Abstract
Background
The intragastric balloon (IGB) is an adjunctive treatment for obesity. This meta-analysis aimed to evaluate the efficacy and safety of IGB treatment by reviewing randomized controlled trials (RCTs).
Methods
A total of 20 RCTs involving 1195 patients were identified. Weight loss results before and after 3 months were analyzed separately. The weight loss results of patients with and without IGB treatment were compared.
Results
Our meta-analysis calculated the following significant effect sizes: 1.59 and 1.34 kg/m2 for overall and 3-month BMI loss, respectively; 14.25 and 11.16 % for overall and >3-month percentage of excess weight loss, respectively; 4.6 and 4.77 kg for overall and 3-month weight loss, respectively; and 2.81, 1.62, and 4.09 % for overall, 3-month, and >3-month percent of weight loss, respectively. A significant effect size was calculated that favored fluid-filled IGBs over air-filled IGBs. Flatulence (8.75 vs. 3.89 %, p = 0.0006), abdominal fullness (6.32 vs. 0.55 %, p = 0.001), abdominal pain (13.86 vs. 7.2 %, p = 0.0001), abdominal discomfort (4.37 vs. 0.55 %, p = 0.006), and gastric ulcer (12.5 vs. 1.2 %, p < 0.0001) were significantly more prevalent among IGB patients than among non-IGB control patients. No mortality was reported from IGB treatment.
Conclusion
IGB treatment, in addition to lifestyle modification, is an effective short-term modality for weight loss. However, there is not sufficient evidence confirming its safety or long-term efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increased prevalence of obesity in the USA is a major public health concern [1]. A combination of calorie-restricted diet, regular physical activity, and behavioral modification with or without pharmacotherapy has been practiced to treat obesity; however, a significant weight loss of 10 to 15 % is rarely achieved or sustained [2].
For morbid obesity, bariatric surgery is the only treatment option with sustainable weight loss and long-term resolution of comorbidities [3]. Preoperative comorbidities are relatively common in severely obese patients and increase the risk of complications at the time of bariatric surgery [4]. However, some patients with moderately increased body mass index (BMI) do not qualify for bariatric surgery [5]. In these patients, an intragastric balloon (IGB) could prepare the patient for bariatric surgery or help them adhere to the new lifestyle modification.
The first generations of IGBs were small (200–220 mm3), air-filled balloons susceptible to spontaneous deflation or erosion by gastric acid. They were associated with severe complications including gastric mucosal erosion and ulceration, small bowel obstruction, and esophageal tears [6–14]. However, second-generation, fluid-filled IGBs have partly resolved these concerns [2, 15–24].
There are few systematic review articles pooling the data on the clinical efficacy and safety of IGBs for treatment of obesity [25–28]. These reviews included studies with non-randomized design [25, 26, 28]. Additionally, another recent meta-analysis suggesting the significant effectiveness of IGB in the treatment of obesity excluded a large number of randomized controlled trials with crossover design [27]. The aim of this systematic review was to determine the efficacy and safety of IGB treatment for weight loss in patients with BMI >27 kg/m2 through a meta-analysis of all qualified randomized controlled trials (RCTs) comparing the weight loss between IGB and conservative treatments. Additionally, the weight loss outcome was compared between fluid-filled and air-filled IGBs.
Methods
Search Strategy
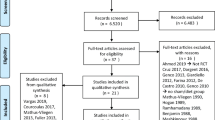
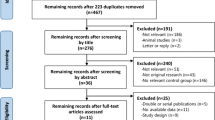
Adhering to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [29], a comprehensive literature search was performed using databases including PubMed, Web of Science, and Scopus for RCTs in English that compared the efficacy of IGB insertion for weight loss with that of conservative treatments, in patients with BMI >27 kg/m2. The search was conducted in October 2015 and included the terms gastric balloon, intragastric balloon, or intra-gastric balloon; gastric bubble, intragastric bubble, or intra-gastric bubble; and weight loss or fat loss. A total of 884 articles were identified and 503 records remained after the removal of duplicates (Fig. 1).
Eligibility
The titles and abstracts of the retrieved papers were screened for eligibility. RCTs reporting weight loss in patients with IGBs compared to that in a non-IGB control group were included. Case reports, review articles, and non-randomized clinical trials were excluded. The full texts of the remaining 25 articles were reviewed.
Quality Assessments
Two independent researchers, blinded to each other, evaluated the quality of each paper using the following parameters: randomization, method of blinding, allocation concealment, description of attrition, intention-to-treat analysis, and report of adverse events. Any conflict was resolved by a third reviewer.
Data Extraction
Data extraction was standardized for all studies and included study characteristics (author, publication year, sample size, and number of subjects in each group), demographic and initial anthropometric measurements [age, gender, and body mass index (BMI) or weight], type of intervention, weight loss outcome, and related complications. Only studies with obtainable mean ± standard deviation (SD) for the outcomes of interest were included in the quantitative analysis.
Definition
In the intervention group, only patients who underwent IGB insertion for the first time were considered for analysis. In the control group, patients who received sham procedures, lifestyle modification, or no treatment were included. If there were more than two parallel groups, the group that underwent sham procedure was considered as the control group for analysis. The primary outcome was the efficacy of IGB for weight loss. As the eligible RCTs reported weight loss results as BMI loss, percentage of excess weight loss (%EWL), weight loss (WL), or percent of weight loss (%WL), we separated the studies, wherever possible, by the units of weight loss. The secondary outcome was the safety of IGB determined by the prevalence of IGB-related adverse events compared to that in the control group (n, %).
Data Analysis
Data was synthesized with the Review Manager (RevMan) software for Windows (Version 5.3, The Nordic Cochrane Centre, Denmark, 2014). This resulted in the pooling of data [mean, standard deviation (SD), and number of patients per group] from the different studies. IGB (intervention) groups and non-IGB (control) groups were compared. The results were calculated as effect size and 95 % confidence intervals (95 % CI). Due to the inclusion of RCTs with crossover design, patients’ data in these studies were included only prior to crossover. Because the time point at which the crossover was performed was 3 to 4 months after initial IGB insertion, the subgroup analysis was performed according to the time point of 3-month or over 3-month treatment with IGB. The resulting weight losses (continuous data) were represented with fixed-effects and random-effects models. To analyze the safety of IGB insertion, we compared the reported number of IGB-related adverse events in the intervention and control groups. For comparison of adverse events (dichotomous data) between intervention and control groups, the Mantel-Haenszel statistical method was used. A p < 0.05 was considered statistically significant.
Heterogeneity and Publication Bias
We performed I 2 testing to assess the heterogeneity of the studies with I 2 ≥ 60 % considered indicative of substantial heterogeneity and deemed significant at p < 0.1. A test for publication bias was not performed because the number of studies within each subgroup was small.
Results
A total of 20 RCTs encompassing 1195 patients were included in this analysis. Fifteen studies randomized their patients (n = 835) to either IGB or sham procedure, 4 studies used behavioral modification for non-IGB patients (n = 310), and 1 study (n = 50) used pharmacotherapy (Sibutramin) in the control group. Lifestyle modification was implemented in 17 RCTs (n = 1119) in addition to IGB treatment. Earlier studies employed air-filled IGBs (7 studies, 266 patients), while more recent studies used IGBs filled with fluid (water or normal saline) (13 studies, 929 patients).
Table 1 summarizes patient characteristics. The studies were published from 1987 to 2015. The sample size ranged from 21 to 326 patients, the age of participants varied between 18 and 65 years, and the average BMI at the time of IGB insertion was between 27 and 50.4 kg/m2. Treatment with IGB lasted from 12 weeks to 6 months. All studies, except 2 (18, 23), showed female predominance.
Efficacy
Tables 2 and 3 summarize weight loss result and study findings by air-filled and fluid-filled IGBs, respectively. As studies measured the weight loss outcome using different scales, a discrete subgroup analysis was performed for each scale (Fig. 2) to calculate effect sizes between intervention and control groups.
Effect Size Based on BMI
There were 7 studies reporting BMI loss by mean ± SD (508 patients). A significant effect size of 1.59 kg/m2 [(95 % CI −0.84, 4.03), p < 0.0001] was calculated, indicating that the intervention was favored over the control. Subgroup analysis revealed an effect size of 2.4 kg/m2 [(95 % CI 1.21, 6.1), p = 0.19] in the 3-month subgroup (3 studies, 115 patients) and an effect size of 1.34 kg/m2 [(95 % CI 0.88, 1.8), p < 0.0001] in the >3-month subgroup (4 studies, 393 patients). Group analysis indicated significant heterogeneity within the 3-month subgroup (I 2 = 98 %, p < 0.0001) and non-significant heterogeneity within the >3-month subgroup (I 2 = 0 %, p = 0.49). The heterogeneity of all the studies was I 2 = 98 % (p < 0.0001).
Effect Size Based on %EWL
There were 4 studies reporting the %EWL by mean ± SD (513 patients). The calculated effect size was 14.25 % [(95 % CI 2.09, 26.41), p = 0.02]. Subgroup analysis revealed an effect size of 20.01 % [(95 % CI −3.5, 43.52), p = 0.1] for the 3-month subgroup (2 studies, 160 patients) and a significant effect size of 11.16 % [(95 % CI 1.49, 20.83), p = 0.02] for the >3-month subgroup (3 studies, 481 patients). There was significant heterogeneity overall (I 2 = 97 %) and within the 3-month subgroup (I 2 = 99 %), and intermediate heterogeneity (I 2 = 78 %) within the >3-month subgroup.
Effect Size Based on WL
There were 6 studies reporting WL by mean ± SD (486 patients). The calculated effect size of 4.6 kg [(95 % CI 1.6, 7.61), p = 0.003] indicated that the intervention was favored over the control. The subgroup analysis revealed an effect size of 4.77 kg [(95 % CI 0.51, 9.2), p = 0.03] in the 3-month subgroup (5 studies, 160 patients). Only 1 study was available for the >3-month subgroup (326 patients). There was significant overall heterogeneity (I 2 = 83 %) and significant heterogeneity within the 3-month subgroup (I 2 = 85 %, p < 0.0001).
Effect Size Based on %WL
There were 5 studies reporting %WL by mean ± SD (486 patients). The effect size calculated of 2.81 % [(95 % CI 0.62, 4.99), p = 0.01] between intervention and control groups indicated a significant intervention effect. Subgroup analysis revealed an effect size of 1.62 % [(95 % CI 1.07, 2.17) p < 0.0001] in the 3-month subgroup (2 studies, 83 patients) and 4.09 % [(95 % CI 2.13, 6.04), p < 0.0001] in the >3-month subgroup (3 studies, 403 patients). Subgroup analysis indicated no heterogeneity in the 3-month subgroup (I 2 = 0%, p = 0.89) and intermediate heterogeneity for the >3-month subgroup (I 2 = 77 %, p = 0.01). The overall heterogeneity was significant (I 2 = 94 %, p < 0.0001).
Effect Size Based on IGB Type
Subgroup analysis of weight loss based on IGB type (air-filled vs. fluid-filled) was performed. A standardized mean difference and an estimated SD for studies with unreported SD were used. When the treatment length was 3 months, the air-filled IGB subgroup (4 studies, 188 patients) had an effect size of 0.26 [(95 % CI −0.12, 0.64), p = 0.19] and the fluid-filled IGB subgroup (8 studies, 434 patients) had a significant effect size of 0.25 [(95 % CI 0.05, 0.45), p = 0.02]. When the IGB treatment duration was over 3 months, there were not enough air-filled IGB studies for analysis; however, an effect size of 0.82 [(95 % CI 0.65, 1), p < 0.0001] was observed for the fluid-filled IGB subgroup (7 studies, 640 patients). There was significant heterogeneity within fluid-filled IGB studies and between air-filled and fluid-filled IGB studies (I 2 > 90 %).
Safety
Studies reported 11 types of complications in both the intervention and control groups. Table 4 compares the incidence of complications between IGB and non-IGB patients. Flatulence (8.75 vs. 3.89 %, p = 0.0006), abdominal fullness (6.32 vs. 0.55 %, p = 0.001), abdominal pain (13.86 vs. 7.2 %, p = 0.0001), abdominal discomfort (4.37 vs. 0.55 %, p = 0.006), gastric ulcer (12.5 vs. 1.2 %, p < 0.0001), and nausea (24.79 vs. 11.43 %, p = 0.46) occurred more frequently in the intervention group than in the control group, respectively. Additionally, some studies reported complications including small bowel obstruction [10], grade D esophagitis [30], gallstone formation [31], gastroesophageal reflux [32], hypoxia at IGB removal [21], and cervical esophageal perforation and pneumonitis after IGB retrieval [33]. These were not significantly different between the two groups.
To compare the incidence of gastric ulcer between the two IGB types, a subgroup analysis was performed revealing a non-significant odds ratio (95 % CI) of 1.67 (0.54, 5.22) for air-filled IGB and a non-significant odds ratio (95 % CI) of 12.01 (0.09, 1522.71) for fluid-filled IGB.
Discussion
IGBs have noticeably evolved over recent years. Earlier generations of IGBs had a limited expanding volume (200–220 ml); current generations have an intragastric capacity of up to 960 ml. Past IGB generations were filled with air, had a low resistance to gastric acid, and the treatments lasted a maximum of 3–4 months [7]. This may explain spontaneous balloon deflation, passage of the balloon into the feces, and insufficient weight loss with air-filled IGBs [7, 12]. However, over the past decade, improved weight loss results have been achieved with BioEnterics intragastric balloons [4, 34]. A recent meta-analysis by the American Society for Gastrointestinal Endoscopy (ASGE) Bariatric Endoscopy Task Force indicated that the Orbera IGB meets the PIVI criteria (5 % TBWL) for the management of non-primary obesity [35].
Previous review articles have attempted to synthesize data regarding the efficacy and safety of IGBs and identify the most appropriate indication for IGB treatment [25, 27, 28]. One review analyzed a small number of studies [27], while the other reviews analyzed a large number of low-quality trials [25, 28]. The significant heterogeneity among included studies may have arisen from demographic variations, differences in the initial BMI of patients, co-administration of non-IGB weight-loss treatments, differences in IGB type, and presence or lack of a sham procedure in the control group [26–28]. Some of the RCTs terminated their follow-up with IGB removal [8, 9, 12, 16, 17, 19, 23, 24, 32, 36, 37] while other RCTs followed their patients after IGB removal [6, 7, 20–22, 30, 31, 38]. This would result in uncertainty regarding potential late complications of IGB.
Efficacy
Our meta-analysis calculated the following significant effect sizes: 1.59 and 1.34 kg/m2 for overall and 3-month BMI loss, respectively; 14.25 and 11.16 % for overall and > 3-month %EWL, respectively; 4.6 and 4.77 kg for overall and 3-month weight loss, respectively; and 2.81, 1.62, and 4.09 % for overall, 3-month, and >3-month %WL, respectively. There was a large effect size favoring fluid-filled IGBs over air-filled IGBs. The larger effect size for >3-month %WL was probably due to the larger heterogeneity within this study group. A meta-analysis by Imaz et al. estimated a net weight loss of 14.7 kg, initial weight loss of 12.2 %, an average of 5.7 kg/m2 BMI loss, and 32.1 % EWL at balloon removal (after 6 months) [28]. Another meta-analysis by Zheng et al. calculated an effect size of 8.9 kg for weight loss, 3.1 kg/m2 for BMI reduction, and 21.0 % for EWL after 6 months of IGB treatment, and an effect size of 1.5 kg for weight loss and 1.2 kg/m2 for BMI reduction with IGB treatment under 6 months [27]. These meta-analyses all favored IGB over non-IGB treatment despite differences in the degree of efficacy. These differences resulted from variations in the studies analyzed, time-point set for subgroup analysis, and method of data combination (fixed effect vs. random effects). Zheng et al. excluded RCTs with crossover design, employed a combination of fixed-effects and random-effects methods, and performed a subgroup analysis based on a 6-month time-point. Our analysis included all RCTs regardless of crossover design (by considering patient data only prior to the crossover), used a random-effects model, and set a different time-point for the duration of IGB treatment (3 months). As the patients in these RCTs were treated with different weight loss interventions after IGB removal, their results after IGB removal were not included in our meta-analysis.
Contemporary fluid-filled IGBs yield better weight loss results than their air-filled predecessors [15, 16]. Our subgroup analysis of RCTs by IGB type (air-filled or fluid-filled) confirmed this finding. Overall analysis of weight loss, regardless of the IGB type, may explain the dilution of IGB superiority over conservative treatment after 3 months. Of the seven RCTs investigating the efficacy of air-filled IGBs and lifestyle modification, six concluded that air-filled IGBs were not effective. To the best of our knowledge, no other systematic review has analyzed the effect of IGB type on weight loss.
Safety
Our analysis revealed that nausea, gastric ulcer, flatulence, abdominal fullness, pain, and discomfort were more common in the intervention groups than in the control groups. Furthermore, rare complications were reported at the time of IGB removal including small bowel obstruction [10], grade D esophagitis [30], gallstone formation [31], gastroesophageal reflux [32], hypoxia with IGB extraction [21], pneumonitis, and esophageal perforation. Reports of balloon deflation, which can contribute to weight loss failure and result in serious complications such as bowel obstruction or necrosis, were more common when air-filled IGBs were used [7, 12, 15, 16]. However, fluid-filled IGBs were associated with higher prevalence of early gastrointestinal symptoms including nausea, vomiting, epigastric pain, and bloating sensation. This could lead to balloon intolerance and early removal [15, 16, 30, 31].
Limitations
The main limitation of this meta-analysis was the large BMI range of included patients ranging from 27 to 50 kg/m2. While IGB is approved in patients with BMI >27 kg/m2, the only eligible RCT that recruited patients with such a low BMI was that of Lee et al. because other studies were non-RCT [23]. Additionally, as a significant heterogeneity existed between the included studies, the results of this meta-analysis should be interpreted with caution. Moreover, due to the crossover design of some studies and the implementation of additional weight loss interventions, patient data were excluded after IGB removal. This reduced the study power of each subgroup and necessitated the division of subgroups at the 3-month time-point to compare weight loss results. Additionally, because the data of air-filled and fluid-filled IGBs were pooled together in our meta-analysis, a diluted effect of IGBs in producing weight loss was to be expected. However, subgroup analysis based on IGB type clearly revealed such a difference. Safety analysis was not the main objective of the included RCTs; therefore, further sham-controlled RCTs are required to determine the net efficacy and long-term safety of new generations of IGB.
Conclusion
IGB treatment, in addition to lifestyle modification, is an effective short-term modality for weight loss in selected patients. However, there is insufficient evidence supporting its long-term efficacy. Fluid-filled IGBs produce considerably better weight loss than traditional air-filled IGBs.
References
WHO. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva, Switzerland: World Health Organization; 2009.
Loveman E, Frampton GK, Shepherd J, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess. 2011;15(2):1–182.
Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;24(3):437–55.
Genco A, Lorenzo M, Baglio G, et al. Does the intragastric balloon have a predictive role in subsequent LAP-BAND((R)) surgery? Italian multicenter study results at 5-year follow-up. Surg Obes Relat Dis. 2014;10(3):474–8.
Paulus GF, de Vaan LE, Verdam FJ, Bouvy ND, Ambergen TA, van Heurn LW. Bariatric surgery in morbidly obese adolescents: a systematic review and meta-analysis. Obes Surg. 2015;25(5):860–78.
Mathus-Vliegen EM, Tytgat GN, Veldhuyzen-Offermans EA. Intragastric balloon in the treatment of super-morbid obesity. Double-blind, sham-controlled, crossover evaluation of 500-milliliter balloon. Gastroenterology. 1990;99(2):362–9.
Hogan RB, Johnston JH, Long BW, et al. A double-blind, randomized, sham-controlled trial of the gastric bubble for obesity. Gastrointest Endosc. 1989;35(5):381–5.
Ramhamadany EM, Fowler J, Baird IM. Effect of the gastric balloon versus sham procedure on weight loss in obese subjects. Gut. 1989;30(8):1054–7.
Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr. 1988;48(3):592–4.
Benjamin SB, Maher KA, EL Jr C, et al. Double-blind controlled trial of the Garren-Edwards gastric bubble: an adjunctive treatment for exogenous obesity. Gastroenterology. 1988;95(3):581–8.
Meshkinpour H, Hsu D, Farivar S. Effect of gastric bubble as a weight reduction device: a controlled, crossover study. Gastroenterology. 1988;95(3):589–92.
Lindor KD, RW Jr H, Ilstrup DM, Jensen MD. Intragastric balloons in comparison with standard therapy for obesity—a randomized, double-blind trial. Mayo Clin Proc. 1987;62(11):992–6.
Garren M, Garren L, Giordano F. The Garren gastric bubble: an Rx for the morbidly obese. Endocr Rev. 1984;1:57–60.
Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet. 1982;1(8265):198–9.
Giardiello C, Borrelli A, Silvestri E, Antognozzi V, Iodice G, Lorenzo M. Air-filled vs water-filled intragastric balloon: a prospective randomized study. Obes Surg. 2012;22(12):1916–9.
De Castro ML, Morales MJ, Del Campo V, et al. Efficacy, safety, and tolerance of two types of intragastric balloons placed in obese subjects: a double-blind comparative study. Obes Surg. 2010;20(12):1642–6.
Mathus-Vliegen EM, Eichenberger RI. Fasting and meal-suppressed ghrelin levels before and after intragastric balloons and balloon-induced weight loss. Obes Surg. 2014;24(1):85–94.
Mohammed M, Anwar R, Mansour AH, Elmasry E, Othman G. Effects of intragastric balloon versus conservative therapy on appetite regulatory hormones in obese subjects. Trends Med Res. 2014;9(2):58–80.
Mathus-Vliegen EM, de Groot GH. Fasting and meal-induced CCK and PP secretion following intragastric balloon treatment for obesity. Obes Surg. 2013;23(5):622–33.
Fuller NR, Pearson S, Lau NS, et al. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity (Silver Spring). 2013;21(8):1561–70.
Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Relat Dis. 2013;9(2):290–5.
Genco A, Maselli R, Frangella F, et al. Effect of consecutive intragastric balloon (BIB(R)) plus diet versus single BIB(R) plus diet on eating disorders not otherwise specified (EDNOS) in obese patients. Obes Surg. 2013;23(12):2075–9.
Lee YM, Low HC, Lim LG, et al. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc. 2012;76(4):756–60.
Farina MG, Baratta R, Nigro A, et al. Intragastric balloon in association with lifestyle and/or pharmacotherapy in the long-term management of obesity. Obes Surg. 2012;22(4):565–71.
Dumonceau JM. Evidence-based review of the Bioenterics intragastric balloon for weight loss. Obes Surg. 2008;18(12):1611–7.
Fernandes M, Atallah AN, Soares BG, et al. Intragastric balloon for obesity. Cochrane Database Syst Rev. 2007(1):CD004931.
Zheng Y, Wang M, He S, Ji G. Short-term effects of intragastric balloon in association with conservative therapy on weight loss: a meta-analysis. J Transl Med. 2015;13:246.
Imaz I, Martinez-Cervell C, Garcia-Alvarez EE, Sendra-Gutierrez JM, Gonzalez-Enriquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008;18(7):841–6.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94.
Mathus-Vliegen EM, Tygat GN. Gastro-oesophageal reflux in obese subjects: influence of overweight, weight loss and chronic gastric balloon distension. Scand J Gastroenterol. 2002;37(11):1246–52.
Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc. 2005;61(1):19–27.
Genco A, Cipriano M, Bacci V, et al. BioEnterics Intragastric Balloon (BIB): a short-term, double-blind, randomised, controlled, crossover study on weight reduction in morbidly obese patients. Int J Obes. 2006;30(1):129–33.
Ponce J, Woodman G, Swain J, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11(4):874–81.
Genco A, Bruni T, Doldi SB, et al. BioEnterics intragastric balloon: the Italian experience with 2,515 patients. Obes0020Surg. 2005;15(8):1161–4.
Abu Dayyeh BK, Kumar N, Edmundowicz SA, et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82(3):425–38 .e5
Rigaud D, Trostler N, Rozen R, Vallot T, Apfelbaum M. Gastric distension, hunger and energy intake after balloon implantation in severe obesity. Int J Obes Relat Metab Disord. 1995;19(7):489–95.
Martinez-Brocca MA, Belda O, Parejo J, et al. Intragastric balloon-induced satiety is not mediated by modification in fasting or postprandial plasma ghrelin levels in morbid obesity. Obes Surg. 2007;17(5):649–57.
Genco A, Cipriano M, Bacci V, et al. Intragastric balloon followed by diet vs intragastric balloon followed by another balloon: a prospective study on 100 patients. Obes Surg. 2010;20(11):1496–500.
Acknowledgment
We would like to express our appreciation to Anabela Rodrigues for her help in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed Consent
Not applicable.
Statement of Human and Animal Rights
Not applicable.
Rights and permissions
About this article
Cite this article
Saber, A.A., Shoar, S., Almadani, M.W. et al. Efficacy of First-Time Intragastric Balloon in Weight Loss: a Systematic Review and Meta-analysis of Randomized Controlled Trials. OBES SURG 27, 277–287 (2017). https://doi.org/10.1007/s11695-016-2296-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2296-8