Abstract
Background
One-year results of the VBLOC DM2 study found that intermittent vagal blocking (VBLOC therapy) was safe among subjects with obesity and type 2 diabetes mellitus (T2DM) and led to significant weight loss and improvements in glycemic parameters and cardiovascular risk factors. Longer-term data are needed to determine whether the results are sustained.
Methods
VBLOC DM2 is a prospective, observational study of 28 subjects with T2DM and body mass index (BMI) between 30 and 40 kg/m2 to assess mid-term safety and weight loss and improvements in glycemic parameters, and other cardiovascular risk factors with VBLOC therapy. Continuous outcome variables are reported using mixed models.
Results
At 24 months, the mean percentage of excess weight loss was 22 % (95 % CI, 15 to 28, p < 0.0001) or 7.0 % total body weight loss (95 % CI, 5.0 to 9.0, p < 0.0001). Hemoglobin A1c decreased by 0.6 percentage points (95 % CI, 0.2 to 1.0, p = 0.0026) on average from 7.8 % at baseline. Fasting plasma glucose declined by 15 mg/dL (95 % CI, 0 to 29, p = 0.0564) on average from 151 mg/dL at baseline. Among subjects who were hypertensive at baseline, systolic blood pressure declined 10 mmHg (95 % CI, 2 to 19, p = 0.02), diastolic blood pressure declined by 6 mmHg (95 % CI, 0 to 12, p = 0.0423), and mean arterial pressure declined 7 mmHg (95 % CI, 2 to 13, p = 0.014). Waist circumference was significantly reduced by 7 cm (95 % CI, 4 to 10, p < 0.0001) from a baseline of 120 cm. The most common adverse events were mild or moderate heartburn, implant site pain, and constipation.
Conclusions
Improvements in obesity and glycemic control were largely sustained after 2 years of treatment with VBLOC therapy with a well-tolerated risk profile.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
While the causes of T2DM are multifactorial, the most important risk factor is obesity. The current guidelines of the American Heart Association, American College of Cardiology, and The Obesity Society for the management of overweight and obesity state that a weight loss of 2.5 to 5.5 kg reduces the risk of developing T2DM by 30 to 60 % [1]. The Diabetes Prevention Program (DPP) Study demonstrated that a modest 7 % total body weight loss (TBL) through intensive lifestyle intervention reduced the incidence of diabetes by 58 % [2]. However, after 10 years of follow-up in DPP, the weight loss in the lifestyle intervention group was partially regained and the reduction in diabetes incidence was somewhat attenuated [3]. The weight regain in DPP with lifestyle intervention is consistent with the results of the majority of behavioral intervention programs for weight loss.
Current bariatric surgical interventions for weight loss have been shown to have a significant effect on glycemic control and diabetes [4]; however, the potential for serious complications and permanent anatomical alterations make bariatric surgery an unacceptable option for many patients with obesity who have, or are at risk for, T2DM. Weight loss drugs are efficacious treatments for many patients; however, many patients do not respond or do not tolerate the drugs in the short term. Cost and patient compliance also limit their effectiveness; additionally, the long-term cardiovascular safety profile of these medications is unclear and the effect on glycemic parameters is often modest. A weight loss treatment that is more durable than pharmacotherapy but less risky than conventional bariatric weight loss surgery would be an attractive option for many patients with obesity who have, or are at risk for, T2DM.
A recent randomized, double-blind, sham-controlled clinical trial of intermittent vagal nerve block (VBLOC) therapy for morbid obesity in 239 subjects showed superiority over sham control in weight loss at 1 year with a low rate of serious complications [5].
The VBLOC DM2 study is a prospective study of 28 subjects with obesity and T2DM receiving VBLOC therapy. After 1 year of therapy, clinically significant reductions in weight and improvements in glycemic parameters were demonstrated [6]. Given the importance of sustained improvement in obesity to control glycemic parameters, longer-term safety and effectiveness data were needed to support VBLOC therapy as a viable option to treat patients with obesity and T2DM. The 2-year results of the VBLOC DM2 study are the focus of this report.
Materials and Methods
Study Design
The VBLOC DM2 study is a prospective, open-label, single-arm study of the safety and effectiveness of VBLOC therapy in subjects with obesity and T2DM. The VBLOC DM2 study was designed as an exploratory investigation to assess the impact of vagal nerve block among obese patients with T2DM. Efficacy measures of interest were improvements from baseline in percentage excess weight loss (%EWL), percent total weight loss (%TWL), fasting plasma glucose, hemoglobin A1c (HbA1c), and systolic, diastolic, and mean arterial blood pressure. All adverse events were recorded and attributed by the investigator as related or not related to the device, procedure, or therapy algorithm. The relatedness of each serious adverse event (SAE) was adjudicated by a Clinical Events Committee. The relatedness and severity of nonserious adverse events (AEs) were attributed by the site investigator.
This study was conducted at five sites in Mexico, Norway, Switzerland, and Australia (two sites). Institutional Review Board approval was obtained from all investigative sites, and informed consent was obtained from all individual participants included in the study.
Inclusion and Exclusion Criteria
Participants were eligible for inclusion if they had T2DM with a body mass index between 30 and 40 kg/m2, HbA1c between 7 and 10 %, were aged 25 to 60 years, had diabetes 12 years or less, absence of significant diabetic complications, and had failed to respond to a diet or exercise program. The most important exclusion criteria were type 1 diabetes mellitus, significant weight loss in the last year (>10 % TWL), clinically significant hiatal hernia, smoking cessation within the last 6 months, or use of a weight loss drug within the last 3 months. Exclusion criteria relevant to diabetes were insulin dependence (short-term insulin use during perioperative period was allowed) and use of GLP-1 receptor agonists. A full list of inclusion and exclusion criteria are in Supplementary Information 1.
Intervention
All subjects received a fully implanted Maestro Rechargeable System. The details of the implantation procedure have been described previously [7]. Briefly, the Maestro Rechargeable System consists of two leads placed laparoscopically on the anterior and posterior abdominal vagal trunks. Leads are connected to a rechargeable neuroregulator placed in a subcutaneous pocket on the lateral chest wall. A mobile charger is used for approximately 30 min every 1 or 2 days to recharge the neuroregulator battery.
Devices were programmed to deliver electrical stimulation at 5000 Hz and 3 to 8 mA for at least 12 h each day, with a goal of 6 mA delivered for at least 14 h per day. Initially, the amplitude was increased over the course of the first several visits, and device parameters (i.e., amplitude and hours of therapy delivered per day) were adjusted to maximize weight loss and minimize adverse events related to sensations of therapy, if necessary.
Visit Schedule and Data Collection
Subjects were seen every week in the first month, every other week through month 3, then monthly in the first year, and every other month in the second year. At each of these visits, subjects received individual weight management counseling, which consisted of education on strategies for weight loss through healthy eating, exercise, and goal tracking. No specific diets or exercise regimens were prescribed prior to implant or during the study. Weight, adverse events, and medication changes were recorded at every visit. Glycemic parameters, lipid parameters (low- and high-density lipoprotein (LDL and HDL, respectively) and triglycerides (TG)) and triplicate blood pressure values were measured at baseline, 1, 4, and 12 weeks, and 6, 12, and 24 months. Hypertension was defined as systolic blood pressure (SBP) ≥130 mmHg and/or diastolic blood pressure (DBP) ≥80 mmHg per the JNC-7 criteria for T2DM [8]. Decisions to reduce or stop any medications were made by the patients’ primary care physician and not the investigational team.
Statistical Analysis
Baseline demographics and medical history information are summarized using descriptive statistics. Continuous variables are summarized with means and standard deviations or confidence intervals. Categorical variables are summarized as frequencies and percentages.
Changes in continuous parameters were assessed using mixed-effects regression models with unstructured covariance structures and random intercepts for each subject. Each model was fit treating time categorically so as not to impose a specific functional form to the trajectory over time. All observed values were included in the mixed models and missing data were treated as missing at random. Mean changes are shown with 95 % confidence intervals as descriptive analyses and p values. All analyses were performed in SAS version 9.3. Graphics were generated using R version 3.1.0.
Weight loss was assessed using the percentage of excess weight loss (%EWL), which is calculated as follows: %EWL = 100 % × [weight loss / excess body weight at implant]. The percentage of total weight loss (%TWL) is also reported as another weight loss measure of interest.
Results
Subject Demographics and Disposition
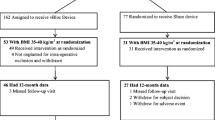
Twenty-eight subjects were enrolled and implanted with a Maestro Rechargeable System. Seventeen of the subjects were female (61 %), the mean age was 51 ± 9 years, the mean weight was 107 ± 16 kg, and the mean BMI was 37 ± 3 kg/m2. Baseline diabetes medications were primarily metformin alone, with 16 subjects on this medication only. Four subjects were on metformin and gliclazide; one subject was on metformin and pioglitazone; one subject was on metformin and glibenclamide; one subject was on gliclazide only; one subject was on glibenclamide only; one subject was on diabex only; one subject was on metformin, gliclazide, and pioglitazone; and two subjects were on no diabetes medication at baseline. At 2 years, 27 subjects (96 %) remained enrolled in the study. Twenty-four subjects (86 %) attended the 2-year visit. Three subjects missed their 2-year visit, and one subject was explanted and withdrawn prior to their 2-year visit.
Weight Loss
Mean estimated weight loss over time is shown in Fig. 1, and weight loss responder thresholds for %EWL and %TWL are shown in Table 1. At 1 year, the estimated mean %EWL was 24 % (95 % CI, 18 to 30, p < 0.0001) or 7.5 % TWL (95 % CI, 5.6 to 9.5, p < 0.0001). At 2 years, weight loss was largely sustained with an estimated mean %EWL of 22 % (95 % CI, 15 to 28, p < 0.0001) or 6.9 % TWL (95 % CI, 4.9 to 9.0, p < 0.0001). At 2 years, 57 % of subjects had achieved at least 20 % EWL or 5 % TWL, 43 % had achieved at least 25 % EWL or 7.5 % TWL, and 30 % had achieved at least 10 % TWL.
Glycemic Control
At baseline, the mean HbA1c was 7.8 %. At 1 year, the mean estimated HbA1c was significantly reduced by 1.0 percentage point (95 % CI, 0.7 to 1.4, p < 0.0001) and had been reduced by 0.6 percentage points (95 % CI, −1.0 to −0.2, p = 0.0026) at 2 years (Fig. 2, Table 2). The proportion of subjects with an HbA1c of 7 % or lower improved from 25 % at baseline to 69 % at 1 year and 63 % at 2 years.
Fasting plasma glucose was significantly decreased from a mean of 151 mg/dL at baseline by 28 mg/dL (95 % CI, 13 to 42, p = 0.0003) at 1 year and by 15 mg/dL (95 % CI, 0 to 29, p = 0.0564) at 2 years (Fig. 3, Table 2).
At 2 years, four subjects missed their visit and one subject had no 2-year follow-up data on diabetes medications. Among the 23 subjects with complete data, 12 subjects (52 %) had not changed their medication regimen, four subjects (17 %) had increased either the number of medications or dosage, and seven subjects (30 %) had decreased either the number of medications or dosage.
Blood Pressure, Lipids, and Waist Circumference
Short-term results for reductions in blood pressure and waist circumference in VBLOC DM2 have been reported previously [6]. The mean systolic blood pressure, diastolic blood pressure, and mean arterial pressure were reduced, but not significantly changed in the full study sample (Table 2). Fifteen of the subjects (54 %) had elevated blood pressure at baseline, and significant improvements in all blood pressure measures were observed among these subjects at 24 months (Table 2). Systolic blood pressure was reduced by 10 mmHg (95 % CI, 2 to 19, p = 0.02), diastolic blood pressure was reduced by 6 mmHg (95 % CI, 0 to 12, p = 0.0423), and mean arterial pressure was reduced by 7 mmHg (95 % CI, 2 to 13, p = 0.014). One subject who had normal blood pressure at baseline became hypertensive and was started on hypertension medication. Seven of the subjects with elevated blood pressure at baseline were on hypertensive medications, and two required an increase in medication dosage by 24 months. LDL was essentially unchanged from a baseline of 111 to 115 mg/dL at 24 months (24-month change 95 % CI, −6 to 17, p = 0.3655). Likewise, HDL was unchanged from a baseline of 45 to 50 mg/dL at 24 months (24-month change 95 % CI, −4 to 13, p = 0.3292), However, TG significantly dropped 64 mg/dL from a baseline of 207 mg/dL by 24 months (95 % CI, −98 to −29, p = 0.0005). Lastly, waist circumference was significantly reduced by 7 cm (95 % CI, 4 to 10, p < 0.0001) from a baseline of 120 cm at 24 months.
Adverse Events
The most common AEs related to the device, procedure, or therapy that occurred in more than one subject are presented in Table 3. The most common related AEs through 2 years were heartburn (29 %), constipation (21 %), pain at the neuroregulator site (18 %), nausea (11 %), and other pain (11 %). Nearly all these AEs were mild or moderate in severity, and there were few events ongoing as of the 24-month visit.
As reported previously, there were no surgical complications following implant [6]. Two SAEs were reported through 24 months. One SAE was due to a revision for pain at the neuroregulator site, and the other was due to a revision following a lead breakage. Both SAEs were considered serious because the hospitalizations following revision were longer than 24 h in duration per the standard of care of the clinical sites. Both SAEs resolved after the revisional procedure and neither were considered life-threatening. There were no deaths, unanticipated adverse device effects, or life-threatening complications through 24 months.
Surgical Interventions
Two revisions and two explants were performed through the 2-year visit. One subject had a revision procedure prior to their 12-month visit to reposition the neuroregulator to resolve pain. The revision resolved the pain temporarily, though the subject was ultimately explanted prior to their 2-year visit to resolve the pain. Another subject required a revision approximately 20 months after implant to resolve a lead failure. One additional explant approximately 22 months after implant was performed based on the subject’s decision and lack of compliance with the therapy.
Discussion
This investigation has demonstrated that the beneficial effects of an intermittent VBLOC seen after 12 months of therapy were maintained at 24 months. The average EWL at 12 months was 24 %, which was sustained with 22 % at 2 years. HbA1c was reduced from baseline by 1 percentage point at 12 months and by 0.6 percentage points at 24 months. The reductions in blood pressure and waist circumference were also similar at 24 months from those seen at 12 months. The two important lipids predictive of cardiovascular risk are LDL and TG. LDL was essentially unchanged in this trial; however, TG were significantly decreased at 24 months compared to baseline. Only 17 % of patients required an increase in diabetes medications through 2 years. Lastly, there were no deaths or life-threatening complications seen in the study.
It is now generally accepted in the medical community that conventional bariatric surgical procedures are effective for achieving sustainable weight loss and improvements in several medical conditions such as type 2 diabetes and hypertension [4, 9, 10]. The average weight loss achieved in this obese population with VBLOC therapy is lower than observational results routinely reported with gastric bypass, sleeve gastrectomy, and gastric banding procedures. However, many potential surgical candidates are unwilling to proceed with conventional bariatric surgery for multiple reasons including the potential for serious complications, long-term sequelae, and permanent alterations to the gastrointestinal tract. Therefore, there is a large unmet need for effective therapies that are safer and less complicated.
In the past few years, several innovative, new technologies have been developed. These include endoscopic gastric plication, endoscopic placement of barrier sleeves, and gastric electrical stimulation [11–13]. However, the majority of the published reports with these devices/procedures are typically based on follow-up periods of 12 months or less, so the long-term efficacy and safety profiles of these treatments are not known.
A recently published ongoing randomized, double-blind, sham-controlled study of 239 subjects, the ReCharge Study, demonstrated similar weight loss results with VBLOC at 12 months to the DM2 trial [5]. The ReCharge Study was the first pivotal trial of a weight loss device to show a significant treatment effect over a control device and is the first controlled study of a weight loss device that resulted in approval from the US Food and Drug Administration. The results of the VBLOC DM2 study suggest that the benefits of VBLOC therapy are largely sustained to 24 months. The efficacy and safety of VBLOC therapy demonstrated in several studies [5–7] would likely be very attractive to many of the patients who historically have not accepted conventional weight loss surgery.
Body weight reduction of as little as 5 % has been shown to benefit the type 2 diabetic patient [14]. While a 5 % reduction in body weight can be achieved by nonsurgical means, the results are often confounded by recidivism, poor patient compliance, and the cost and side effects of the pharmacologic agents as well as the progression of diabetes and its complications.
Several limitations of the study should be acknowledged. The study did not include a control group. However, the recently published results of the randomized, sham-controlled ReCharge study of VBLOC therapy in patients with obesity demonstrated superiority of VBLOC over sham control in weight loss [5]. Given that the weight loss observed in this study was nearly identical to that of the treatment arm of the ReCharge study, it is reasonable to assume that the degree of weight loss observed in the VBLOC DM2 study should be largely attributed to the effect of VBLOC therapy. It should be noted that the ReCharge study enrolled fewer than 10 % of patients with T2DM, so only the effect of VBLOC therapy on weight loss has been demonstrated in randomized controlled trials. Another limitation of this study was that the number of subjects included in this study was small. A randomized controlled trial would be needed to demonstrate that VBLOC therapy provides additional benefit in T2DM beyond that of standard of care. However, durable weight loss has been shown in other contexts to slow the progression of diabetes and reduce the incidence of diabetes.
Conclusion
Two-year follow-up of the VBLOC DM2 study among patients with obesity and T2DM demonstrated continued efficacy and safety of VBLOC therapy. Longer-term follow-up suggests that VBLOC therapy provides significant and sustained weight loss and improvement in glycemic control. VBLOC therapy continues to be well-tolerated through 24 months of follow-up.
References
Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–38.
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
Diabetes Prevention Program Research G, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85.
Ikramuddin S, Blackstone RP, Brancatisano A, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312:915–22.
Shikora S, Toouli J, Herrera MF, et al. Vagal blocking improves glycemic control and elevated blood pressure in obese subjects with type 2 diabetes mellitus. J Obes. 2013;2013:245683.
Camilleri M, Toouli J, Herrera MF, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143:723–31.
The seventh report of the joint national committee on the prevention, detection, evaluation, and treatment of high blood pressure (JNC-7). Bethesda, National Institutes of Health (US); 2003.
Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–9.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76.
Brethauer SA, Harris JL, Kroh M, et al. Laparoscopic gastric plication for treatment of severe obesity. Surg Obes Relat Dis. 2011;7:15–22.
de Jonge C, Rensen SS, Verdam FJ, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves type 2 diabetes. Obes Surg. 2013;23:1354–60.
Miras M, Serrano M, Duran C, et al. Early experience with customized, meal-triggered gastric electrical stimulation in obese patients. Obes Surg. 2015;25:174–9.
Fujioka K. Benefits of moderate weight loss in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:186–94.
Acknowledgments
We acknowledge Drs. Daniel Bessessen, James Freston, Miguel Herrera, Melissa Martinson, and Frank Moody for participation as members of the Data and Safety Monitoring Board and Clinical Events Committee. We also acknowledge Jody Dew for safety data reporting, Lisa Thackeray and Teresa Yurik for statistical analysis, and Brenda Bachmann, Jane Collins, Ruth Hutchinson, and Magnus Strommen for study coordination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
EnteroMedics Inc. fully sponsored the VBLOC DM2 study. The study design, data collection, and data integrity are ensured by the sponsor, though data analysis was conducted by an independent contractor.
Conflict of Interest
Drs. Shikora, R. Brancatisano, and Billington have received support as consultants from EnteroMedics Inc. Drs. Tweden and Knudson are employees of EnteroMedics Inc. Drs. Herrera and Pantoja received support for proctoring from EnteroMedics Inc. Drs. Toouli, Kow, and A. Brancatisano have received support for traveling in the past from EnteroMedics Inc. Dr. Billington reports that he is a consultant for EnteroMedics and NovoNordisk and has received grant support from Covidien. Drs. Kulseng, Zulewski, and Johnsen report no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 34 kb)
Rights and permissions
About this article
Cite this article
Shikora, S.A., Toouli, J., Herrera, M.F. et al. Intermittent Vagal Nerve Block for Improvements in Obesity, Cardiovascular Risk Factors, and Glycemic Control in Patients with Type 2 Diabetes Mellitus: 2-Year Results of the VBLOC DM2 Study. OBES SURG 26, 1021–1028 (2016). https://doi.org/10.1007/s11695-015-1914-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1914-1