Abstract
Background
We have previously reported on the benefits of Pre-Surgical Exercise Training (PreSET) on physical fitness and social interactions in subjects awaiting bariatric surgery (BS). However, data are needed to know whether these benefits are maintained post-BS.
Objectives
The purpose of this paper was to evaluate the effect of PreSET on physical activity (PA) level, physical fitness, PA barriers, and quality of life (QoL) 1 year (1-Y) after BS.
Methods
Of the 30 participants randomized into two groups (PreSET and usual care), 25 were included in the final analysis. One year after BS, time spent in different PA intensities and number of steps were assessed with an accelerometer. Before BS and until 1-Y after BS, physical fitness was assessed with symptom-limited cardiac exercise test, 6-min walk test (6MWT), and sit-to-stand, half-squat, and arm curl tests. QoL, PA barriers, and PA level were evaluated with questionnaires.
Results
The number of steps (7460 vs 4287) and time spent in light (3.2 vs 2.2 h/day) and moderate (0.6 vs 0.3 h/day) PA were higher in the PreSET group 1-Y after BS. The changes in 6MWT heart cost (1.3 vs 0.6 m/beats/min), half-squat test (38.8 vs 10.3 s), and BMI (− 16.8 vs − 13.5 kg/m2) were significantly greater in the PreSET group compared to those in the usual care group. No other significant difference between groups was observed.
Conclusion
The addition of the PreSET to individual lifestyle counseling seems effective to improve PA level and submaximal physical fitness 1-Y after BS. Studies with larger cohorts are now required to confirm these results.
The trial was registered at clinicaltrials.gov (NCT01452230).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vast majority of patients awaiting bariatric surgery (BS) are inactive before surgery, and despite the massive weight loss and a slight improvement in physical activity (PA) level during the first year, most of them remain inactive after BS [1, 2]. Evidence is mounting to support the importance of PA before and after BS to improve physical fitness, glucose metabolism, body composition, and quality of life [3,4,5,6]. However, optimal support to help BS patients needs to be developed.
The pre-surgical period could be an ideal starting point for PA intervention. According to Bond et al., the motivation to practice regular moderate PA and the engagement in various PA intensities increased significantly closer to the candidate’s surgery date [7]. In addition, prospective studies reported that most patients remain in their pre-BS PA level categories, after BS. In other words, patients with a higher PA level before BS have a higher PA level after BS [1, 2]. Recent studies reported that pre-BS PA interventions can improve physical fitness, PA level, embarrassment to performing PA, enjoyment, self-efficacy, and quality of life in subjects awaiting BS [3, 4, 6, 8, 9]. However, long-term studies showing maintenance of benefits are necessary to justify the relevance of pre-BS PA interventions. A recent publication from the Bari-Active trial tested a 6-week face-to-face PA counseling versus usual care and reported that the intervention group maintained their pre-BS PA improvement 6 months following BS [10]. However, no results are currently available for longer periods after BS, nor on other outcomes (e.g., quality of life, physical fitness) or other modalities of intervention (e.g., supervised exercise training).
We recently reported that adding a Pre-Surgical Exercise Training program (PreSET) to an interdisciplinary lifestyle intervention results in improvements in physical fitness, social interactions, and embarrassment 12 weeks after the intervention [6]. The main objective of this study was to compare changes from baseline to 1 year (1-Y) after BS in PA, physical fitness, PA barriers, and quality of life between the PreSET and the usual care groups.
Materials and Methods
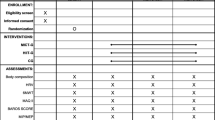
Considering that methods have been largely described elsewhere [6, 11], here is only a brief overview.
Experimental Design
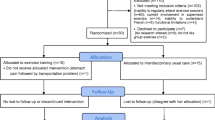
The trial was a randomized controlled study using an allocation list generated by a computer random sequence, stratified by sex and maximal aerobic capacity (> or ≤ 7 metabolic equivalent of task (MET)) and kept in sealed envelopes. Anthropometric data, body composition, physical fitness, PA barriers, quality of life, and sociodemographic data were collected before BS at three time points (before (T1) and 12 weeks after the PreSET (T2) and 2 weeks before BS (T3)), and after BS at four time points (3 (T4), 6 (T5), 9 (T6), and 12 months (T7) after BS). A symptom-limited cardiac exercise test was performed before (T1), 12 weeks after the PreSET (T2), and 1-Y after BS (T7). Objective assessment of PA level with accelerometers was performed 1-Y after BS (T7). The study was conducted after approval from the Ethics Review Board of research on humans of the Centre Hospitalier Universitaire de Sherbrooke (CHUS) and Université de Sherbrooke. All participants gave informed consent. The trial was registered at clinicaltrials.gov (NCT01452230).
Participants
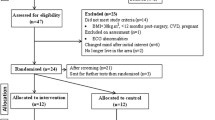
Our sample consisted of 30 adult candidates to BS who were randomized between the PreSET and the usual care group. Only patients with BMI ≥ 35 kg/m2 and comorbidities or ≥ 40 kg/m2, aged between 18 and 65 years and without uncontrolled neuropsychiatric illnesses receive a laparoscopic Roux-en-Y gastric bypass or sleeve gastrectomy at the CHUS. Patients were approached for participation in the study if they were expected to be operated within 3 to 6 months and practiced less than two weekly supervised exercise sessions. We excluded persons with (i) inabilities to regularly attend supervised exercise sessions, (ii) medical contraindications for PA, (iii) functional limitations not allowing them to complete the 6-min walking test (6MWT), (iv) inability to speak fluently the language in which the intervention was provided (French), or (v) uncontrolled neuropsychiatric illnesses. After enrollment, the only exclusion criterion was to have an injury or an incident resulting in more than 2 weeks of an inability to perform PA.
Interventions
All participants benefited from individual counseling sessions every 6–8 weeks before BS during at least 6 months and at 3, 6, 9, and 12 months after BS with a dietitian and a PA specialist, and 52% of participants (without significant difference between groups) participated to voluntary group educational sessions, called the “Motivated’s Club” on PA and nutrition and psychological issues related to weight management [12]. In addition, the PreSET group underwent three weekly 80-min sessions consisting of 10 min of warm-up, 30 min of endurance activity at 55 to 85% of the heart rate reserve (treadmill, walking circuit, arm-ergocycle, elliptical, dance/aerobic exercise), 20 to 30 min of strength exercises with small equipment (dumbbells, elastic bands, medicine balls and sticks), and 10 min of a cool-down period, with monthly aquagym session, which lasted until 2 weeks before BS. Additional details on the PreSET have been provided elsewhere [11].
Measures
The number of minutes of walking, moderate and vigorous PA, and sitting time during the last 7 days was self-reported by participants in the International PA Questionnaire-Short Form (IPAQ-SF) [13]. To objectively assess PA, participants were asked to wear a triaxial accelerometer (Actigraph® GT3X+, Pensacola, FL, USA) at their right hip during all waking hours 7 days after the 1-Y assessment. It appears to be an accurate tool to estimate free-living PA and has already been used in BS participants [14, 15]. Only the data from participants with a valid wear time period, i.e., 4–7 consecutive days with 9 h per day of wear time, were considered for the analysis. The accelerometer data was extracted using the Actilife software v.6.13.3 and saved in 10-s epochs. The Freedson Combination (1998) formula was used to calculate total energy expenditure (kcal/day), and cutoffs were used to classify PA intensities, since the latter has not been established in BS patients yet (sedentary time < 100 counts per minute (cpm), light physical activity (LPA) = 100–1951 cpm, moderate PA (MVPA) = 1952–5724 cpm, and vigorous (VPA) > 5724 cpm) [16]. Participants were categorized as active (≥ 150 min per week of moderate to vigorous PA) or inactive (< 150 min per week of moderate to vigorous PA) according to accelerometer data. No wear time was defined as 180 min of consecutive zeros [17]. A diary was completed by participants to validate when they started and finished wearing the accelerometer each day.
Physical fitness was assessed with symptom-limited cardiac exercise test (Cornell 0, 5, 10 or Bruce protocols), 6MWT, and sit-to-stand, half-squat, and arm curl tests according to standardized protocols described elsewhere [6, 11]. Briefly, during the 6MWT, participants had to walk as far as they could for 6 min by going back and forth in a 30-m corridor. The 6MWT heart cost (relative exercise intensity) was calculated using the 6MWT distance divided by 6MWT mean HR [11]. For the sit-to-stand test, participants had to stand up and sit down on a chair as many times as possible in 30 s, and during the half-squat test, participants had to maintain as long as possible this position: back against the wall, arms crossed on chest, and flexion of 90° between thighs and calves. Finally, the highest number of flexion/extension of the dominant arm within a 30-s time frame with a dumbbell of 2.3 kg for women and 3.6 kg for men was performed during the arm curl test.
The physical exercise belief questionnaire [18] was used to assess PA benefits and perceived psychological barriers for 16 items divided into four categories: benefits of exercise, confidence, embarrassment, and fear of injury. The scores to these questionnaires were transformed into percentages of maximal score.
Weight-related quality of life (WRQOL) was assessed with the Laval questionnaire [19]. It contains 44 questions divided into six domains: symptoms, activity/mobility, personal hygiene/clothing, emotions, social interactions, and sexual life. Final scores are expressed in percentage of maximal score and total score was created by adding all the subscales’ scores.
Body weight, height, and neck circumference were measured using standard protocols [11]. A bipedal bioimpedance scale (Tanita TBF-300A®) was used to assess body composition [6]. Resting blood pressure and heart rate measurement were performed after 5 min of rest in a sitting position with an automatic blood pressure device (Omron HEM 74) [6].
Statistical Considerations
Baseline characteristics were compared between groups with Mann-Whitney for scale data or chi-square tests for nominal data.
Given the number of time assessment (n = 7) and given its robustness with missing data, we favored mixed model analyses to assess the effect of the PreSET. Our model had the following fixed effects: groups, time, and the interaction between group and time. We considered a random intercept with a diagonal residual covariance matrix (allowing for a different residual variance over time). For BMI, due to convergence reasons, a simplified model was used with an identity matrix multiplied by a scalar as the residual covariance matrix. We did post hoc tests to verify if there were differences between T1 and each of the other times. Normal residual distribution was checked to valid statistical models. The Holm-Bonferroni method was used to adjust p values according to the number of comparisons.
Mann-Whitney tests were used for accelerometer parameters (e.g., PA time, steps per day, and energy expenditure) to test the differences between groups 1-Y after BS. The number of active and inactive participants in each group was compared with the Fisher exact test. Variables were presented using the mean and its standard deviation (SD). The attendance rate was calculated by dividing the number of sessions performed (at home or at the hospital) by the number of sessions required (3×/week) until 2 weeks before BS [11]. SPSS (version 23.1; IBM Corporation, Chicago, IL) was employed to perform all statistical analyses. The null hypothesis was rejected at p < 0.05.
Results
Population
The study flow chart is presented in Fig. 1. The baseline characteristics of the participants are presented in Table 1. No significant baseline difference was noted between the PreSET and the usual care groups.
Physical Activity Intervention Attendance
The PreSET participants (n = 13) followed the exercise training program for 32.6 ± 8.0 weeks before BS (range 27–51 weeks). The duration of the training differs from one participant to another due to different waiting times before BS. The PreSET group attended a median of 70 (45–90) % of the total recommended exercise sessions (3×/week) from the baseline of the PreSET until 2 weeks before BS. Seven participants (47%) attended more than 70% of the sessions.
Physical Activity Level
Changes in the declared PA level were not significantly different between groups following BS (see Table S1). Accelerometers were worn on average for 6.6 ± 1.3 days and 13.7 ± 2.0 h per day without a significant difference between groups. Significant differences were observed between the PreSET and usual care groups 1-Y after BS for the number of steps per day and the duration of light and moderate PA per day, as well as for total energy expenditure (see Fig. 2). The duration of vigorous PA (0.02 ± 0.10 vs 0.01 ± 0.00 h per day p = 0.42) and sedentary time (10.4 ± 1.2 vs 10.7 ± 1.6 h per day; p = 0.62) were not different between groups. The number of active participants in the PreSET group was significantly higher 1-Y after BS compared to the usual care group (75.0 vs 27.3%; p = 0.03).
Physical Fitness
Figure 3A outlines a trend for a greater change in the 6MWT distance in the PreSET group after BS (1-year mean change (97.8 ± 56.8 vs 46.9 ± 70.3 m in usual care). The PreSET group showed better improvement of the 6MWT heart cost compared to the usual care group (global p value = 0.02) at 3 (0.5 ± 0.4 vs 0.1 ± 0.6 m/beats/min), 6 (0.8 ± 0.5 vs 0.5 ± 0.4 m/beats/min), and 12 months post-BS (1.3 ± 0.5 vs 0.6 ± 0.4 m/beats/min) (see Fig. 3b). The PreSET group had a greater increase in the half-squat test compared to the usual care group (global p value = 0.02) (1-Y mean change 38.8 ± 49.6 vs 10.3 ± 21.4 s) (see Fig. 3c). No significant difference between groups was observed for changes in the arm curl and sit-to-stand tests, as well as for the maximal aerobic capacity (METS) obtained with the symptom-limited cardiac exercise (see Table S1).
Pre- and post-bariatric surgery changes in the PreSET (n = 13) and usual care (n = 12) groups. p* values compared with T1 unadjusted, p″ values compared with T1 adjusted with Holmes, 6MWT = 6-min walk test, BMI = body max index, PreSET = Pre-Surgical Exercise Training. T1 = baseline, T2 = 12 weeks after PreSET, T3 = 2 weeks before BS, T4 = 3 months after BS, T5 = 6 months after BS, T6 = 9 months after BS, T7 = 12 months after BS
Physical Exercise Beliefs and Barriers and WRQOL
No significant difference between groups was found for changes in the belief in exercise benefits (global p value = 0.44), confidence in athletic ability (global p value = 0.19), embarrassment during PA (global p value = 0.32), fear of injury (global p value = 0.77), or WRQOL (global p value ≥ 0.28) (see Table S1).
Anthropometric Data and Vital Signs
The PreSET group had a more important decrease in BMI at 9 (− 17.8 ± 7.0 vs − 12.8 ± 5.0 kg/m2) and 12 months (− 16.8 ± 4.4 vs − 13.5 ± 5.3 kg/m2) after BS (global p value = 0.05) compared to the usual care group (see Fig. 3d). Compared to the usual care group, the PreSET also had a significant larger decrease in fat-free mass at 6 (− 8.7 ± 3.5 vs − 5.8 ± 3.7 kg), 9 (− 10.5 ± 4.1 vs − 5.8 ± 3.5 kg), and 12 (− 10.6 ± 4.3 vs − 6.6 ± 3.9 kg) months after BS (global p value = 0.03) (see Table S1). No significant difference was observed between groups for changes in the other anthropometric measures (i.e., neck circumference, fat mass), as well as for resting heart rate and blood pressure (see Table S1).
Discussion
We have previously shown that the PreSET, in addition to interdisciplinary lifestyle management, improves physical fitness, social interactions, and embarrassment on the short term (12 weeks) [6]. This study is the first to examine the long-term effects of supervised exercise training compared to usual care in subjects awaiting BS.
One year after BS, the PreSET group had a higher number of steps per day (7460.4 ± 3869.4 vs 4287.5 ± 2248.5 steps/day) and number of hours per day of light (3.2 ± 0.9 vs 2.2 ± 1.1 h/day) and moderate PA (0.6 ± 0.4 vs 0.3 ± 0.2 h/day) compared to the usual group. Our results support those of Bond et al. who concluded that pre-BS PA intervention can positively impact the level of PA after BS [10]. Otherwise, our accelerometer results are not supported by self-reported PA data. However, limited concordance between objective and self-reported assessments of PA had been already noted in BS patients, and this underlines the need for objective measures [20].
One year after BS, we can still observe a larger improvement in submaximal physical fitness in the PreSET group, since the 6MWT heart cost, and the half-squat test showed better improvements in this group. The 6MWT distance change at 1-Y is larger in the PreSET group compared to that in the usual care group (97.8 ± 56.8 vs 46.9 ± 70.3 m); however, the significant difference disappeared after Holmes adjustments. The better improvement of submaximal physical fitness after BS in the PreSET is also supported by the difference in PA level between groups.
In contrast, we noted no difference between groups in maximal METS obtained with the symptom-limited cardiac exercise. Several explanations can be advanced to explain this result. First, as supported by our accelerometer data, the time spent in vigorous PA is low and not different between the groups 1-Y post-BS, reducing opportunities to improve maximal aerobic capacity. Second, the important massive weight loss in the first year after BS may have masked the smaller effects of PA on maximal aerobic capacity. Indeed, several studies reported an improvement in maximal aerobic capacity after BS [21, 22]. Unfortunately, this improvement is not related to a real improvement in physical fitness, since only the relative maximal aerobic capacity (mL/min/kg) improved after BS, as opposed to the absolute capacity (mL/min) [21]. Data are required to see whether a difference between our groups can be observed on the longest term in the weight loss maintenance phase. Finally, our limited sample size can also explain why we cannot detect a difference between groups.
Whereas a short-term effect on social interactions and PA embarrassment has been demonstrated in our previous study after the PreSET [6], we have not been able to demonstrate a long-term significant difference between groups concerning PA barriers and WRQOL. Our small sample size, as well as the important change noted in WRQOL after BS, could explain this lack of significant difference [23, 24]. In addition, we have assumed that the greatest attention, and encouragements provided by the PA specialist, and group interactions during the PreSET could partly explain the short-term improvement of social interactions. However, those elements are no longer present after BS since the PreSET intervention stops at the time of BS.
Although observational studies showed an association between PA and weight loss after BS, several experimental studies providing supervised exercise training after BS found no significant weight loss difference between the intervention and usual care groups [25,26,27,28], probably due to the strong influence of BS on weight loss and the fact that structured exercise may affect non-exercise PA [28]. However, we reported a larger decrease in BMI in the PreSET group compared to that in the usual care group (− 16.8 ± 4.4 vs − 13.5 ± 5.3 kg/m2). According to our results, this difference could be explained surprisingly by the larger loss of fat-free mass in the PreSET group. Nevertheless, the bioimpedance method used in this study is not the gold standard to assess body composition, particularly in individuals with severe obesity [29, 30]. Additional studies and the use of different exercise modalities and support are required to clarify the impact of PA on weight loss and body composition after BS, but we strongly believe that the major interest of PA after BS is to contribute to weight loss maintenance.
The major strengths of this study were the collection of 1-Y post-BS data as well as the study design (randomized controlled trial). However, several limitations have to be considered. Our small sample size limited our ability to detect significant differences between groups on some outcomes and may not represent the whole obese population awaiting BS. In addition, the high number of participants excluded because they were unable to attend regular exercise sessions (n = 80) limits the generalizability of our results and the potential effectiveness of the intervention. Due to the distance of patients’ home from the bariatric surgery center, supervised exercise training at the hospital is not always the best option for patients and alternative delivery modalities like telehealth could be used for physical activity interventions [31]. The duration of the training also differs from one participant to another due to different waiting times before BS. Data on energy intake and type of PA performed in post-BS (aerobic vs resistance) are missing, specifically that to explain the difference of fat-free mass loss between groups. Finally, baseline accelerometer data are missing to study the change of energy expenditure after BS and assess baseline differences between groups.
Considering all these results, we can conclude that a PreSET in addition to an interdisciplinary lifestyle counseling seems promising to increase PA level and physical fitness 1-Y after BS and is associated with a greater decrease in BMI. Studies with larger cohorts are now needed to confirm these results. Two- and three-year post-BS data are also required to understand the impact of a PreSET during the weight loss maintenance phase.
References
King WC, Hsu JY, Belle SH, et al. Pre- to postoperative changes in physical activity: report from the longitudinal assessment of bariatric surgery-2. Surg Obes Relat Dis. 2012;8(5):522–32.
King WC, Chen JY, Bond DS, et al. Objective assessment of changes in physical activity and sedentary behavior: pre- through 3 years post-bariatric surgery. Obesity. 2015;23(6):1143–50.
Baillot A, Audet M, Baillargeon JP, et al. Impact of physical activity and fitness in class II and III obese individuals: a systematic review. Obes Rev. 2014;15(9):721–39.
Bond DS, Thomas JG, King WC, et al. Exercise improves quality of life in bariatric surgery candidates: results from the Bari-Active trial. Obesity. 2015;23(3):536–42.
Woodlief TL, Carnero EA, Standley RA, et al. Dose response of exercise training following roux-en-Y gastric bypass surgery: a randomized trial. Obesity. 2015;23(12):2454–61.
Baillot A, Mampuya WM, Dionne IJ, et al. Impacts of supervised exercise training in addition to interdisciplinary lifestyle management in subjects awaiting bariatric surgery: a randomized controlled study. Obes Surg. 2016;26(11):2602–10.
Bond DS, Evans RK, DeMaria E, et al. Physical activity and quality of life improvements before obesity surgery. Am J Health Behav. 2006;30(4):422–34.
Bond D. Bari-Active: a preoperative intervention to increase physical activity. Obes Surg. 2011;21(8):1042.
Bond DS, Graham Thomas J, Vithiananthan S, et al. Changes in enjoyment, self-efficacy, and motivation during a randomized trial to promote habitual physical activity adoption in bariatric surgery patients. Surg Obes Relat Dis. 2016;12(5):1072–9.
Bond DS, Thomas JG, Vithiananthan S, et al. Intervention-related increases in preoperative physical activity are maintained 6-months after bariatric surgery: results from the bari-active trial. Int J Obes. 2017;41(3):467–70.
Baillot A, Mampuya WM, Comeau E, et al. Feasibility and impacts of supervised exercise training in subjects with obesity awaiting bariatric surgery: a pilot study. Obes Surg. 2013;23(7):882–91.
Kamga-Ngande CN, Carpentier AC, Nadeau-Marcotte F, et al. Effectiveness of a multidisciplinary program for management of obesity: the Unite d'Enseignement, de Traitement et de Recherche sur l'Obesite (UETRO) database study. Metab Syndr Relat Disord. 2009;7(4):297–304.
Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
Santos-Lozano A, Marin PJ, Torres-Luque G, et al. Technical variability of the GT3X accelerometer. Med Eng Phys. 2012;34(6):787–90.
Berglind D, Willmer M, Eriksson U, et al. Longitudinal assessment of physical activity in women undergoing Roux-en-Y gastric bypass. Obes Surg. 2015;25(1):119–25.
Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81.
King WC, Li J, Leishear K, et al. Determining activity monitor wear time: an influential decision rule. J Phys Act Health. 2011;8(4):566–80.
Larsen JK, Geenen R, van Ramshorst B, et al. Binge eating and exercise behavior after surgery for severe obesity: a structural equation model. Int J Eat Disord. 2006;39(5):369–75.
Therrien F, Marceau P, Turgeon N, et al. The laval questionnaire: a new instrument to measure quality of life in morbid obesity. Health Qual Life Outcomes. 2011;9:66.
Bond DS, Jakicic JM, Unick JL, et al. Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity. 2010;18(12):2395–7.
Lund MT, Hansen M, Wimmelmann CL, et al. Increased post-operative cardiopulmonary fitness in gastric bypass patients is explained by weight loss. Scand J Med Sci Sports. 2016;26(12):1428–34.
de Souza SA, Faintuch J, Sant'anna AF. Effect of weight loss on aerobic capacity in patients with severe obesity before and after bariatric surgery. Obes Surg. 2010;20(7):871–5.
Karlsson J, Taft C, Ryden A, et al. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes. 2007;31(8):1248–61.
Kolotkin RL, Crosby RD, Gress RE, et al. Two-year changes in health-related quality of life in gastric bypass patients compared with severely obese controls. Surg Obes Rel Dis. 2009;5(2):250–6.
Castello V, Simões RP, Bassi D, et al. Impact of aerobic exercise training on heart rate variability and functional capacity in obese women after gastric bypass surgery. Obes Surg. 2011;21(11):1739–49.
Shah M, Snell PG, Rao S, et al. High-volume exercise program in obese bariatric surgery patients: A randomized, controlled trial. Obesity. 2011;19(9):1826–34.
Coen PM, Tanner CJ, Helbling NL, et al. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. J Clin Invest. 2015;125(1):248–57.
Carnero EA, Dubis GS, Hames KC. Jakicic JM. Coen PM, et al. Randomized trial reveals that physical activity and energy expenditure are associated with weight and body composition after RYGB. Obesity: Houmard JA; 2017. [Epub ahead of print]
Buckinx F, Reginster JY, Dardenne N, et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord. 2015;16:60.
Boneva-Asiova Z, Boyanov MA. Body composition analysis by leg-to-leg bioelectrical impedance and dual-energy X-ray absorptiometry in non-obese and obese individuals. Diabetes Obes Metab. 2008;10(11):1012–8.
Baillot A, Boissy P, Tousignant M, et al. Feasibility and effect of in-home physical exercise training delivered via telehealth before bariatric surgery. J Telemed Telecare. 2017;23(5):529–35.
Acknowledgements
We would like to gratefully thank research professionals (Vicki Lebrun, Marie-Michèle Rosa-Fortin), the kinesiologist (Anouk Landry), and trainees of Université de Sherbrooke (Canada) (Gabrielle Lebel, Rachel Lapointe, Alexandrine Boucher, Sofia Vasquez) who contributed to the supervised exercise training sessions and data collection.
Funding
This study was funded by the Canadian Institutes of Health Research (CIHR, Grant No. OPB-131592 to MFL). At the time of the study, AB was the recipient of a scholarship from the Department of Medicine of Université de Sherbrooke. At the time of the study, MFL was the recipient of a National Researcher Award from the Fonds de recherche du Québec–Santé (FRQ-S). The Research Center of the Centre hospitalier universitaire de Sherbrooke is a FRQ-S-funded research center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Table S1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Baillot, A., Vallée, CA., Mampuya, W.M. et al. Effects of a Pre-surgery Supervised Exercise Training 1 Year After Bariatric Surgery: a Randomized Controlled Study. OBES SURG 28, 955–962 (2018). https://doi.org/10.1007/s11695-017-2943-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2943-8