Abstract
Background
Metabolic syndrome (MetS) is an important etiologic and prognostic factor for cancer, but few studies have assessed hospitalization outcomes among patients with both conditions.
Methods
Data was obtained from the Healthcare Cost and Utilization project Nationwide Inpatient Sample (HCUP-NIS). Study variables were assessed using ICD-9 codes on adults aged 40 years and over admitted to a US hospital between 2007 and 2011 with primary diagnosis of either breast, colorectal, or prostate cancer. We examined in-hospital mortality, post-surgical complications, and discharge disposition among cancer patients with MetS and compared with non-MetS patients.
Results
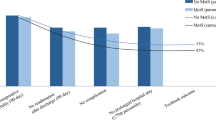
Hospitalized breast (OR: 0.31, 95% CI: 0.20–0.46), colorectal (OR: 0.41, 95% CI: 0.35–0.49), and prostate (OR: 0.28, 95% CI: 0.16–0.49) cancer patients with MetS had significantly reduced odds of in-hospital mortality. The odds of post-surgical complications among breast (OR: 1.20, 95% CI: 1.03–1.39) and prostate (OR: 1.22, 95% CI: 1.09–1.37) cancer patients with MetS were higher, but lower by 7% among colorectal cancer patients with MetS. Additionally, breast (OR: 1.21, 95% CI: 1.11–1.32) and colorectal (OR: 1.06, 95% CI: 1.01–1.11) cancer patients with MetS had significantly higher odds for discharge to a skilled nursing facility compared with those without MetS, but this was not statistically significant among prostate cancer patients.
Conclusions
Adverse health outcomes were significantly higher among hospitalized patients with a primary diagnosis of cancer and MetS. Future studies are needed to identify clinical strategies for detecting and managing patients with MetS to reduce the likelihood of poor inpatient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incidence rates for cancer and metabolic syndrome (MetS) have continued to increase dramatically in the USA and globally [34]. MetS is defined clinically as a cluster of interrelated biochemical conditions that include abdominal obesity, insulin resistance, dyslipidemia, and hypertension, and this condition has been associated with significantly increased risk for coronary heart disease, stroke, and type 2 diabetes [8, 24, 27, 33]. Recent epidemiologic evidence also suggests that MetS is an important etiologic factor for the development of cancer [47], as well as poor prognosis for common cancer types including breast and colorectal cancer [20]. The prevalence of both MetS and cancer increases dramatically with age [46], and with the rapid aging of the US population, adverse health outcomes for millions of adults, as well as healthcare costs, are likely to be significant [11].
Currently, at least a third of the general US adult population meet the current clinical criteria for MetS based on analysis of the National Health and Nutritional Survey (NHANES 2003–2012), with prevalence increasing to about 50% among those ages 60 years and older [1]. Individual components such as obesity [15], diabetes [6, 37], and hypertension [19, 23] have long been shown to increase the risk of medical complications, overall and cancer-specific mortality. However, health outcomes among hospitalized patients, who are likely older, more vulnerable, and with more severe health-related conditions, have not been well evaluated. Prior studies have reported strong positive associations between MetS and incidence and mortality due to breast [12, 20], colorectal [5, 20], liver [20], and bladder cancers [20], but hospitalized patients, particularly those with the most common cancer types in US adults (breast, prostate, and colorectal cancer), need further study. Hence, the purpose of this study is to examine health outcomes, specifically in-hospital mortality, post-surgical complications, and discharge disposition, among hospitalized cancer patients with a clinical diagnosis of MetS.
Methods
Study Population
This cross-sectional study was conducted among breast, colorectal, and prostate cancer patients ages 40 years and older admitted to a US hospital between 2007 and 2011. Clinical data were obtained from the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS), which covers over 1000 hospitals in the USA and includes data on over seven million hospital stays. Further details about NIS can be obtained from http://www.hcupus.ahrq.gov/nisoverview.jsp.
Clinical and Individual Variables
International Classification of Disease, ninth edition ICD-9 codes for breast (ICD-9 codes: 174.x), colorectal (ICD-9 codes: 153.x, 154.0–154.3, 154.8), and prostate cancer (ICD-9 code: 185) recorded during admissions were assessed. Patients in whom the diagnosis of interest was not coded in first two diagnostic fields were excluded in order to exclude patients with underlying conditions. In addition, data on race, age, gender, area-level income, residential region, and insurance status were obtained from the NIS dataset. As cancer stage data is not captured in the dataset, a proxy cancer stage variable was created using the clinical criteria of disease staging, with patients categorized as metastatic when ICD-9 code indicated metastatic disease to other organs (196.x, 197.x, 198.x, 199.0–199.1) and non-metastatic when those codes were absent. Several studies have used similar staging criteria using the HCUP-NIS database [4]. Hospital length of stay was calculated by subtracting the number of days between admission and discharge, with same-day stays coded as 0. Surgical treatment for breast, colorectal, and prostate cancer was classified using diagnostic and procedure codes similar to those used in prior studies with HCUP NIS data (ICD-9 codes: breast: 85.41–85.48, 85.20–85.23; colorectal: 17.33–17.36, 17.39, 45.7×, 45.80–45.82, 48.42–48.43, 48.49, 48.50–48.52, 48.63–48.65; prostate: 60.2–60.6) [2, 3, 32].
Study Exposure
Metabolic syndrome (MetS) was defined based on diagnosis codes (ICD-9 code: 277.7) or having at least three out of five components of MetS, namely, high blood pressure (ICD-9 codes: 401–405), BMI ≥ 30 (ICD-9 codes: V85.3-V85.4, 278.01, 278.03), altered fasting glucose (ICD-9 codes: 250.00, 250.02, 250.10, 250.12, 250.20, 250.22, 250.30, 250.32, 250.40, 250.42, 250.50, 250.52, 250.60, 250.62, 250.70, 250.72, 250.80, 250.82, 250.90, 250.92), low HDL cholesterol (ICD-9 codes: 272.5–272.6), and high triglycerides (ICD-9 codes: 272.1–272.4). This definition was chosen to approximate the definition published by the US National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) [7] previously examined in the NIS database [31] and Surveillance, Epidemiology, and End Results-Medicare database [42]. A modified Deyo comorbidity index [4] was created to account for the number of comorbid conditions present upon admission among patients, and those included myocardial infarction, chronic heart failure, peripheral vascular disease, cerebrovascular disease, dementia, cardio-pulmonary disease, rheumatic disease, peptic ulcer disease, mild liver disease, hemiplegia, paraplegia, renal disease, liver disease, and HIV/AIDS.
Outcome Measures
The main study outcomes assessed for cancer patients were in-hospital mortality, post-surgical complications, and discharge disposition. In-hospital mortality was defined as deaths occurring during hospitalization; post-surgical complication was defined using ICD-9 codes associated with mechanical wounds, infection, renal, gastrointestinal, cardiovascular or pulmonary complications, as well as intra-operative complications. Discharge disposition is based on whether patients are discharged to recover at home or in other facilities such as nursing homes, and was classified into the following: (1) routine discharge: discharged to home or self-care; (2) discharge to a skilled nursing facility; (3) expired/died; and (4) other—classified as discharged due to any other reasons not stated above [10, 16].
Statistical Analysis
Descriptive statistics was conducted using Chi-square tests for categorical variables and t test for continuous variables, and the proportion of cancer in patients with MetS was assessed. Multivariable logistic regression analysis was used to determine the association between MetS and in-hospital mortality, complications, and discharge disposition separately for breast cancer in women only, prostate cancer in men only, and colorectal cancer in both men and women. All models were adjusted for age, race, stage, income, insurance and residential region, number of comorbidities, treatment, stage, and length of stay. Additionally, models for discharge disposition and in-hospital mortality were also adjusted for complications, and models for colorectal cancer were also adjusted for gender. All statistical analyses were performed with SAS 9.4 (Cary, NC).
Results
MetS was present in 5.2, 6.9, and 5.5% of hospitalized breast (70,916), colorectal (152,952), and prostate (87,623) cancer patients, respectively (Table 1). MetS was more prevalent in the older age groups of 60–69 years (7.3% in breast, 8.6% in colorectal, and 6.2% in prostate patients) compared with younger age groups (1.4% in breast, 2.6% in colorectal, and 2.9% in prostate cancer). For breast (8.% vs. 4.5%), colorectal (7.4% vs. 6.7%), and prostate (7.6% vs. 4.9%) cancer patients, MetS prevalence was higher among Blacks compared to Whites. In addition, patients residing in the lowest socio-economic status (SES) regions had higher prevalence of MetS compared with those in the highest SES regions across all three cancer types. Regardless of cancer type, patients on Medicare had much higher MetS prevalence compared with those with private or other insurance types.
After adjusting for demographics, SES, insurance, and stage (Table 2), breast (OR: 0.31, 95% CI: 0.20–0.46) and prostate (OR: 0.28, 95% CI: 0.16–0.49) cancer patients with MetS had a 70–80% lower odds of in-hospital deaths, while colorectal cancer patients experienced about 60% reduced odds (OR: 0.41, 95% CI: 0.35–0.49). However, increasing number of comorbidities was associated with about 43% increased odds of in-hospital mortality across the three cancer types (breast: OR: 1.43, 95% CI: 1.31–1.57; colorectal: OR: 1.46, 95% CI: 1.41–1.51; prostate: OR: 1.44, 95% CI: 1.31–1.58), excluding comorbid conditions included in the definition of MetS. There was a 20–22% increased odds of post-surgical complications among breast (OR: 1.20, 95% CI: 1.03–1.39) and prostate (OR: 1.22, 95% CI: 1.09–1.37) cancer patients with MetS, but a 7% decreased odds among colorectal cancer patients (OR: 0.93, 95% CI: 0.88–0.99) with MetS (Table 3). Increasing number of comorbid conditions also increased the odds of post-surgical complications among breast cancer patients (OR: 1.10, 95% CI: 1.03–1.17), but reduced the odds among colorectal cancer patients (OR: 0.90, 95% CI: 0.88–0.92). Breast (OR: 1.21, 95% CI: 1.11–1.32) and colorectal (OR: 1.06, 95% CI: 1.01–1.11) cancer patients with MetS were significantly more likely to be discharged to a skilled nursing facility compared with those without MetS (Table 4). Prostate cancer patients with MeS were also 10% more likely to be discharged to skilled nursing facilities; however, this was not statistically significant.
Discussion
In a large dataset of hospitalized cancer patients, we observed that 5 to 7% of breast, colorectal, and prostate cancer patients met the criteria for MetS using the NCEP ATP III definition [7]. The observed prevalence of MetS among hospitalized cancer patients increased with age and was much higher among Blacks and Hispanics compared with Whites, and higher among residents of lower SES regions compared with higher SES regions. These demographic patterns are similar to those observed in the general US population [1], and other studies have reported a prevalence of about 9% among patient populations in the HCUP NIS [31]. About a third of the general US adult population currently meets the criteria for MetS, with significantly increasing trends observed in the past few decades [41]. Differences in the availability of relevant data items in routine healthcare claim databases such as the HCUP likely contributed to our observed lower estimate. Nevertheless, significant increases in the prevalence of MetS are expected to continue due to demographic changes (due to the aging of the US population) and trends in lifestyle risk factors (such as obesity, physical activity, and diet). These risk factors also independently contribute to cancer risk and prognosis, making the assessment of the impact of metabolic syndrome on cancer outcomes highly relevant.
We observed that breast, colorectal, and prostate cancer patients with MetS had lower odds of in-hospital mortality, but higher odds of post-surgical complications and discharge to skilled nursing facilities. Other studies have reported a positive association between MetS and cancer mortality [17, 25, 43] with result showing increased risk of cancer-related mortality among individuals with MetS, and other studies have reported increased risk of post-surgical complications among patients with MetS [30]. Our finding of an inverse association between MetS and in-hospital mortality may be due to several factors: (1) differences in the specific MetS criteria used, (2) tumor-specific differences in the association between MetS and cancer mortality that may be masked by examining overall cancer mortality, and/or (3) differences in study population examined. In this study, we focused on hospitalized cancer patients, who are likely to be at more advanced stages of disease and hospitalized for surgery or treatment of other cancer-related complications. This study population is also unique in several ways. For instance, the severity of the cancer diagnosis and complicated treatment process may have led to mis-classification of MetS status if relevant data on diabetes, cholesterol, or BMI are not routinely captured/ recorded in the medical records. In addition, an underlying MetS condition may be considered secondary to the primary cancer diagnosis during admission, and thus not addressed or evaluated during hospitalization. In addition, since MetS is associated with poorer hospitalization outcomes [9, 36, 38], this may have resulted in our observed higher odds of post-surgical complications and non-routine discharge, in which case mortality outcomes will occur outside of the hospital setting and thus will not be captured in the dataset.
There were increased odds of post-operative complications among breast and prostate cancer patients with MetS, but decreased odds for patients with colorectal cancer. This may be due to differences in the types of surgical procedures. A major innovation in recent surgical techniques was the development of laparoscopic surgery for colon and prostate cancer [21, 29, 45]. This less-invasive surgery type has been associated with significantly reduced rates of post-surgical complications, and a recent study observed that MetS was associated with poorer post-surgical outcomes following radical prostatectomy compared with those who received laparoscopic surgery [39]. We also observed significantly higher odds of breast and colorectal cancer patients with MetS to be discharged to skilled facilities. Our findings corroborate the existing literature showing that cancer patients with perioperative events, comorbidities, and surgical complications were at higher risk for rapid postoperative functional decline, leading to discharge to skilled facilities [16, 18, 44]. Taken together, these results suggest that the inverse association observed between MetS and in-hospital mortality is likely an artifact of the greater likelihood for these patients to experience post-surgical complications and to be discharged to nursing facilities.
Given that millions of US adults currently meet the criteria for MetS, especially at older ages when the risk of chronic diseases like cancer is also highest, it is inevitable that a significant proportion of hospitalized patients will experience complications or adverse health outcomes more severe than indicated due to their current health condition. The question of whether health outcomes among cancer patients can be improved by controlling or eliminating MetS awaits large prospective studies. However, there is growing recognition of the importance of MetS as a significant public health issue, due to its independent association with worse health outcomes, as well as its role as an etiologic and/or prognostic risk factor for many other chronic diseases including cardiovascular diseases, stroke, chronic kidney diseases, and Alzheimer’s disease. MetS is also associated with increased healthcare utilization, longer hospital stay, increased healthcare costs, and need for post-hospitalization care. A critical need will be to identify which approaches should be taken to address or resolve components of MetS among pre-, peri-, or post-operative patients, who are at increased risk of complications. Hospitalized cancer patients with MetS may benefit from holistic clinical approaches to recognize components of MetS and its potential impact on health outcomes, and management strategies combining nutritional changes (e.g., low-carbohydrate dietary patterns shown to improve HDL, glucose, and HbA1C levels [13, 14, 22], prescribed physical activity (e.g., 30-min moderate physical activity 3–4 days/week to improve blood pressure and glycemic control [28, 40]), and/or the use of statins as lipid-lowering agents [35]. Future studies will be required to formally evaluate whether these combined behavioral/lifestyle approaches among cancer patients may improve post-operative outcomes and improve survival.
Several strengths and limitations are relevant to these study results. First, there is very little direct evidence regarding inpatient outcomes among adults with clinical diagnoses of MetS and cancer, despite the high prevalence of MetS in US adults, the common risk factors shared by both conditions, and the vulnerability of hospitalized patients. Second, use of the Nationwide Inpatient Survey provided objective clinical claims data on MetS and cancer diagnosis on a large sample of US adults, with baseline socio-demographic data that allowed us to adjust for potential confounders. As with most studies based on administrative claim database, the study was also subject to several limitations. First, our operational definition of MetS relied exclusively on ICD-9 codes documented in the NIS database and may be vulnerable to misclassification and under-classification especially for MetS. The definition of MetS is ever-evolving [26] and included measurements of cholesterolemia, triglyceridemia, fasting plasma glycemia, and waist circumference. These may not always be assessed during admission for a primary diagnosis of cancer or may not be consistently recorded as diagnosis codes, potentially explaining the lower prevalence of MetS in our study sample. MetS was defined here based on the presence of diagnosis codes for at least three out of five components, following the definition proposed by the US National Cholesterol Education Program Adult Treatment Panel III [7] and similar to the methodology reported by other investigators [1, 31, 42]. Second, due to the cross-sectional nature of the dataset, we are unable to directly assess causality or account for individuals who have MetS components that are well controlled with medication. Nevertheless, we observed significantly worse health outcomes among patients with clinical diagnoses of MetS and cancer that warrant the early identification of these patients and formulation of clinical strategies to manage or eliminate components of MetS prior to surgery. Future studies are also needed to provide critical information regarding prevention and treatment strategies most appropriate for cancer patients, especially those who are sicker and hospitalized.
In conclusion, hospitalized patients with MetS and clinical diagnosis of breast, colorectal, and prostate cancer were less likely to experience in-hospital mortality, but were more likely to experience post-surgical complications and non-routine discharge to skilled nursing facilities. Clinical strategies for timely identification and control of MetS components may go a long way in reducing these adverse health outcomes among hospitalized patients.
References
Aguilar M, Bhuket T, Torres S, et al. PRevalence of the metabolic syndrome in the united states, 2003-2012. JAMA. 2015;313(19):1973–4. https://doi.org/10.1001/jama.2015.4260.
Akinyemiju T, Sakhuja S, Vin-Raviv N. Racial and socio-economic disparities in breast cancer hospitalization outcomes by insurance status. Cancer Epidemiol. 2016a;43:63–9. https://doi.org/10.1016/j.canep.2016.06.011.
Akinyemiju T, Waterbor JW, Pisu M, et al. Availability of healthcare resources and colorectal cancer outcomes among non-Hispanic white and non-Hispanic black adults. J Community Health. 2016b;41(2):296–304. https://doi.org/10.1007/s10900-015-0096-z.
Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, et al. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiol. 2015;39(5):745–51. https://doi.org/10.1016/j.canep.2015.07.010.
Aleksandrova K, Boeing H, Jenab M, et al. Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer Prev Res (Phila). 2011;4(11):1873–83. https://doi.org/10.1158/1940-6207.capr-11-0218.
Anand N, Chong CA, Chong RY, et al. Impact of diabetes on postoperative outcomes following colon cancer surgery. J Gen Intern Med. 2010;25(8):809–13. https://doi.org/10.1007/s11606-010-1336-7.
Anon. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143.
Athyros VG, Ganotakis ES, Elisaf MS, et al. Prevalence of vascular disease in metabolic syndrome using three proposed definitions. Int J Cardiol. 2007;117(2):204–10. https://doi.org/10.1016/j.ijcard.2006.04.078.
Bai YM, Li CT, Tsai SJ, Tu PC, Chen MH, Su TP (2016). Metabolic syndrome and adverse clinical outcomes in patients with bipolar disorder. BMC Psychiatry 16(1):448
Balentine CJ, Naik AD, Robinson CN, et al. Association of high-volume hospitals with greater likelihood of discharge to home following colorectal surgery. JAMA surgery. 2014;149(3):244–51. https://doi.org/10.1001/jamasurg.2013.3838.
Berger NA, Savvides P, Koroukian SM, et al. Cancer in the elderly. Trans Am Clin Climatol Assoc. 2006;117:147–56.
Bhandari R, Kelley GA, Hartley TA, et al. Metabolic syndrome is associated with increased breast cancer risk: a systematic review with meta-analysis. Int J Breast Cancer. 2014;2014:189384. https://doi.org/10.1155/2014/189384.
Boden G, Sargrad K, Homko C, et al. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142(6):403–11.
Brehm BJ, Seeley RJ, Daniels SR, et al. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88(4):1617–23. https://doi.org/10.1210/jc.2002-021480.
Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. https://doi.org/10.1056/NEJMoa021423.
Cholankeril G, Hu M, Tanner E, et al. Skilled nursing facility placement in hospitalized elderly patients with colon cancer. Eur J Surg Oncol. 2016;42(11):1660–6. https://doi.org/10.1016/j.ejso.2016.06.005.
Colangelo LA, Gapstur SM, Gann PH, et al. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomark Prev. 2002;11(4):385–91.
Cramer JD, Patel UA, Samant S, et al. Discharge destination after head and neck surgery: predictors of discharge to Postacute care. Otolaryngol Head Neck Surg. 2016;155(6):997–1004. https://doi.org/10.1177/0194599816661514.
Dyer AR, Stamler J, Berkson DM, et al. High blood-pressure: a risk factor for cancer mortality? Lancet (London, England). 1975;1(7915):1051–6.
Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–11. https://doi.org/10.2337/dc12-0336.
Finkelstein J, Eckersberger E, Sadri H, et al. Open versus laparoscopic versus robot-assisted laparoscopic prostatectomy: the European and US experience. Rev Urol. 2010;12(1):35–43.
Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–90. https://doi.org/10.1056/NEJMoa022207.
Grossman E, Messerli FH, Boyko V, et al. Is there an association between hypertension and cancer mortality? Am J Med. 2002;112(6):479–86.
Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–36. https://doi.org/10.1161/atvbaha.107.151092.
Hsing AW, Sakoda LC, Chua Jr S. Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86(3):s843–57.
Johnson LW, Weinstock RS. The metabolic syndrome: concepts and controversy. Mayo Clin Proc. 2006;81(12):1615–20. https://doi.org/10.4065/81.12.1615.
Koren-Morag N, Goldbourt U, Tanne D. Relation between the metabolic syndrome and ischemic stroke or transient ischemic attack: a prospective cohort study in patients with atherosclerotic cardiovascular disease. Stroke. 2005;36(7):1366–71. https://doi.org/10.1161/01.str.0000169945.75911.33.
Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab Physiologie appliquee, nutrition et metabolisme. 2007;32(1):76–88. https://doi.org/10.1139/h06-113.
Lepor H. Open Versus Laparoscopic Radical Prostatectomy. Rev Urol. 2005;7(3):115–27.
Lohsiriwat V, Pongsanguansuk W, Lertakyamanee N, et al. Impact of metabolic syndrome on the short-term outcomes of colorectal cancer surgery. Dis Colon Rectum. 2010;53(2):186–91. https://doi.org/10.1007/DCR.0b013e3181bdbc32.
Memtsoudis SG, Kirksey M, Ma Y, et al. Metabolic syndrome and lumbar spine fusion surgery: epidemiology and perioperative outcomes. Spine. 2012;37(11):989–95. https://doi.org/10.1097/BRS.0b013e31823a3a13.
Milenkovic M, Russo CA, Elixhauser A. Hospital stays for prostate cancer, 2004: statistical brief #30. In: healthcare cost and utilization project (HCUP) statistical briefs. Rockville: Agency for Healthcare Research and Quality (US); 2006.
Nilsson PM, Engstrom G, Hedblad B. The metabolic syndrome and incidence of cardiovascular disease in non-diabetic subjects--a population-based study comparing three different definitions. Diabet Med. 2007;24(5):464–72. https://doi.org/10.1111/j.1464-5491.2007.02142.x.
O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. https://doi.org/10.1111/obr.12229.
Ott C, Schmieder RE. The role of statins in the treatment of the metabolic syndrome. Curr Hypertens Rep. 2009;11(2):143–9.
Ounhasuttiyanon A, Lohsiriwat V. Metabolic syndrome and outcome after breast reconstruction. Gland Surg. 2014;3(1):85–7. https://doi.org/10.3978/j.issn.2227-684X.2014.02.07.
Ranc K, Jorgensen ME, Friis S, et al. Mortality after cancer among patients with diabetes mellitus: effect of diabetes duration and treatment. Diabetologia. 2014;57(5):927–34. https://doi.org/10.1007/s00125-014-3186-z.
Shehab A, Al-Dabbagh B, Almahmeed W, et al. Prevalence, characteristics, and in-hospital outcomes of metabolic syndrome among patients with acute coronary syndrome in the United Arab Emirates. Open Cardiovasc Med J. 2012;6:81–7. https://doi.org/10.2174/1874192401206010081.
Shiota M, Takeuchi A, Sugimoto M, et al. The differential impact of body mass index and the feature of metabolic syndrome on oncological outcomes following different surgical procedures in Japanese men with prostate cancer. Ann Surg Oncol. 2016; https://doi.org/10.1245/s10434-016-5705-2.
Sigal RJ, Kenny GP, Wasserman DH, et al. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27(10):2518–39.
Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59(10):1410–5. https://doi.org/10.1136/gut.2010.213553.
Trabert B, Wentzensen N, Felix AS, et al. Metabolic syndrome and risk of endometrial cancer in the united states: a study in the SEER-medicare linked database. Cancer Epidemiol Biomark Prev. 2015;24(1):261–7. https://doi.org/10.1158/1055-9965.epi-14-0923.
Trevisan M, Liu J, Muti P, et al. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomark Prev. 2001;10(9):937–41.
Trinh QD, Bianchi M, Sun M, et al. Discharge patterns after radical prostatectomy in the United States of America. Urol Oncol. 2013;31(7):1022–32. https://doi.org/10.1016/j.urolonc.2011.10.007.
Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6(7):477–84. https://doi.org/10.1016/s1470-2045(05)70221-7.
Veronica G, Esther RRM. Aging, metabolic syndrome and the heart. Aging Dis. 2012;3(3):269–79.
Zhou JR, Blackburn GL, Walker WA. Symposium introduction: metabolic syndrome and the onset of cancer. Am J Clin Nutr. 2007;86(3):s817–9.
Funding
This study was funded by grant K01TW010271 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics and Consent Statement
This study was considered exempt by the Institutional Review Board at the University of Alabama at Birmingham, as the HCUP-NIS database is a publicly available and non-identifiable data source.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Statement of Informed Consent
This study used the HCUP-NIS database which is a publicly available and non-identifiable secondary data source. For this type of study, additional informed consent is not required.
Statement of Human and Animal Rights
This article does not contain any experiments with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Akinyemiju, T., Sakhuja, S. & Vin-Raviv, N. In-Hospital Mortality and Post-Surgical Complications Among Cancer Patients with Metabolic Syndrome. OBES SURG 28, 683–692 (2018). https://doi.org/10.1007/s11695-017-2900-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2900-6