; SPSS Inc., Chicago, IL. USA). Continuous data are expressed as median and interquartile (25th–75th) range or mean and SD, unless otherwise indicated. Differences between preoperative and postoperative parameters were compared by Wilcoxon paired rank test. For all tests, a two-sided p < 0.05 was considered statistically significant. Sample size was calculated setting a power of 0.9 with quantitative variable (i.e., number of total reflux episodes, weight kg, AET%, LES resting pressure mmHg), assuming the hypothesis of 25% parameters improvement following D-SLEEVE. To reach a significance set at p < 0.05 for clinical and instrumental items, enrolment of ≥ 7 patients for each group was needed.
Results
Postoperative evaluations were performed at a median interval of 2 weeks before D-SLEEVE and 12 months (11–13) afterward. The preoperative (131 kg [97–152], BMI = 46 [37–52]) and postoperative (96 kg [76–102], BMI = 31.2 [26,27,28,29,30,31,32,33,34,35,36,37,38,39]) anthropology was statistically different (p < 0.05) with 59% excess weight loss (EWL) (Fig. 2). When compared to previously published group of 25 consecutive SG [17] (130.8 kg (119–156), BMI = 46.1 (38–58 vs 98 kg (72–110), BMI = 34.7 (28–46), pre- and postoperative, respectively) without fundoplication, weight loss was not statistically different at 1-year follow up (EWL% 59 vs. 56, p = NS) (Fig. 3).
All patients were positive at preoperative GERDQ score (i.e. > 9), while after surgery, the incidence of symptoms related to reflux was modified in all patients (i.e., no perception of heartburn, regurgitation, and epigastric pain); postoperatively, in fact, GERDQ score resulted in the normal range in all patients (p < 0.05). Endoscopically mild sliding type 1 hiatal hernia (< 2 cm) and LA grade A esophagitis was revealed in 11 (68.7%) and 9 (56.2%) patients, respectively. Postoperative endoscopic control detected persistent LA grade A esophagitis in one patient (6.2%, p < 0.05), while type 1 hiatal hernia was not reported. No esophageal motility disorders were detected at preoperative HRiM.
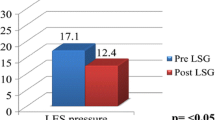
Table 1 shows a detailed pre- and postoperative assessment at HRiM. The median LES length was not statistically increased postoperatively (3.9 vs. 4.2 cm, respectively; p = 0.08). Preoperative EGJ morphology was equally distributed in type 1 (16/32, 50%) and type 2 (16/32, 50%) (Fig. 4). Postoperatively, improved with only 12.5% (4/32) type 2 crural distance (Fig. 5). Median LES resting pressure was statistically increased postoperatively (from 8.2 [6–10] to 22.5 mmHg [19–24] p < 0.05). Mean EGJ-CI increased postoperatively from 9 [7–11] to 23 mmHg*cm [21–26] (p < 0.05) (Fig. 6).
Example of two preoperative swallows at high-resolution impedance manometry (HRiM) with effective peristalsis, normal sphincters relaxation, altered esophagogastric junction with a small hiatal hernia < 2 cm (EGJ type II), and a bolus that fully clears. MII, multichannel intraluminal impedance; HRM, high-resolution manometry; BEP, bolus entrance point; BExP, bolus exit point; UES, upper esophageal sphincter, LES, lower esophageal sphincter; IP, intragastric pressure
Example of two normal postoperative swallows at high-resolution impedance manometry (HRiM) with effective peristalsis, normal sphincters relaxation, normal esophagogastric junction and a bolus that fully clears. MII, multichannel intraluminal impedance; HRM, high-resolution manometry; BEP, bolus entrance point; BExP, bolus exit point; UES, upper esophageal sphincter, LES, lower esophageal sphincter; IP, intragastric pressure
Median percentage of ineffective peristaltic waves at high-resolution manometry decreased from 56 to 46% after D-SLEEVE (p = 0.205). The median percentage of impedance complete bolus transit did not change from 90 to 85 (p = 0.285) after sleeve. Postoperatively, no bolus retrograde movement at manometric MII was observed after swallow.
Pre- and postoperative median MII-pH time of monitoring was similar (1210 and 1150 min, respectively). The median registration for the recumbent position was similar in the preoperative (410 min) and postoperative (420 min) evaluation. The preoperative mean esophageal acid exposure time (AET) pH < 4 was 5.0%. Postoperative AET was in the normal range (pH < 1.2%, p < 0.05); specifically, pH postoperative evolution is summarized in Table 2 (Fig. 7).
Table 3 shows detailed findings regarding the effects of D-SLEEVE at MII-pH. The procedure produced a decrease of mean total reflux episodes (58 vs. 32; p < 0.05) detected at MII. Specifically, a significant reduction of mean postoperative retrograde movement was detected for both acid (38 vs. 21; p < 0.05) and non-acid (20 vs. 11; p < 0.05) reflux episodes (Fig. 8). Esophageal bolus clearance time (BCT) increased after D-SLEEVE (14 to 24 s; p < 0.05). Mean SI and SAP (preoperative values 70% and 95%, respectively) were not associated postoperatively (35% and 30%, respectively).
After surgery, we reported a decrease of mean total reflux episodes (58 vs. 32; p < 0.05) detected at multichannel intraluminal impedance. A significant reduction of mean postoperative retrograde movement was detected for both acid (38 vs. 21; p < 0.05) and non-acid (20 vs. 11; p < 0.05) reflux episodes
Discussion
Initial reports emphasized an augmented risk of new onset postoperative GERD after SG [24,25,26]. In agreement with this, several authors reported an increased exposure to acid following SG [5], associated to decreased postoperative LES pressure due to surgical damage at Helvetius muscular collar fibers [5], elimination of His angle [27], and increased intraluminal pressure [28]. To better understand physiological change of SG associated to GERD, we recently investigated how a SG, with a regular intraluminal space (e.g., not narrow at incisura angularis), preserving the antrum, and distant at least 1 cm from the His angle, does not impair LES. The manometric and ph-impedance evaluation excluded “de novo GERD” but highlighted an increased acid exposition and post prandial retrograde movement after sleeve. Given these results in a cohort without preoperative hiatal hernia and/or GERD, it is possible to assume a potential postoperative increase of GERD in patient at risk.
Roux-en-Y gastric bypass is currently considered the standard technique to treat obese patients with GERD and for a long time had been the only surgical option. Recently, some authors reported different combinations of antireflux procedures with SG, with encouraging results [29,30,31].
However, to our knowledge, these studies were limited on symptoms and/or endoscopic findings without any functional instrumental investigation, able to demonstrate whether fundoplication acts as an antireflux barrier above a sleeved stomach.
Though a number of physiological variables may be changed after SG, here, we have identified a critical role for combining an antireflux surgery in contrasting an altered EGJ with a specific need of pre- and postoperative accurate investigation of physiology. Among different possible antireflux techniques (i.e., Nissen [29], Dor [32], Nissen-Rossetti [33]), we designed a simple procedure driven to have a limited impact on physiologic antireflux mechanisms. Indeed, D-SLEEVE does not impair Laimer-Bertelli membrane, endoabdominal and infra-diaphragmatic (trasversalis) fasciae, phreno-esophageal ligament, and posterior retrogastric fat. Moreover, no dissection of esophagus is necessary. The procedure is limited to a short division of proximal hepatogastric ligament to expose the right crus. The EGJ overlap is obtained with the anterior gastric wall, able to relax during swallow while increasing cardiac pressure when gastric lumen is replete [12]. To avoid postoperative dysphagia, fundoplication should lay at cardiac Z-line level, involving superior part of gastric fundus. We use endoscopy to intraoperatively check level and geometry of the plication [34].
Taken together, our results demonstrated that a functional fundoplication in combination with SG is effective in controlling mild GERD. Indeed, the entire cohort benefited of D-SLEEVE with a complete resolution of symptoms related to reflux and normalization of GERDQ score (p < 0.05) and decrease of postoperative esophagitis (p < 0.05). This is not surprising after D-SLEEVE, given LES showed a significant increase of mean and resting pressure compared to either baseline (p < 0.05).
Postoperatively, no hiatal hernia was detected at endoscopy and distance of LES/fundoplication from crura increased. This was probably due to either decrease of intra-abdominal pressure following weight loss or to the difficulty of determining distance from crura after fundoplication. This procedure, indeed, does not alter the EGJ so that distance should not change, being crura and esophagus not dissected.
Noteworthy, an advantage of D-SLEEVE lays on potential decreased risk of postoperative fistula rate. The procedure avoids stapling the most proximal portion of stomach and the suture line run below the fundoplication; vascularization at His angle is not altered, preserving last short gastric vessel and gastro-phrenic ligament [35, 36]. Last, we may speculate that leaving in place the last short gastric vessels, crura and retrogastric ligaments may potentially reduce the risk of twisting, slippage, or chest migration [37].
At the same time, according to our results, D-SLEEVE acts as an effective bariatric procedure with similar restrictive capacity to conventional SG. In particular, in comparable cohort of consecutive patients underwent by the same group standard SG, there was no difference on weight loss at a short-term 1-year follow-up (p = NS) [10]. This is consistent with similar intraluminal gastric volume at intraoperative endoscopy and postoperative Rx swallow. One potential draw-back of this technique is to leave an excessive gastric fundus in place, which may dilate at long term with potential risk of long-term weight regain. Some authors could argue that adding a fundoplication may potentially mitigate the grelin reduction or other metabolic effects, which was not investigated in this study [38,39,40]. A longer follow-up is needed to establish whether D-SLEEVE provides similar outcomes on weight loss. Nowadays, it seems advisable, to obtain an adequate gastric removal, to achieve an extensive posterior mobilization, and to fashion a short anterior valve including the most proximal portion of fundus. We suggest creating the fundoplication once gastric dissection is completed, before the stapling. A generous over-sawn running suture may help to reduce an excessive fundus when needed.
An indirect indicator of the restrictive effect of the technique was the postoperative increase of intragastric pressure and BCT. This was not surprising, as D-SLEEVE similarly to SG is an effective procedure with consistent reduction of total gastric capacity volume (i.e. ≃ 70–90%). Non-distensible walls create a rapid increase of intragastric pressure when the remnant stomach is full. Main advantage of D-SLEEVE is to overcome this inconvenience by adding pressure on LES, restoring a pressure delta from stomach to esophagus that acts as a natural barrier to prevent late on set GERD.
Specifically, reflux was absent after D-SLEEVE, according to data from manual review of MII-pH traces; patients who underwent D-SLEEVE had statistically decreased ATE, within the normal range. After a careful analysis, the number of reflux episodes decreased for both acid and non-acid type, both normalized postoperatively. Moreover, symptom associations (e.g., SI and SAP) to retrograde movements switched after the procedure with no postoperative correlation.
Observing at MII-pH statistically different results in patients underwent D-SLEEVE; presence of fundoplication might have a relevant impact on these reductions. One limitation of this study is the lack of a direct comparison of standard SG vs D-SLEEVE, in the setting of a randomized trial, being standard SG in which the authors believe not currently indicated for patients with symptomatic GERD and/or esophagitis [41]. Moreover, this study did not include patients with hiatal hernia and may require additional crura repair and/or reduction of the esophagus into the abdomen, or sufficiently benefit from postoperative decreased abdominal pressure following weight reduction; thus, no conclusion should be extrapolated for patients with concomitant hiatal hernia and GERD.
At present, it seems reasonable to assert that D-SLEEVE is able to control GERD in patients with mild symptoms and/or esophagitis, reestablishing normal delta pressure of asymptomatic not operated patients. If this mechanism is associated to reduced intra-abdominal pressure due to weight loss, a more controlled diet or other reasons that will be able to control GERD need further evaluations at longer-term follow-up.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
04 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11695-021-05244-w
References
Angrisani L, Santonicola A, Hasani A, et al. Five-year results of laparoscopic sleeve gastrectomy: effects on gastroesophageal reflux disease symptoms and co-morbidities. Surg Obes Relat Dis. 2016;12(5):960–8. https://doi.org/10.1016/j.soard.2015.09.014.
Rosenthal RJ, International Sleeve Gastrectomy Expert Panel, Diaz AA, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19.
Helvetius M. Observation sur le estomac de la Homme. Historie de l'Accademie Royale des Sciences. 1719; p. 337–45.
Soricelli E, Casella G, Baglio G, et al. Lack of correlation between gastroesophageal reflux disease symptoms and esophageal lesions after sleeve gastrectomy. Surg Obes Relat Dis. 2018;14(6):751–6.
Braghetto I, Korn O. Late esophagogastric anatomic and functional changes after sleeve gastrectomy and its clinical consequences with regards to gastroesophageal reflux disease. Dis Esophagus. 2019;32(6). https://doi.org/10.1093/dote/doz020.
Sebastianelli L, Benois M, Vanbiervliet G, et al. Systematic endoscopy 5 years after sleeve gastrectomy results in a high rate of Barrett’s esophagus: results of a multicenter study. Obes Surg. 2019;29:1462–9.
Doulami G, Triantafyllou S, Natoudi M, et al. 24-h multichannel intraluminal impedance pHmetry 1 year after laparoscopic sleeve gastrectomy: an objective assessment of gastroesophageal reflux disease. Obes Surg. 2017;27:749–53.
Sharma A, Aggarwal S, Ahuja V, et al. Evaluation of gastroesophageal reflux before and after sleeve gastrectomy using symptom scoring, scintigraphy, and endoscopy. Surg Obes Relat Dis. 2014;10:600–5.
Braghetto I, Gonzalez P, Lovera C, et al. Duodenogastric biliary reflux assessed by scintigraphic scan in patients with reflux symptoms after sleeve gastrectomy: preliminary results. Surg Obes Relat Dis. 2019;S1550-7289(19):30100–5. https://doi.org/10.1016/j.soard.2019.03.034.
Del Genio G, Tolone S, Limongelli P, et al. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg. 2014;24(1):71–7. https://doi.org/10.1007/s11695-013-1046-4.
Foschi D, De Luca M, Sarro G, Bernante P, Zappa MA, Moroni R, Navarra G, Foletto M, Ceriani V, Piazza L, Di Lorenzo N. Linee Guida di Chirurgia dell’Obesità. Ed. Società Italiana di Chirurgia dell’Obesità e delle Malattie Metaboliche (S.I.C.OB.), 2016.
Del Genio G, Tolone S, Del Genio F, et al. Impact of total fundoplication on esophageal transit: analysis by combined multichannel intraluminal impedance and manometry. J Clin Gastroenterol. 2012;46(1):e1–5.
Pizza F, Rossetti G, Limongelli P, et al. Influence of age on outcome of total laparoscopic fundoplication for gastroesophageal reflux disease. World J Gastroenterol. 2007;13(5):740.
Shikora SA, Kim JJ, Tarnoff ME, et al. Laparoscopic Roux-en-Y gastric bypass: results and learning curve of a high-volume academic program. Arch Surg. 2005;140(4):362–7.
Genta RM, Spechler SJ, Kielhorn AF. The Los Angeles and Savary-Miller systems for grading esophagitis: utilization and correlation with histology. Dis Esophagus. 2011;24:10–7.
Amato G, Limongelli P, Pascariello A, et al. Association between persistent symptoms and long-term quality of life after laparoscopic total fundoplication. Am J Surg. 2008;196(4):582–6.
Del Genio G, Limongelli P, Del Genio F, et al. Sleeve gastrectomy improves obstructive sleep apnea syndrome (OSAS): 5 year longitudinal study. Surg Obes Relat Dis. 2016;12(1):70–4. https://doi.org/10.1016/j.soard.2015.02.020.
Tolone S, Limongelli P, del Genio G, et al. Gastroesophageal reflux disease and obesity: do we need to perform reflux testing in all candidates to bariatric surgery? Int J Surg. 2014;12:173–7.
del Genio G, Tolone S, Rossetti G, et al. Objective assessment of gastroesophageal reflux after extended Heller myotomy and total fundoplication for achalasia with the use of 24-hour combined multichannel intraluminal impedance and pH monitoring (MII-pH). Dis Esophagus. 2008;21(7):664–7. https://doi.org/10.1111/j.1442-2050.2008.00847.x.
Tutuian R, Vela MF, Balaji NS, et al. Esophageal function testing with combined multichannel intraluminal impedance and manometry: multicenter study in healthy volunteers. Clin Gastroenterol Hepatol. 2003;1(3):174–82.
Tolone S, Savarino E, Zaninotto G, et al. High-resolution manometry is superior to endoscopy and radiology in assessing and grading sliding hiatal hernia: a comparison with surgical in vivo evaluation. United European Gastroenterol J. 2018;6(7):981–9. https://doi.org/10.1177/2050640618769160.
Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(10):160–74.
del Genio G, Tolone S, Rossetti G, et al. Total fundoplication does not obstruct the esophageal secondary peristalsis: investigation with pre- and postoperative 24-hour pH-multichannel intraluminal impedance. Eur Surg Res. 2008;40(2):230–4.
Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett's esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. 2017;13(4):568–74. https://doi.org/10.1016/j.soard.2016.11.029.
Chiu S, Birch DW, Shi X, et al. Effect of sleeve gastrectomy on gastroesophageal reflux disease: a systematic review. Surg Obes Relat Dis. 2011;7(4):510–5.
Deitel M, Gagner M, Erickson AL, et al. Third international summit: current status of sleeve gastrectomy. Surg Obes Relat Dis. 2011;7(6):749–59.
Kleidi E, Theodorou D, Albanopoulos K, et al. The effect of laparoscopic sleeve gastrectomy on the antireflux mechanism: can it be minimized? Surg Endosc. 2013;27(12):4625–30. https://doi.org/10.1007/s00464-013-3083-4.
Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450–6.
Nocca D, Skalli EM, Boulay E, et al. Nissen sleeve (N-sleeve) operation: preliminary results of a pilot study. Surg Obes Relat Dis. 2016;12(10):1832–7. https://doi.org/10.1016/j.soard.2016.02.010.
Sánchez-Pernaute A, Talavera P, Pérez-Aguirre E, et al. Technique of Hill’s gastropexy combined with sleeve gastrectomy for patients with morbid obesity and gastroesophageal reflux disease or hiatal hernia. Obes Surg. 2016;26(4):910–2. https://doi.org/10.1007/s11695-016-2076-5.
Olmi S, David G, Cesana G, et al. Modified sleeve gastrectomy combined with laparoscopic Rossetti fundoplication and vascularization assessment with Indocyanine green. Obes Surg. 2019;29:3086–8. https://doi.org/10.1007/s11695-019-03970-w.
Moon RC, Teixeira AF, Jawad MA. Safety and effectiveness of anterior fundoplication sleeve gastrectomy in patients with severe reflux. Surg Obes Relat Dis. 2017;13(4):547–52. https://doi.org/10.1016/j.soard.2016.10.008.
Olmi S, Caruso F, Uccelli M, et al. Laparoscopic sleeve gastrectomy combined with Rossetti fundoplication (R-sleeve) for treatment of morbid obesity and gastroesophageal reflux. Surg Obes Relat Dis. 2017;13(12):1945–50.
Rossetti G, del Genio G, Maffettone V, et al. Laparoscopic reoperation with total fundoplication for failed Heller myotomy: is it a possible option? Personal experience and review of literature. Int Surg. 2009;94(4):330–4.
Gagner M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surg Endosc. 2019;34:396–407. https://doi.org/10.1007/s00464-019-06782-2.
Demeusy A, Sill A, Averbach A. Current role of staple line reinforcement in 30-day outcomes of primary laparoscopic sleeve gastrectomy: an analysis of MBSAQIP data, 2015-2016 PUF. Surg Obes Relat Dis. 2018;14(10):1454–61. https://doi.org/10.1016/j.soard.2018.06.024.
Del Genio GM, Collard JM. Acute complications of antireflux surgery. In: Ferguson MK, Fennerty MB, editors. Managing failed anti-reflux therapy. London: Springer Verlag; 2006. p. 67–77.
Karamanakos SN, Vagenas K, Kalfaretzos F. Weight loss, appetite suppression and changes in fasting and postprandial ghrelin and peptide-YY levels after roux-en-Y gastric bypass and sleeve gastrectomy a prospective, double blind study. Ann Surg. 2008;247:401–7.
Dimitriades E, Daskalakis M, Kampa M, et al. Alterations in gut hormones following laparoscopic sleeve gastrectomy [published online ahead of print October 26, 2012]. Ann Surg. https://doi.org/10.1097/SLA.0b013e31826e1846.
Melissas J, Daskalakis M, Koukouraki S, et al. Sleeve gastrectomy-a “food limiting” operation. Obes Surg. 2008;18(10):1251–6.
Oor JE, Roks DJ, Ünlü Ç, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg. 2016;211(1):250–67.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethics Approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
del Genio, G., Tolone, S., Gambardella, C. et al. Sleeve Gastrectomy and Anterior Fundoplication (D-SLEEVE) Prevents Gastroesophageal Reflux in Symptomatic GERD. OBES SURG 30, 1642–1652 (2020). https://doi.org/10.1007/s11695-020-04427-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04427-1