Abstract
Background
Laparoscopic sleeve gastrectomy (LSG) is currently the leading bariatric procedure and targets, among other obesity classes, patients with BMI 30–35 kg/m2, which are reaching alarming proportions.
Methods
Between February 2010 and August 2015, data on 541 consecutive patients with BMI 30–35 kg/m2 undergoing LSG were prospectively collected and analyzed.
Results
Mean age was 32 ± 8 years (13–65) and 419 (77.4 %) were women. Preoperative weight was 92.0 ± 8.8 kg (65–121) and BMI was 32.6 ± 1.5 kg/m2 (30–35). Comorbidities were detected in 210 (39 %) patients. Operative time was 74 ± 12 min (40–110) and postoperative stay was 1.7 ± 0.22 days (1–3). There were no deaths, leaks, abscesses or strictures and the rate of hemorrhage was 1.2 %. At 1 year, 98 % were followed and BMI decreased to 24.7 ± 1.6, the percentage of total weight loss (% TWL) was 24.1 ± 4.7 while the percentage of excess BMI loss (%EBMIL) reached 106.1 ± 24.1. At 5 years, 76 % of followed patients achieved a ≥50 % EBMIL.
Conclusion
With appropriate surgical expertise, LSG in patients with BMI 30–35 kg/m2 achieved excellent outcomes with a zero fistula rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic sleeve gastrectomy (LSG) exponentially grew worldwide over the recent 3 years and is the leading weight loss procedure today in many countries, representing more than 70 % of all bariatric procedures [1, 2].
The indications of LSG recently released by several surgical societies to include patients with BMI 30–35 kg/m2 that are reaching pandemic proportions worldwide [3–5]. There is actual evidence that a BMI 30–35 kg/m2 increases the risks of various comorbid conditions, malignancies such as colorectal and breasts cancers, negatively impacts on both physical and psychological quality of life, and consequently deserves effective treatment [6–9].
Similarly to other authors, we have previously reported excellent outcomes without any substantial surgical risks in patients with BMI 30–35 kg/m2 undergoing LSG [10, 11]. Although, the major advantage of LSG is technical simplicity, gastric leaks can occur and remain a dreaded and life-threatening complication [12, 13].
In the current study, we report on operative outcome and results in 541 consecutive patients with BMI 30–35 kg/m2 with special reference to gastric leaks.
Materials and Methods
We performed a retrospective study of a prospectively maintained database including all consecutive obese patients with BMI 30–35 kg/m2 who have undergone LSG between February 2010 and August 2015. Eligible non-classical criteria were patients with BMI 30–35 kg/m2 who failed to achieve adequate weight loss through proper conservative methods ((life-style modification and medications) and lasting for ≥5 years or have obesity-related comorbidities [14]. Patients with a history of a prior bariatric procedure or undergoing concomitant abdominal surgery were excluded. The risks, benefits, and long-term consequences of LSG were discussed in detail during the initial encounter with the surgeon and the dietician. Written informed consent was obtained preoperatively from all patients. The cost of the procedure was at the patients’ expense and the paid package included the fees for a 1-year follow-up.
All patients were submitted to a preoperative anesthesiology workup including appropriate multidisciplinary counseling [14].
Collected data included patient demographics and perioperative outcomes. Follow-up data included weight loss parameters, change in comorbidity status, and body image satisfaction scoring. Remission of type 2 diabetes was defined as fasting plasma glucose level <126 mg/dL and HbA1c level <6.5 % requiring no medications. Remission of dyslipidemia and hypertension were defined as normal lipid panel and blood pressure <135/85 mmHg without medication. Remission of sleep apnea syndrome was considered when stopping continuous positive airway pressure or absence of symptoms strongly suggesting sleep apnea. Partial improvement was considered when considering the number or dosage of the drugs used for the treatment of comorbidities or partial regression of symptoms.
Ideal body weight was calculated based on a BMI of 25 kg/m2. All patients received preoperative low-molecular-weight heparin and antibiotic prophylaxis.
Data analysis was carried out using the SPSS software version 21. Results are reported as mean ± SD or as percentages when appropriate.
Surgical Technique
The technique used for LSG is based on a 5-port approach [10]. Using the Ligasure (Covidien, Minneapolis, MN, USA) vessel sealing device, the vessels of the gastric greater curvature were ligated starting at 4 cm proximal to the pylorus and proceeding to the angle of His where all the attachments of the fundus to the left crus were carefully released to avoid any laceration or thermal injury at this level. The LSG was created by applying sequential firings of 60-mm Endo GIA staplers loaded with a green (4.8 mm) cartridge (Covidien, Norwalk, CT, USA) tightly abutting a 36-French (F) bougie and starting at 4 cm proximal to the pylorus to the angle of His. A loose transection, was performed at the top end of the gastric tube avoiding excessive stretching and laceration in the gastric wall next to the staple line. An intraoperative methylene blue test was performed to exclude a leak. Increasing systolic blood pressure to 130 mmHg while decreasing the pneumoperitoneum pressure allowed the achievement of hemostasis at the staple line by cautery or over suturing. The specimens were retrieved from the 15 mm-port and were checked for adequate stapling. Attempts of excessive blowing or stretching of the retrieved specimens always resulted in tears in the gastric wall just adjacent to and not into the staple line. No abdominal drainage was left in place.
Early ambulation was strongly encouraged. Patients resumed a clear fluid on postoperative day 1 and were discharged on postoperative day 2 unless a complication occurred. Upon discharge, detailed dietary instructions were provided. Patients returned to the outpatient clinic at 1 and 4 weeks following surgery, then every 3 months for the first year to monitor complications, food tolerance, appetite, weight loss, eating behavior, and comorbidity status [14]. After the first year, the follow-up was decreased to every 6 months. Telephone calls, emails, and messages were also used to monitor foreigners 189/541 (35 %) or patients who traveled away and could not visit regularly the outpatient clinic. The percentage of excess BMI loss (%EBMIL) is calculated by dividing the change in BMI from baseline by excess BMI which corresponds to the actual BMI minus the ideal BMI (25 kg/m2).
Results
Demographics
Out of a total of 3512 bariatric procedures, 541(15 %) consecutive obese patients with BMI 30–35 kg/m2 were enrolled in the study. Data were collected prospectively. Patient demographics are shown in Table 1. The study population comprised 419 (77.4 %) young women; 112 (26.7 %) had undergone a prior cosmetic surgery. Thirty seven patients (6.8 %) had undergone a previous cholecystectomy. Comorbidities were detected in 210 (39 %) patients with an average of 2.8 ± 1.1 comorbidities.
Perioperative outcome
1Intraoperative and postoperative data are shown in Table 2. Mean operative time was 74 ± 12 min (40–110) and postoperative stay was 1.7 ± 0.22 days (1–3). The mean number of sequential firings of 60-mm endo GIA stapler cartridges was 5 ± 0.45 cartridges (4–6). Patients were monitored postoperatively in the recovery room and were then transferred to the surgical wards. None were admitted to the intensive care unit. Seven (1.3 %) patients experienced postoperative intra-abdominal bleeding and were treated conservatively with a mean of 2.28 ± 0.03 (2–4) packed red cell units. One patient was diagnosed with a symptomatic splenic infarction at the upper pole and had spontaneous resolution of symptoms. Two patients presented pulmonary atelectasia and infection and were both successfully managed with antibiotics. No leaks, abscesses, or strictures were reported during the postoperative period. No deaths occurred within 30 days of surgery and the overall postoperative morbidity was 1.85 %. Sixteen (3.1 %) patients underwent a laparoscopic cholecystectomy for new onset symptomatic cholelithiasis during the first postoperative year.
Weight and comorbidities changes
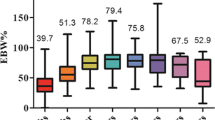
The changes in weight loss parameters are summarized in Table 5. One-year follow-up visits were achieved by 486/496 (98 %) of patients that have completed 1 year postoperatively. This time represented the nadir of weight loss with a mean BMI of 24.7 ± 1.6 kg/m2, mean BMI loss (BML) of 7.9 ± 1.7 kg/m2, percentage of total weight loss (%TWL) of 24.1 ± 4.7 %, and percentage of excess BMI loss (%EBMIL) of 106.1 ± 24.1 % (Fig. 1). Out of the 102 patients that completed 5 years postoperatively, 52(51 %) patients were lost to follow-up. At 5 years, 38/50 (76 %) of followed patients achieved a ≥50 % of EBMIL. None of the patients had a BMI drop below 20 kg/m2. Evolution of comorbidity status and of metabolic syndrome components is shown in Tables 3 and 4. On a satisfaction scale of 1 (very poor) to 5 (excellent), the patient body image satisfaction scoring for patients achieving 1 year of follow-up was 4.2 ± 0.7.
Discussion
LSG exponentially grew worldwide and represents today the leading procedure in the treatment of morbid obesity. However, the current era equally demands effective therapy for the moderately obese population, i.e patients with a BMI 30–35 kg/m2 that have reached alarming proportions worldwide [3–5]. The impact of a BMI 30–35 kg/m2 on health has been widely investigated and has been shown to cause multiple diseases, and a decrease in quality of life as well as life expectancy [6–9]. These statements are in accordance with the high prevalence of comorbidities in the current series as well as the body image deterioration noted given the high rate of prior cosmetic surgeries. Body image dissatisfaction also played a major role in the decision for LSG in our predominantly young female population (Table 5).
The BMI cutoffs for bariatric surgery classically excluded patients with BMI 30–35 kg/m2. However, these cutoffs were arbitrarily established 20 years ago by the NIH at a time when a few open surgical options with high complication rates were available. Recently, the indications released by several surgical societies include patients with BMI 30–35 kg/m2 [4, 15]. They have pointed out the inapplicability of the predefined BMI cutoffs given that BMI is a poor predictor of body or visceral adiposity and that novel minimally invasive surgical approaches are currently available [16]. We have previously performed a series of LSG in patients with BMI 30–35 kg/m2 and evaluated short-term risks and benefits. LSG was technically straightforward and resulted in excellent outcomes without substantial surgical risks [10]. The current series confirms our previously published results in the short and long term and outlines the absence of high gastric leak in this population when LSG is performed by experienced surgeons.
Several studies have shown that LSG allows a reduction in the preoperative BMI of nearly 7 to 10 points [1, 11, 17–19]. This is in concordance with our results in which the preoperative BMI was reduced by 7.9 points and dropped to the optimal BMI category of (20.0–24.9 kg/m2) whereas none of the patients suffered from excessive weight loss and malnutrition at the nadir point of weight loss [20]. Notably, since the cost of 1-year follow-up visits was included in the initial package, patients were better motivated and only 2 % of patients were lost to follow-up at this point which compares favorably with other published reports [11, 18, 21]. Remarkably, this optimal BMI category is rarely obtained when LSG is performed on morbidely obese patients [21, 22]. The elevated %EBMIL values in this study as compared to those of morbidely obese patients are explained by the relatively low baseline BMI values and are in agreement with other studies [11, 18, 21]. Like others, this efficiency was maintained at 5 years with 76 % of followed patients achieving a ≥50 % of EBMIL [18]. In parallel comorbidities substantially improved or resolved and were associated with improved psychological status.
Although LSG is technically attractive, the procedure is burdened by the risk of gastric leaks that occurred in up to 7 % of cases [12, 22]. High gastric leak mechanisms are technically dependent occurring mostly during the initial experience (distal stenosis, inadequate stapling, laceration or thermal injury) or tissue-dependent occurring during the healing process. In this study, the conjunction of technical ease, advanced experience, youth, absence of organ dysfunction, and low BMI may have contributed to this final outcome which is in accordance with three recently published series showing a gastric leak risk of 0 to 0.025 % in this population [11, 18, 19]. Again, the correlation between BMI and the risk of leaks was recently validated by a risk prediction model study in a series of 5871 LSG [23].
Conclusion
This study shows that with appropriate surgical technique, LSG can achieve excellent outcomes without substantial surgical risks in patients with BMI of 30–35 kg/m2. Similarly to other authors and based on clinical effectiveness, cost-effectiveness, safety, equity, and ethics, we believe that there is no current justification for preventing this group from taking advantage of this life-changing treatment. Ultimately, a day-case LSG booming in ambulatory surgery centers should be anticipated for these patients [24].
References
Sroka G, Milevski D, Shteinberg D, et al. Minimizing hemorrhagic complications in laparoscopic sleeve gastrectomy—a randomized controlled trial. Obes Surg. 2015;25(9):1577–83.
Gagner M, Deitel M, Erickson AL, et al. Survey on laparoscopic sleeve gastrectomy at the fourth international consensus summit on sleeve. Obes Surg. 2013;23(12):2013–7.
Clinical Issues Committee A. Bariatric surgery in class I obesity (body mass index 30–35 kg/m(2)). Surg Obes Relat Dis. 2013;9(1):e1–10.
Busetto L, Dixon J, De Luca M, et al. Bariatric surgery in class I obesity. Obes Surg. 2014;24(4):487–519.
Cerci M, Bellini MI, Russo F, et al. Bariatric surgery in moderately obese patients: a prospective study. Gastroenterol Res Pract. 2013;2013:276183.
Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316(2):104–8.
Flegal KM, Kit BK, Orpana H, et al. Association of all cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82.
Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78.
Keating CL, Peeters A, Swinburn BA, et al. Utility-based quality of life associated with overweight and obesity: the Australian diabetes, obesity, and lifestyle study. Obesity (Silver Spring). 2013;21(3):652–5.
Noun R, Chakhtoura G, Nasr M, et al. Laparoscopic sleeve gastrectomy for mildly obese patients (body mass index 30–35 kg/m2). Operative and short-term results. J Obes. 2012;2012:813650.
Park JY, Kim YJ. Efficacy of laparoscopic sleeve gastrectomy in mildly obese patients with body mass index of 30–35 kg/m2. Obes Surg. 2015;25(8):1351–7.
Márquez MF, Ayza MF, Lozano RB, et al. Gastric leak after laparoscopic sleeve gastrectomy. Obes Surg. 2010;20(9):1306–11.
Chouillard E, Chahine E, Schoucair N, et al. Roux-En-Y Fistulo-Jejunostomy as a salvage procedure in patients with post-sleeve gastrectomy fistula. Surg Endosc. 2014;28(6):1954–60.
National Institute for Health and Care Excellence. Identification, assessment, and management of overweight and obesity. Clinical Guidelines 189. BMJ 2014. 2014;349:g6608.
Dixon JB, Zimmet P, Alberti KG. Bariatric surgery: an IDF statement for obese type 2 diabetes. Surg Obes Relat Dis. 2011;7(4):433–47.
Livingston EH. Inadequacy of BMI as an indicator for bariatric surgery. JAMA. 2012;307(1):88–9.
Boza C, Salinas J, Salgado N, et al. Laparoscopic sleeve gastrectomy as a stand-alone procedure for morbid obesity: report of 1,000 cases and 3-year follow-up. Obes Surg. 2012;22(6):866–71.
Hong J-S, Kim W-W, Han S-M. Five years results of laparoscopic sleeve gastrectomy in Korean patients with lower body mass index (30–35 kg/m2). Obes Surg. 2015;25(5):824–9.
Maiz C, Alvarado J, Quezada N, et al. Bariatric surgery in 1119 patients with preoperative body mass index <35 (kg/m2) : results at 1 year. Surg Obes Relat Dis. 2015;11(5):1127–32.
Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9.
Sakran N, Raziel A, Goitein O, et al. Laparoscopic sleeve gastrectomy for morbid obesity in 3003 patients: results at a high-volume bariatric center. Obes Surg. 2016.
Slim R, Smayra T, Chakhtoura G, et al. Endoscopic stenting of gastric staple line leak following sleeve gastrectomy. Obes Surg. 2013;23(11):1942–5.
Aminian A, Brethauer SA, Sharafkhah M, et al. Development of a sleeve gastectomy risk calculator. Surg Obes Relat Dis. 2015;11(4):758–64.
Rebibo L, Dhahri A, Badaoui R, et al. Laparoscopic sleeve gastrectomy as day-case surgery. Surg Obes Relat Dis. 2015;11(2):335–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Noun, R., Slim, R., Nasr, M. et al. Results of Laparoscopic Sleeve Gastrectomy in 541 Consecutive Patients with Low Baseline Body Mass Index (30–35 kg/m2). OBES SURG 26, 2824–2828 (2016). https://doi.org/10.1007/s11695-016-2224-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2224-y