Abstract
Background
The number of bariatric procedures performed in Japan is increasing. There are isolated reports of bariatric surgery, but there have been no nationwide surveys including long-term data.
Methods
We retrospectively reviewed data for patients who underwent bariatric and metabolic surgery throughout Japan and reviewed outcomes. Surveys were sent to ten institutions for number of procedures, preoperative patient weight and preoperative obesity-related comorbidities, and data at 1, 3, and 5 years postoperatively. Improvement of type 2 diabetes mellitus at 3 years after surgery was stratified by baseline ABCD score, based on age, body mass index, C-peptide level, and duration of diabetes.
Results
Replies were received from nine of the ten institutions. From August 2005 to June 2015, 831 patients, including 366 males and 465 females, underwent bariatric procedures. The mean age was 41 years, and mean BMI was 42 kg/m2. The most common procedure was laparoscopic sleeve gastrectomy (n = 501, 60 %) followed by laparoscopic sleeve gastrectomy with duodenojejunal bypass (n = 149, 18 %). Laparoscopic Roux-en-Y gastric bypass was performed in 100 patients (12 %), and laparoscopic adjustable gastric banding was performed in 81 (10 %). At 3 years postoperatively, the remission rate of obesity-related comorbidities was 78 % for diabetes, 60 % for hypertension, and 65 % for dyslipidemia. Patients with complete remission of diabetes at 3 years postoperatively had a higher ABCD score than those without (6.4 ± 1.6 vs 4.2 ± 2.0, P < 0.05).
Conclusions
Bariatric and metabolic surgery for Japanese morbidly obese patients is safe and effective. These results are comparable with the results of previous studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of obese people in the world has continued its upward trend in recent years. It is reported that more than 2.1 billion people (approximately 30 % of the world’s population) are overweight or obese [1]. Obesity must be addressed on a global scale urgently. Numerous studies have demonstrated elevated risk of metabolic complications of obesity with relatively low levels of body mass index (BMI) among Asian populations because Asians are more prone to central obesity [2]. Although the prevalence of morbid obesity is low in Japan (0.25–0.3 %) compared with the USA and European countries [3, 4], it is increasing.
The number of bariatric and metabolic surgical procedures performed is increasing each year. According to the statistics of the International Federation for the Surgery of Obesity and Metabolic Disorders, the number of procedures that was about 40,000 per year in 1998 [5] increased to 146,000 in 2003 [6], 344,000 in 2008 [7], and 469,000 in 2013 [8]. Despite this worldwide trend, bariatric surgery is still not common in Japan. Bariatric procedures were approved for payment by the national health insurance system in 2014 and, prior to that time, were not reimbursed at all. The surgical volume is relatively low, and the Japanese Society for the Surgery of Obesity and Metabolic Disorders collects data for all cases of bariatric and metabolic surgery throughout Japan. There are isolated reports of bariatric surgery, but there have been no nationwide surveys including long-term data [9–13]. We undertook this study to review long-term outcomes of bariatric and metabolic surgery in Japan.
Methods
Bariatric and metabolic surgery was performed in 16 facilities in Japan as of November 2015. An e-mail survey was sent to the ten institutions which have performed bariatric and metabolic surgery for more than 3 years. Seven institutions are university hospitals, and three are private hospitals. We distributed a survey to collect data for number of procedures, preoperative weight, and the presence of obesity-related comorbidities preoperatively, and at 1, 3, and 5 years postoperatively. Both early (≦30 days) and late (>30 days) complications were evaluated. The institutional review board at each site approved the study, and all patients provided written informed consent. Criteria for remission and improvement in obesity-related comorbidities including type 2 diabetes mellitus, hypertension, and dyslipidemia are shown in Table 1, using criteria from Brethauer SA. et al. [14]. Data for patients at each institution were collected from the records at each of the responding institutions. There is no national database for this information.
In addition, we examined the remission rate for type 2 diabetes mellitus at 3 years after surgery stratified by baseline ABCD score. The ABCD score, which is composed of age, BMI, C-peptide level, and duration of type 2 diabetes mellitus, has been reported to be useful in predicting the success of type 2 diabetes mellitus treatment using metabolic surgery [15]. The ABCD score was slightly modified from the original based on further analysis as shown in Table 2 [16]. If deemed necessary, the survey was followed by e-mail communications to obtain a response. This survey did not collect personal identifying information for any patient.
For calculations of weight loss and percent excess BMI loss resulting from each surgical procedure, weighted averages were calculated from the averages and the number of procedures in each institution. We calculated the percent excess BMI loss as follows:
Statistical Analysis
All statistical analyses were performed using SPSS version 23 (SPSS Inc., Chicago, IL), with baseline comparison made using the Mann–Whitney U test. Continuous variables were expressed as the mean (and standard deviation, SD), with differences expressed as mean (and SD). A two-sided p value of <0.05 was considered statistically significant.
Results
Procedures Performed
Replies were received from nine of the ten institutions surveyed. From August 2005 to June 2015, 831 patients, including 366 males and 465 females, underwent bariatric and metabolic surgical procedures. All procedures were completed laparoscopically without conversion to open surgery. One of the nine responding institutions had performed more than 500 procedures, one had more than 100, three had 30–55, two had 20–25, and the remaining one had less than 10. Four different procedures were performed in this series. The most commonly performed procedure was laparoscopic sleeve gastrectomy (LSG) (n = 501), and the second was laparoscopic sleeve gastrectomy with duodenojejunal bypass (LSG/DJB) (n = 149). Laparoscopic Roux-en-Y gastric bypass (LRYGB) was performed in 100 patients, and laparoscopic adjustable gastric banding (LAGB) was performed in 81 patients. The mean age was 41 years, the mean preoperative weight was 114 kg, and the mean preoperative BMI was 42 kg/m2. The preoperative type 2 diabetes mellitus prevalence was 50 %, hypertension 62 %, and dyslipidemia 74 %.
Weight Loss Outcome
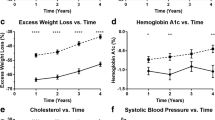
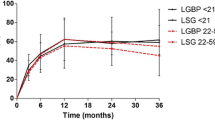
The results of percent total weight loss and percent excess BMI loss after each procedure are shown in Figs. 1 and 2. After LSG, percent total weight loss was 29 % at 1 year (n = 447), 28 % at 3 years (n = 183), and 26 % at 5 years (n = 57). Percent excess BMI loss was 72 % at 1 year, 71 % at 3 years, and 63 % at 5 years.
After LRYGB, percent total weight loss was 33 % at 1 year (n = 92), 33 % at 3 years (n = 53), and 29 % at 5 years (n = 38). Percent excess BMI loss was 83 % at 1 year, 82 % at 3 years, and 74 % at 5 years.
After LAGB, percent total weight loss was 21 % at 1 year (n = 73), 19 % at 3 years (n = 46), and 19 % at 5 years (n = 31). Percent excess BMI loss was 48 % at 1 year, 52 % at 3 years, and 53 % at 5 years.
After LSG/DJB, percent total weight loss was 24 % at 1 year (n = 144), 26 % at 3 years (n = 60), and 32 % at 5 years (n = 19). Percent excess BMI loss was 70 % at 1 year, 78 % at 3 years, and 76 % at 5 years.
Based on this study, patients who underwent procedures other than LSG/DJB tended to regain weight gradually after 3 years.
Obesity-Related Comorbidity Outcomes
Type 2 Diabetes Mellitus
A total of 415 patients had type 2 diabetes mellitus preoperatively. We followed 136 patients at the third year after surgery. Details for these patients are shown in Table 3. We examined improvement of type 2 diabetes mellitus at the third year after surgery stratified by baseline ABCD score in 129 of 136 patients. The baseline ABCD score for type 2 diabetes mellitus according to the surgical procedure performed is shown in Table 4. Remissions, including both complete and partial remissions, were in total 78 % (101/129) and included 85 % (55/65) in the LSG group, 71 % (30/42) in the LSG/DJB group, 92 % (12/13) in the LRYGB group, and 44 % (4/9) in the LAGB group. The remission rate for patients with a preoperative ABCD score five points or less was 57 % (12/21) for LSG, 68 % (19/28) for LSG/DJB, 100 % (1/1) for LRYGB, and 0 % (0/5) for LAGB. The patients with complete type 2 diabetes mellitus remission after metabolic surgery at 3 years had a higher ABCD score than those without (6.4 ± 1.6 vs 4.2 ± 2.0, P < 0.05).
Hypertension
A total of 516 patients had preoperative hypertension. Of these patients, we followed 177 for 3 years after surgery. Details for these patients are shown in Table 3. The number of patients with hypertension was 97 in the LSG group, 39 in the LSG/DJB group, 17 in the LRYGB group, and 24 in the LAGB group. Remissions, including both complete and partial remission, were in total 60 % (106/177) and included 66 % (64/97) for patients who underwent LSG, 46 % (18/39) for LSG/DJB, 88 % (15/17) for LRYGB, and 38 % (9/24) for LAGB.
Dyslipidemia
The number of patients with dyslipidemia is 615. Of these, we followed 175 3 years postoperatively. The details for these patients are shown in Table 3. The number of patients with dyslipidemia was 94 in the LSG group, 31 in the LSG/DJB group, 20 in the LRYGB group, and 30 in the LAGB group. The remission rate was in total 65 % (114/175) and included 63 % (59/94) for patients undergoing LSG, 68 % (21/31) for LSG/DJB, 90 % (18/20) for LRYGB, and 53 % (16/30) for LAGB.
Complications
Complications occurred in 137 cases for an overall complication rate of 16 %. The details of these complications are shown in Tables 5 and 6. Early complications (within 30 days of surgery) occurred after 77 cases (9 %), of which 35 (24 patients) required reoperation. These included bleeding in 19 (14 intra-abdominal, 3 at the anastomotic site, and 2 at the trocar site), leakage in 11, organ/space surgical site infection in three, small bowel injury in one, and sleeve stenosis in one. Early sleeve stenosis occurred in one patient. In that patient, laparoscopic strictureplasty was performed on postoperative day 19. This was not effective, and laparoscopic revision LRYGB was required on postoperative day 21. Postoperative mortality occurred in one patient, who died of multiple organ failure due to postoperative bleeding after LAGB.
Late complications (more than 30 days after surgery) occurred in 60 patients (7 %), of whom 18 required reoperation or interventions. These included internal hernia in three (two in the mesenteric space and one in Petersen’s space), intractable gastroesophageal reflux disease (GERD) after LSG in two and LSG/DJB in two, sleeve stenosis or stricture in four, bowel perforation due to marginal ulcer in two, band slippage in two, bowel obstruction in two, and esophageal dilation after LAGB in one. Intractable GERD required revision surgery which was refractory to proton pump inhibitor administration that occurred in four patients (as described below). Three out of the four patients of sleeve stenosis or stricture were treated revision surgery (as described below), and the remaining one patient was treated with endoscopic placement of a self-expandable and retrievable stent.
Revision Surgery
Revision surgery was performed in 22 patients (3 %), including insufficient weight loss in 13 patients, intractable GERD in four patients, sleeve stenosis or stricture in four patients, and the need for esophageal dilation after LAGB in one. The primary operation associated with insufficient weight loss was LSG in 10 patients, and LSG/DJB and LRYGB and LAGB were one patient each. The revision procedures included laparoscopic biliopancreatic diversion (BPD/DS) with repeat sleeve gastrectomy in three patients, LRYGB in four patients, BPD/DS in two patients, laparoscopic repeat sleeve gastrectomy in two patients, LSG in one patient, and a band on pouch in one patient. The procedures for intractable GERD included laparoscopic LRYGB in two patients and laparoscopic seromyotomy in two patients. One of the two patients who underwent seromyotomy did not improve, and LRYGB was then performed.
Discussion
This is a retrospective review of the long-term outcomes and effects of bariatric and metabolic surgery based on a nationwide survey in Japan. This study was conducted by the Japanese Society for the Surgery of Obesity and Metabolic Disorders and reviewed 831 laparoscopic bariatric and metabolic operations performed in nine institutions. Obesity and its associated metabolic disorders are strongly linked to both morbidity and mortality [17]. Bariatric surgery is the most effective treatment for morbid obesity. These operations not only result in weight loss surgery, but also serve as metabolic procedures which result in excellent long-term sustained weight loss and a reduction of comorbidities [18].
Bariatric surgery has not been popular in Japan until recently. Obesity is steadily increasing in Japan due to factors such as a lack of exercise, adoption of a more Western diet, and changing lifestyles. Although the incidence of morbid obesity is small in Japan (0.25–0.3 %), the number of morbidly obese people is considerable (over 300,000 people) and cannot be ignored. Obesity-related comorbidities have become widely recognized. Reimbursement for LSG by government health insurance began in April 2014, and the number of bariatric procedures performed has increased dramatically. The LSG has been established as a safe and effective procedure, and many studies have also confirmed it to be effective for long-term weight loss and metabolic disorders [18–20]. LSG is the most commonly performed operation, in 501 patients (60 %), in this series.
However, there are systematic reviews and meta-analyses showing that LRYGB has a significantly higher percentage of excess weight loss and better resolution of obesity-related comorbidities compared with LSG [18, 20, 21]. Seki et al. reported long-term outcomes of LSG in morbidly obese Japanese patients [12] and showed that LSG is safe, effective, and acceptably durable up to 5 years, but the percentage excess weight loss for super-obese patients (BMI > 50 kg/m2) was significantly lower than that in the reference obesity group (BMI < 50 kg/m2). The authors conclude that other surgical options such as LRYGB and LSG/DJB may be required for super-obese patients. LRYGB is considered to be the gold standard for bariatric surgery because of its efficacy and the duration of effects, but there is an important problem regarding access to the excluded stomach for endoscopic screening after surgery, since gastric cancer is a common disease in Japan, and is routinely screened for in mass population screening programs [22]. Kasama et al. introduced the LSG/DJB as a modification of the duodenal switch (“short-limb” DS) in 2009 [23]. They reported that LSG/DJB, as a previously standardized procedure with malabsorptive effects, is safe and feasible, particularly for patients with a risk of gastric cancer, and provides similar outcomes compared with LRYGB in short-term follow-up. Seki et al. reported the short-term outcomes of LSG/DJB for type 2 diabetes mellitus [13], with a complete remission rate of 69 % and a remission rate including complete and partial remissions of 82 % at 1 year after LSG/DJB. LSG/DJB for obese individuals with type 2 diabetes mellitus compensates for the shortcomings of LRYGB and has strong anti-diabetic effects which are at least comparable to LRYGB. If the surgical team has sufficient experience to perform intestinal bypass procedures safely for super-obese patients or those with severe metabolic disorders, we recommend either LRYGB or LSG/DJB. We evaluated outcomes regardless of the degree of obesity in this survey, but in our results as well, LSG/DJB and LRYGB achieved a better weight loss outcome compared with LSG and LAGB.
Optimal outcomes for the remission of type 2 diabetes mellitus after metabolic surgery will occur if patients who are best suited to the surgery are selected and those who will predictably have a poor result are notified that although they are categorized as being less likely to have a remission, they may still have multiple benefits from undergoing surgery. Therefore, we need preoperative information on the association between possible predictors and long-term outcomes in order to advise our patients. Various predictive factors for type 2 diabetes mellitus were discussed. The DiaRem score as a preoperative predictor of type 2 diabetes mellitus remission following LRYGB was recently proposed by Still et al. based on age, use of insulin, HbA1c, and type of anti-diabetes medications [24]. However, controversies remain regarding the results of these studies. Lee WJ et al. previously identified age, BMI, C-peptide, and duration of diabetes to be four independent predictors for short-term type 2 diabetes mellitus remission after metabolic surgery [25]. Dixon JB et al. devised the ABCD score, combining four important domains, patient age, BMI, C-peptide level, and duration of diabetes that predict the remission of type 2 diabetes mellitus and validated its use by showing that it is a good predictor of success after metabolic surgery for the treatment of type 2 diabetes mellitus [15]. The ABCD score used in this study was slightly modified from the original after further analysis as shown in Table 2 [16]. A 4-point score, ranging from 0 (lowest value) to 3 (maximal value), was given to BMI, C-peptide, and duration of diabetes according to analysis. For age, only a 1-point score was given. The scores for each variable were added, so that the total score ranged from 0 to 10 points. Lee MH et al. reported long-term diabetes remission after metabolic surgery [26] and reported that patients with prolonged complete type 2 diabetes mellitus remission after surgery had a higher ABCD score than those without (7.8 ± 1.7 vs 5.6 ± 2.4, P < 0.05). We evaluated the preoperative ABCD score in patients with type 2 diabetes mellitus. In this study, patients with complete type 2 diabetes mellitus remission after metabolic surgery at 3 years had a higher ABCD score than those without (6.4 ± 1.6 vs 4.2 ± 2.0, P < 0.05). The type 2 diabetes mellitus remission rates after bariatric surgery, by procedure, were in order of LRYGB, LSG, LSG/DJB, and LAGB. Prior to data analysis, some investigators involved in this study expected that the type 2 diabetes mellitus remission rate for LSG/DJB would be better than for other operations. Possible reasons for the difference in the results compared to expectations are that LSG/DJB was selectively used in low BMI patients (27.5 ≦ BMI < 35 kg/m2) associated with more severe type 2 diabetes mellitus. In patients with five points or less ABCD score, the type 2 diabetes mellitus remission rate in patients undergoing LSG/DJB was the highest of the procedures performed (68 %) except LRYGB for which there was only one patient.
The hypertension remission rates by procedure were in the order of LRYGB, LSG, LSG-DJB, and LAGB. Dyslipidemia remission rates were in order of LRYGB, LSG-DJB, LSG, and LAGB. The metabolic improvement effect of LRYGB is the highest compared to the other procedures. The prevalence of gastric cancer is comparatively high, and the anatomical difficulty for cancer screening in the remnant stomach by upper GI endoscopy has been considered as a non-negligible problem in Japan. However, the infection rate with Helicobacter pylori has decreased recently in Japan. LRYGB will be able to become a reasonable choice as in other nations.
Conclusion
Bariatric and metabolic surgery for morbidly obese patients in Japan is safe, effective, and durable. The results of this Japan nationwide survey appear to be comparable to similar surveys in European countries and the USA. Patients with a preoperative ABCD score six points or more are more likely to have complete remission of type 2 diabetes mellitus following bariatric and metabolic surgery.
References
Ng M, Fleming T, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–81.
Dixon JB, Zimmet P, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Arq Bras Endocrinol Metabol. 2011;55:367–82.
Yoshiike N, Matsumura Y, Zaman M, et al. Descriptive epidemiology of body mass index in Japanese adults in a representative sample from the national nutrition survey 1990–1994. Int J Obes Relat Metab Disord. 1998;22:684–7.
Ohshiro Y, Ueda K, Nishi M, et al. A polymorphic marker in the leptin gene associated with Japanese morbid obesity. J Mol Med. 2000;78:516–20.
Scopinaro N. The IFSO and obesity surgery throughout the world. International Federation for the Surgery of Obesity. Obes Surg. 1998;8:3–8.
Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14:1157–64.
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19:1605–11.
Angrisani L, Santonicola A, et al. Bariatric Surgery Worldwide 2013. Obes Surg; 2015
Ohta M, Kitano S, Kasama K, et al. Results of a national survey on laparoscopic bariatric surgery in Japan, 2000-2009. Asian J Endosc Surg. 2011;4(3):138–42.
Sasaki A, Wakabayashi G, Yonei Y. Current status of bariatric surgery in Japan and effectiveness in obesity and diabetes. J Gastroenterol. 2014;49(1):57–63.
Yamamoto H, Kaida S, Yamaguchi T, et al. Potential mechanisms mediating improved glycemic control after bariatric/metabolic surgery. Surg Today. 2016;46(3):268–74.
Seki Y, Kasama K, Hashimoto K. Long-term outcome of laparoscopic sleeve gastrectomy in morbidly obese Japanese patients. Obes Surg. 2016;26:138–45.
Seki Y, Kasama K, Umezawa A, et al. Laparoscopic sleeve gastrectomy with duodenojejunal bypass for type 2 diabetes mellitus. Obes Surg. 2016.
Brethauer SA, Kim J, el Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11(3):489–506.
Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36(1):20–6.
Lee WJ, Almulaifi A, Tsou JJ, et al. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score. Surg Obes Relat Dis. 2015;11(5):991–6.
Sjöström L, Narbro K, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52.
Buchwald H, Avidor Y, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Golomb I, Ben David M, Glass A. Long-term metabolic effects of laparoscopic sleeve gastrectomy. JAMA Surg. 2015;150(11):1051–7.
Yip S, Plank LD, Murphy R. Gastric bypass and sleeve gastrectomy for type 2 diabetes: a systematic review and meta-analysis of outcomes. Obes Surg. 2013;23(12):1994–2003.
Li J, Lai D, Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg. 2016;26(2):429–42.
Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9.
Kasama K, Tagaya N, Kanehira E, et al. Laparoscopic sleeve gastrectomy with duodenojejunal bypass: technique and preliminary results. Obes Surg. 2009;19(10):1341–5.
Still CD, Wood GC, Benotti P, et al. A probability score for preoperative prediction of type 2 diabetes remission following RYGB surgery. Lancet Diabetes Endocrinol. 2014;2:38–45.
Lee WJ, Hur KY, Lakadawala M, et al. Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis. 2013;9(3):379–84.
Lee MH, Lee WJ, Chong K, et al. Predictors of long-term diabetes remission after metabolic surgery. J Gastrointest Surg. 2015;19(6):1015–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no competing interests.
Ethical Approval
All procedures performed in our study involving human participants were in accordance with the ethical standards of the institutional and/or Japanese national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in this study.
Additional information
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
Haruta, H., Kasama, K., Ohta, M. et al. Long-Term Outcomes of Bariatric and Metabolic Surgery in Japan: Results of a Multi-Institutional Survey. OBES SURG 27, 754–762 (2017). https://doi.org/10.1007/s11695-016-2361-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2361-3