Abstract
Background
Bariatric surgery can improve glucose metabolism in obese patients with diabetes, but the factors that can predict diabetes remission are still under discussion. The present study aims to examine the impact of preoperative beta cell function on diabetes remission following surgery.
Materials and Methods
We investigated a cohort of 363 obese diabetic patients who underwent bariatric surgery. The impact of several preoperative beta cell function indexes on diabetes remission was explored through bivariate logistic regression models.
Results
Postoperative diabetes remission was achieved in 39.9 % of patients. Younger patients (p < 0.001) and those with lower HbA1c (p = 0.001) at the baseline evaluation had higher odds of diabetes remission. Use of oral anti-diabetics and insulin therapy did not reach statistical significance when they were adjusted for age and HbA1c. Among the evaluated indexes of beta cell function, higher values of insulinogenix index, Stumvoll first- and second-phase indexes, fasting C-peptide, C-peptide area under the curve (AUC), C-peptide/glucose AUC, ISR (insulin secretion rate) AUC, and ISR/glucose AUC predicted diabetes remission even after adjustment for age and HbA1c. Among them, C-peptide AUC had the higher discriminative power (AUC 0.76; p < 0.001).
Conclusions
Patients’ age and preoperative HbA1c can forecast diabetes remission following surgery. Unlike other studies, our group found that the use of oral anti-diabetics and insulin therapy were not independent predictors of postoperative diabetes status. Preoperative beta cell function, mainly C-peptide AUC, is useful in predicting diabetes remission, and it should be assessed in all obese diabetic patients before bariatric or metabolic surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus is a metabolic disease characterized by a progressive decline in pancreatic beta cell function and increased peripheral insulin resistance, leading to poor glycaemic control and multiple micro and macrovascular complications [1].
Obesity is closely related to the development of type 2 diabetes mellitus, and its incidence is growing worldwide [2, 3]. Bariatric surgery has proven itself as an effective strategy to induce diabetes remission in obese patients [4, 5], and it has achieved better results than pharmacologic therapy [6]. According to the American Diabetes Association (ADA), surgery should be considered for adults with body mass index (BMI) > 35 kg/m2 and diabetes, especially if diabetes is difficult to control with lifestyle measures and pharmacotherapy [7]. Bariatric or metabolic surgery in individuals with lower BMIs has been increasingly discussed, and it was already conducted with successful results [8–10].
In order to better select diabetic obese patients to bariatric surgery, numerous studies have tried to identify preoperative characteristics that can foresee an improved glycaemic control after the surgical procedure. Patient age, use of insulin therapy, diabetes duration, and HbA1C levels have been recognized as predictors of diabetes remission [11–13]. Additionally, C-peptide [14, 15] and the C-peptide area under the curve (AUC) during an oral glucose tolerance test (OGTT) [16] have been also identified as useful predictors. These last findings suggest that beta cell function at the time of the surgery is an important determinant of postoperative diabetes status. However, the predictive accuracy of these two measures has never been compared neither other beta cell function indexes have been investigated.
The present study aims to investigate the preoperative factors associated with diabetes remission in obese patients, focusing mainly on validated surrogate markers of beta cell function. In addition, we intend to compare these markers to determine which of them performs the best.
Materials and Methods
Study Design and Participants
A retrospective cross-sectional study was conducted in a population of obese patients evaluated in the multidisciplinary group for surgical treatment of obesity in Centro Hospitalar São João, Porto, Portugal. A total of 1184 patients underwent bariatric surgery between January 2010 and July 2014. Among them, we have identified 363 type 2 diabetic patients that were enrolled in this study — 95 (26.2 %) performed laparoscopic adjustable gastric band (LAGB), 203 (55.9 %) Roux-en-Y gastric bypass (RYGB), and 65 (17.9 %) sleeve gastrectomy (SG). Clinical, anthropometric (height, weight, BMI, abdominal, and waist circumferences), and analytic measures (HbA1C, fasting plasmatic glucose, insulin and C-peptide, lipid profile, uric acid, and serum cortisol) were obtained before surgery and at the follow-up visits.
Type 2 Diabetes Definition and Remission Criteria
To define type 2 diabetes mellitus, we used the 2015 ADA guidelines criteria: fasting plasma glucose ≥7.0 mmol/l or HbA1C ≥ 6.5 % (48 mmol/mol), or 2-h post-load plasmatic glucose ≥11.1 mmol/l during an OGTT, or use of hypoglycaemic agents [7]. Complete diabetes remission was defined according to Buse et al. [17] criteria: (1) normal glycaemic measures (HbA1C < 5.7 % [39 mmol/mol] and fasting plasma glucose <5.6 mmol/l), (2) at least 1 year’s duration, and (3) no active pharmacologic therapy or ongoing procedures.
Insulin Resistance and Beta Cell Function Indexes
To evaluate insulin resistance/sensitivity, fasting and OGTT-derived indexes were used: homeostasis model assessment of insulin resistance (HOMA-IR) [18] and Matsuda index [19], respectively. In order to study beta cell function, we have used several indexes described in the literature. Homeostatic model assessment of beta-cell function (HOMA-%B) was considered as referred in Matthews et al. [18]. Insulinogenic index (IGI) was obtained by the equation ΔInsulin(0-30min)/Δglucose(0-30min) [20]. First and second phase insulin secretion indexes described by Stumvoll et al. [21, 22] were also assessed. C-peptide AUC, insulin AUC, C-peptide/glucose AUC, and insulin/glucose AUC ratios [23] were calculated by the trapezoidal rule during the OGTT0-120min. The Insulin SECretion (ISEC) deconvolution software program was used to determine the insulin secretion rate (ISR) using age, sex, weight, height, and C-peptide concentrations [24, 25]. To calculate indexes that included insulin concentrations, patients on insulin therapy (40 individuals) were excluded due to the confounding effect of exogenous administration. Patients with severe renal failure (seven individuals) were excluded too.
Statistical Analyses
Categorical variables were expressed as frequencies and percentages and were compared by chi-square test. Continuous variables were presented as means and standard deviations and its comparison was performed using Student’s t test. Normal distribution was evaluated using Shapiro-Wilk test or skewness and kurtosis. Pearson’s correlation coefficient was used to assess association between continuous variables. Differences between three or more groups were evaluated by ANOVA test, followed by the Bonferroni test when findings with the ANOVA model were significant. Independent predictors of diabetes remission were identified by binary logistic regression and then included in several predictive models. Models that presented a p > 0.05 on the Hosmer and Lemeshow test were considered a good fit, and then their predictive performance was measured with the area under the curve of the receiver operating characteristic (ROC) curve. Reported p values are two-tailed, and p < 0.05 was considered significant. Analyses were performed with the use of SPSS Statistics 23®.
Results
Baseline Characteristics
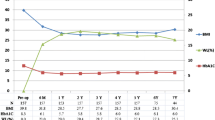
Within the 363 patients with type 2 diabetes criteria that underwent bariatric surgery, 295 (81.3 %) were females. The mean age of the enrolled patients was 51.2 ± 9.64 years. Average preoperative weight and BMI were 116.1 ± 19.0 kg and 44.8 ± 6.19 kg/m2, respectively. Regarding diabetes, the mean preoperative HbA1C was 6.63 ± 1.15 % (49.0 ± 12.6 mmol/mol). A total of 282 (78.6 %) patients were on oral hypoglycaemic agents (26.4 % of them needed more than one oral anti-diabetic drug) and 40 (11.1 %) were on insulin therapy. Fasting plasma insulin (r = 0.21; p = 0.001) and C-peptide levels (r = 0.39; p < 0.001) increased continually with an escalating BMI (Fig. 1).
Postoperative Follow-up
Bariatric surgery provided a statistically significant (p < 0.001) decrease in the weight and BMI (mean reductions of 31.0 ± 14.5 kg and 12.1 ± 5.59 kg/m2, respectively). RYGB led to a greater weight loss percentage both to SG (32.1 ± 7.54 vs. 26.8 ± 8.71 %; p < 0.001) and LAGB (32.1 ± 7.54 vs. 14.8 ± 7.86 %; p < 0.001). Surgery was associated with a mean reduction of 10.8 ± 21.7 and 7.5 ± 13.8 mmHg on systolic and diastolic blood pressure (p < 0.001), respectively, and to a decrease in the use of anti-hypertensive agents (p < 0.001). An improved lipid profile was observed: reduction of 0.53 ± 1.16 mmol/l on total cholesterol (p < 0.001), 0.49 ± 0.97 mmol/l on LDL cholesterol (p < 0.001), 0.63 ± 0.84 mmol/l on triglycerides (p < 0.001), and an increase of 0.17 ± 0.27 mmol/l on HDL cholesterol (p < 0.001). Regarding glycaemic control, HbA1C had a 1.10 ± 1.20 % (12.0 ± 13.1 mmol/mol) reduction after surgery (p < 0.001); and the number of patients taking oral anti-diabetics (p < 0.001) and insulin therapy (p = 0.004) decreased as well.
Diabetes Remission
The comparison of preoperative features between patients that achieved remission and those who did not is shown in Table 1. Postoperative complete diabetes remission was attained in 39.9 % of patients. These patients were younger (mean difference of 6.48 ± 1.12 years; p < 0.001), had lower systolic blood pressure (mean difference of 4.50 ± 2.19 mmHg; p = 0.039), had lower HbA1C (mean difference of 0.90 ± 0.16 % [9.80 ± 1.70 mmol/mol]; p < 0.001), and lower fasting plasma glucose (mean difference of 1.35 ± 0.30 mmol/l; p < 0.001). Furthermore, patients on remission took fewer oral anti-diabetics (p = 0.001) and used less insulin therapy (p < 0.001) preoperatively. Baseline BMI did not differ between both groups (p = 0.401). Calculated indexes of insulin resistance (HOMA-IR) and insulin sensitivity (Matsuda index) were not different as well (p = 0.961 and p = 0.502, respectively). On the other hand, most beta cell function indexes were significantly higher in patients that achieved remission: HOMA-%B (p = 0.003), IGI (p = 0.005), Stumvoll first- and second-phase (p = 0.004 for both indexes), fasting C-peptide (p = 0.036), C-peptide AUC (p = 0.001), C-peptide/glucose AUC (p = 0.001), ISR AUC (p = 0.006), and ISR/glucose AUC (p = 0.003). Patients on remission have lost more 6.28 ± 1.25 kg over the first postoperative year than non-remitting patients (p < 0.001). After adjustment for age, basal HbA1c and percentage of weight loss, RYGB conducted to a higher probability of diabetes remission when compared to LAGB (OR = 3.03; confidence interval - CI 1.13–8.11; p = 0.028). No differences were identified between LAGB and SG (OR = 1.19; CI 0.35–4.01; p = 0.783).
Binary Logistic Regression Models
Baseline features that achieved statistical significance between remission and non-remission groups were included in a binary logistic regression model as potential candidates to remission predictors. Fasting plasma glucose was excluded due to its collinearity with HbA1C. Data is displayed in Table 2. Younger age (OR 0.93; p < 0.001) and lower HbA1C (p = 0.001) at baseline were significant predictors, while systolic blood pressure (p = 0.794), use of oral anti-diabetics (p = 0.092), and insulin therapy (p = 0.811) did not reach statistical significance when they were adjusted for the other included variables.
In what concerns beta cell function indexes, higher values of IGI (p = 0.02), Stumvoll first- and second-phase indexes (p = 0.021), fasting C-peptide (p = 0.037), C-peptide AUC (p = 0.002), C-peptide/glucose AUC (p = 0.006), ISR AUC (p = 0.009), and ISR/glucose AUC (p = 0.027) were identified as independent predictors of diabetes remission even after correcting for age and HbA1C, while HOMA-%B was not (p = 0.416) (data not shown in the table). Those indexes identified as predictors were included in eight different models to evaluate which of them performed the best (Table 3). Models II and III did not represent a good fit to predict our study outcome (p < 0.05 in the Hosmer and Lemeshow test). The remaining models were then compared to assess their predictive value. Model V, that included C-peptide AUC, was the most useful one to predict diabetes remission (AUC 0.76; p < 0.001), followed by Model VII (AUC 0.75; p < 0.001), which included ISR AUC. Models I, IV, VI, and VIII provided an acceptable discrimination power as well (AUCs ≥0.70; p < 0.001).
Conclusions
We have shown that patients with a better pancreatic beta cell function had an increased chance of diabetes remission after bariatric surgery. Of the 12 evaluated surrogate markers of pancreatic function, models that included IGI, fasting plasma C-peptide, plasma C-peptide AUC, C-peptide/glucose AUC, ISR AUC, and ISR/glucose AUC successfully predicted postoperative glycaemic control. Among them, C-peptide AUC had the higher discriminating power. Additionally, age and baseline HbA1C were independent predictors too. Surgical procedures led to improved blood pressure, lipid, and glycaemic profiles. We have also seen a significant reduction in the use of anti-hypertensive medication, anti-dyslipidaemics, oral anti-diabetic drugs, and insulin therapy.
Our group observed a significant correlation between BMI and baseline insulin, and C-peptide levels. Both have decreased after bariatric surgery. This finding supports that elevated insulin and C-peptide levels reflect obesity and type 2 diabetes mellitus underlying insulin resistance and that bariatric surgery increases insulin sensitivity. Sjoström et al. [26] found that baseline insulin levels were predictive of cardiovascular disease reduction after bariatric surgery, highlighting the importance of evaluating beta cell function before the surgical procedure.
We found a 1-year diabetes remission rate of approximately 40 %, proving that bariatric surgery is very effective in glucose metabolism control in obese patients [27]. Our results are in agreement with other studies that reported remission rates between 21 and 63 % in the first postoperative year [11, 13]. Several authors have proposed various contributors to improved diabetes status after bariatric surgery: decreased caloric intake and weight loss, changes in gut physiology, improved pancreatic function and insulin sensitivity, altered bile acid metabolism, and changes in gut microbiota [28, 29].
Bariatric surgery has been increasingly seen as a metabolic surgery and not only as a mean to reach weight reduction. This procedure has been associated with a better control of hypertension and diabetes and reduced cardiovascular events and cardiovascular related deaths [26, 30]. In what concerns diabetes, bariatric surgery has been performed with good results even in class I (BMI between 30 and 35 kg/m2) obese diabetic patients [8]. In order to select patients best suited to bariatric surgery, identifying preoperative predictors of diabetes remission can lead to improved outcomes. Still et al. [12] proposed a score to predict the probability of diabetes remission following RYGB (DiaRem Score) that included age, preoperative HbA1C, use of insulin, and the type of oral anti-diabetic medication. Beta cell function was not included in this score nor evaluated in that study. Recently, studies have brought to light the importance of beta cell function, showing that higher plasma C-peptide levels and plasma C-peptide AUC correlated with an increased probability of diabetes remission [15, 16].
Progressive pancreatic beta cell failure is a part of the natural course of diabetes and starts long before its diagnosis. Therefore, it can be expected that patients with less preoperative dysfunction of the beta cell have higher odds of diabetes remission after surgery. Our study has proved by showing that patients with higher indexes of beta cell function had a higher chance of remission. All C-peptide derived indexes (fasting C-peptide, C-peptide AUC, C-peptide/glucose AUC, ISR AUC, and ISR/glucose AUC) were independent predictors of diabetes remission, even after adjustment for age and HbA1C. On the other hand, all insulin derived indexes, except for IGI, were of no value in predicting postoperative glucose control. We hypothesize this finding could be explained by the fact that C-peptide provides a more reliable estimation of beta cell function than insulin. This occurs because a variable percentage, up to 80 % in some studies, of the insulin that is secreted by pancreas is removed during the first pass through the liver, while there is a negligible C-peptide clearance by this organ [31].
Several authors identified the use of oral anti-diabetics and insulin therapy as having a negative effect on the attainment of diabetes remission [12, 13, 32]. Our results do not support this conclusion. None of those studies evaluated beta cell function indexes. As so, these two variables may only reflect an advanced stage of diabetes and a poor pancreatic reserve and they may not be true diabetes remission predictors per se. Our findings may also explain why a longer disease duration and the presence of diabetes complications have been found to reduce the odds of remission [11, 13]. Reinforcing our hypothesis, Ramos-Levi [33] found that diabetes duration was a predictor of diabetes remission only as long as C-peptide levels were not considered in a multivariate analysis model.
Our study had a larger population than other series on this matter and included patients that underwent either restrictive or malabsorptive procedures. The use of logistic regression allowed us to recognize beta cell function as an independent predictor of better glycaemic control without the interference of age and baseline HbA1C. As potential limitations, diabetes duration should have been recorded and assessed as a potential predictor. Longer follow-up would have been useful to look up for possible recurrence predictors. DiGiorgi et al. [34] showed a 24 % re-emergence rate of diabetes 3 years after RYGB. Furthermore, our study has an odd distribution of sexes with a higher prevalence of females, and the pre-operative relatively good glycaemic control of our population limits the generalization of our findings to patients with more decompensated diabetes.
Future research should address the implementation and dissemination of the assessment of beta cell function indexes in routine practice, when considering bariatric surgery on long-term management of diabetes. Studies evaluating the usefulness of remission predictors on class I (BMI between 30 and 35 kg/m2) obese patients undergoing bariatric surgery should also be considered.
In conclusion, preoperative beta cell function is an independent predictor of diabetes remission after bariatric surgery and should be evaluated prior to surgical procedure in all diabetic patients. Of the 12 assessed indexes, C-peptide AUC was the most useful in predicting diabetes remission and should be included in future prediction scores. Moreover, the importance of beta cell function on disease remission reinforces doctors not to postpone bariatric surgery too long since waiting until beta cell failure has ensued will worsen the surgery outcome.
References
Ferrannini E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: problems and prospects. Endocr Rev. 1998;19(4):477–90.
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–81.
Eckel RH, Kahn SE, Ferrannini E, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? Diabetes Care. 2011;34(6):1424–30.
Dixon JB, le Roux CW, Rubino F, et al. Bariatric surgery for type 2 diabetes. Lancet. 2012;379(9833):2300–11.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1955;222(3):339–50 .discussion 50-2
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
American Diabetes Association Guidelines. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(supplement 1):S1–93.
Cohen RV, Pinheiro JC, Schiavon CA, et al. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35(7):1420–8.
American Society of Metabolic and Bariatric Surgery statements/guidelines. Bariatric surgery in class I obesity (body mass index 30-35 kg/m(2)). Surg Obes Relat Dis. 2013;9(1):e1–10.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med. 2011;28(6):628–42.
Iacobellis G, Xu C, Campo RE, et al. Predictors of short-term diabetes remission after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2015;25(5):782–7.
Still CD, Wood GC, Benotti P, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol. 2014;2(1):38–45.
Robert M, Ferrand-Gaillard C, Disse E, et al. Predictive factors of type 2 diabetes remission 1 year after bariatric surgery: impact of surgical techniques. Obes Surg. 2013;23(6):770–5.
Aarts EO, Janssen J, Janssen IM, Berends FJ, Telting D, de Boer H. Preoperative fasting plasma C-peptide level may help to predict diabetes outcome after gastric bypass surgery. Obes Surg. 2013;23(7):867–73.
Lee WJ, Chong K, Ser KH, et al. C-peptide predicts the remission of type 2 diabetes after bariatric surgery. Obes Surg. 2012;22(2):293–8.
Mallipedhi A, Min T, Prior SL, et al. Association between the preoperative fasting and postprandial C-peptide AUC with resolution of type 2 diabetes 6 months following bariatric surgery. Metab Clin Exp. 2015;64(11):1556–63.
Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133–5.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70.
Park JY, Kim YJ. Prediction of diabetes remission in morbidly obese patients after Roux-en-Y gastric bypass. Obes Surg. 2016;26(4):749–56.
Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23(3):295–301.
Stumvoll M, Van Haeften T, Fritsche A, et al. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care. 2001;24(4):796–7.
Ahren B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol / Eur Fed Endocr Soc. 2004;150(2):97–104.
Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Prog Biomed. 1996;50(3):253–64.
Eaton RP, Allen RC, Schade DS, et al. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab. 1980;51(3):520–8.
Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56.e5.
Cho YM. A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes Metabol J. 2014;38(6):406–15.
Argyropoulos G. Bariatric surgery: prevalence, predictors, and mechanisms of diabetes remission. Curr Diab Rep. 2015;15(4):15.
Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93.
Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54(6):1649–56.
Jimenez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256(6):1023–9.
Ramos-Levi AM, Matia P, Cabrerizo L, et al. Statistical models to predict type 2 diabetes remission after bariatric surgery. J Diabetes. 2014;6(5):472–7.
DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6(3):249–53.
Acknowledgments
We would like to thank Sofia Souteiro, Industrial Engineering and Management student, and Luis Silva, Software Engineer, for their help with the ISEC program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Grant Support
No grant support to declare.
Funding
No funding to declare.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Souteiro, P., Belo, S., Neves, J.S. et al. Preoperative Beta Cell Function Is Predictive of Diabetes Remission After Bariatric Surgery. OBES SURG 27, 288–294 (2017). https://doi.org/10.1007/s11695-016-2300-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2300-3