Abstract
Background
No previous studies have validated the use of portable monitoring (PM) for the diagnosis of obstructive sleep apnea (OSA) in morbidly obese individuals. Our aim was to investigate the accuracy of PM for detecting respiratory events in morbidly obese patients that will be undergoing bariatric surgery.
Methods
This was a prospective study involving patients with body mass index (BMI) ≥35 kg/m2 who were recruited from the Sleep Clinic of Universidade Federal de São Paulo. Sleep-disordered breathing (SDB) was evaluated during full-night polysomnography (PSG). PM use was randomized and used on two consecutive nights: (1) at home (STDHome) and (2) at the sleep laboratory with PSG (PSG_STDLab).
Results
Although 58 participants initially underwent the recordings, 26 (45 %) were excluded because of technical problems. The patients’ mean age was 42.9 ± 10.9 (SD) years, and 56 % were female. The mean BMI was 40.8 ± 5.2 kg/m2. All patients had high risk for OSA, as defined by the Stop-Bang questionnaire, and the mean apnea-hypopnea index (AHI) was 46.9 ± 30.4/h. The intraclass coefficient of the correlation between AHI_PSG and AHI_STDLab was r = 0.92 (p = 0.0001); the intraclass coefficient for AHI_PSG and AHI_STDHome was r = 0.84 (p = 0.0001). The Kappa index was 0.87 (p > 0.0001) for severe cases. The sensitivity and the positive predictive value increased with the disease severity. A Bland-Altman analysis showed good agreement between the investigated methods.
Conclusions
PM is an efficacious method for diagnosing OSA in obese patients who have a high clinical probability of the disease. The method displays good sensitivity and specificity in severe cases; nevertheless, the high rate of data loss must be taken into account.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The epidemic of obesity is increasing worldwide, and it is considered the primary risk factor for obstructive sleep apnea (OSA). OSA affects 3 to 7 % of the overall population [1]. A recent study in the city of São Paulo described an OSA prevalence of 32.9 %, and there was an even higher frequency of OSA among obese males (64.1 % in individuals with body mass index [BMI] ≥35 kg/m2) [2].
In individuals with a BMI equal to or greater than 40 kg/m2, the prevalence of OSA varies from 42 to 48 % in males and from 8 to 38 % in females [3]. In a previous study, Carneiro et al. [4] described a 64 % prevalence of OSA (apnea-hypopnea index [AHI] >5) in morbidly obese patients who were assessed for bariatric surgery. The prevalence of OSA was 55.7 % in females; in males, it was even higher (77.4 %). The demand for bariatric surgery has risen dramatically in recent years. The total number of obesity surgeries performed in the USA and Canada reached 220,000 in 2008 and 2009. OSA has been related to adverse outcomes during the surgical period, such as respiratory failure, bleeding, and other clinical complications. Therefore, it is mandatory to diagnose OSA during the pre-operatory assessment to establish early treatment and minimize adverse outcomes after surgery.
Previous studies have shown that portable monitoring provides reasonable diagnostic accuracy for OSA in patients with a high-probability pretest [5, 6]. However, studies evaluating the use of portable monitoring in patients with comorbidities, such as morbid obesity, are still lacking. Lesser et al. demonstrated good accuracy for OSA diagnosis in obese pediatric patients (aged 9–18 years) using portable monitor (PM) compared with full-night polysomnography (PSG) [3].
Therefore, the OSA diagnostic accuracy of PM in morbidly obese patients needs to be confirmed and technical problems that affect its diagnostic evaluation need to be assessed. The aim of this study was to investigate PM’s accuracy for diagnosing OSA in morbidly obese candidates for bariatric surgery compared with the gold-standard method of full-night PSG.
Methods
Participants
This prospective study evaluated patients older than 18 years from both genders who were referred to the Respiratory Sleep Disorders clinic during pre-operatory assessment for bariatric surgery. Inclusion criteria included symptoms suggestive of OSA, such as intense and loud snoring, witnessed apneas during sleep, and excessive somnolence. Patients with other sleep disorders, such as narcolepsy, restless legs syndrome, insomnia, severe cardiovascular diseases, and neuromuscular diseases, or patients who were being treated for OSA, were on oxygen therapy, or were using alcohol or other drugs were excluded. The study was approved by the Research Ethics Committee at the Federal University of Sao Paulo (Universidade Federal de São Paulo—CEP 0290/11) and was registered at ClinicalTrials.gov (NCT01455077). All participants signed informed consent forms. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Protocol
In the sleep laboratory, before the PSG recording, the Stop-Bang questionnaire [7] and the Epworth Sleepiness Scale (ESS) [8] were applied. Body mass index (BMI), neck circumference, and blood pressure were also measured during the clinical evaluation.
All of the patients were randomized to two sleep assessments using PM equipment (Stardust II, Philips-Respironics, Inc., USA): (1) one at home for one night (STDHome) and (2) one in the sleep laboratory simultaneously with PSG (PSG_STDLab).
These evaluations provided three apnea-hypopnea index (AHI) values: AHI_STDHome, AHI_STDLab, and AHI_PSG. All evaluations were completed within a period of 2 weeks.
Polysomnography
A full-night PSG in the sleep laboratory was performed with Embla equipment (N7000, Embla Systems, Inc., Broomfield, CO, USA). The PSG montage included electroencephalogram, electrooculogram, electromyogram (chin and tibialis anterior muscles), nasal airflow (thermistor and nasal pressure), respiratory effort (inductance plethysmography of thorax and abdomen), oxyhemoglobin saturation (SpO2), snoring, and body position. A trained technician visually performed the sleep scoring according to the Rechtschaffen and Kales criteria [9]. The respiratory event scoring was based on the American Academy of Sleep Medicine (AASM) criteria [10]. Apnea was defined as a complete cessation of airflow during sleep lasting ≥10 s (and was further classified as central, obstructive, or mixed). Hypopnea was defined by a clear amplitude reduction of the nasal cannula during sleep (<50 %) that was associated with either an oxygen desaturation of >3 % or an arousal. Hypopnea events last 10 s or longer. Arousals [11] and leg movements [12] were quantified according to the American Sleep Disorders Association Task Force.
Portable Monitoring with the Stardust II
The STD is a PM designed for diagnosing OSA. It records five physiological parameters: airflow (nasal pressure transducer), respiratory effort (piezoelectric sensor), body position (both devices (PSG and PM) are positioned at the height of the sternum), and SpO2 + heart rate (via probe tape placed on the finger). A research assistant instructed the patient about using the STD at home. The instructions included verbal and written sections to illustrate the correct placement of the monitor, along with a practical demonstration. During training, patients were asked to indicate the time for “lights off” (when the patient goes to bed to sleep), “lights on” (when the patient wakes up in the morning), and any time of night the patient remained awake for more than 15 min. Patients returned the STD device the morning after the study at home. A trained technician applied the sensors used for both STD and PSG recording in the sleep laboratory. The cannulas were placed in the nostril, and the thoracic belts were trapped side by side. Nighttime desaturation was evaluated using oximeters on different fingers of the same hand.
For PM scoring, apnea events were required to show an airflow cessation ≥10 s (central, obstructive, or mixed) [13]. Hypopnea was defined as (1) a decrease (>50 %) from baseline in the amplitude of the nasal cannula during sleep or (2) a clear amplitude reduction of the nasal cannula during sleep (<50 %) associated with an oxygen desaturation of >3 %. Hypopnea events were those that lasted 10 s or longer. AHI was calculated using the total recording time as denominator. A trained technician scored all STD recordings, and another scored the PSGs. The technicians were blinded to each other’s analyses and to the volunteers’ clinical conditions.
Registers that had more than 60 % of the total recording time with good technical quality on all channels were considered for analysis. Recordings were excluded because of failure in data downloading, poor signal from the cannula, poor recording of oximetry, or poor respiratory effort. The recordings that met these criteria and had a sleep efficiency greater than 50 % on the PSG were considered approved for our protocol and were submitted to comparative analysis. None of the patients had complaints of discomfort or difficulty using the STD.
Statistical Analysis
We calculated the sample size using the method described by Hulley [14]. To have a specified power of 0.80 and type I error rate of 0.05, we assumed that the true correlation would be above 0.50 using a two-sided test and zalfa*/2 of 1.96. The sample size required was of 30 patients.
Statistical analysis was performed using SPSS software (version 17.0 for Windows). The demographic variables were presented as descriptive statistics. For comparisons between groups, Student’s t test was used. The PSG variables that were compared with the STD variables obtained in the laboratory during simultaneous recording and the STD variables obtained at home were analyzed using the general linear model with repeated measures. To determine significance, p values of 0.05 was considered. To evaluate agreement, Bland-Altman graphics, intraclass correlation coefficients, and kappa coefficients between the AHI methods (PSG, STDLab, and STDHome) were determined.
The sensitivity, specificity, negative and positive predictive values (AHI = 5, 15, and 30), and receiver operating characteristic (ROC) curves of the AHI of STDLab and STDHome were calculated using the AHI that was obtained from the PSG.
Diagnostic Agreement
Agreement was defined according to Santos-Silva [15] and showed in Table 1. The rates of diagnostic agreement and disagreement were calculated in a manner similar to that described by White and colleagues [16], with one modification: while those authors used an AHI value ≥40 as the cutoff for severe OSA in their assessment of diagnostic agreement, we used an AHI ≥30 as the generally accepted cutoff for severe OSA.
Results
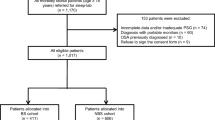
A total of 58 obese patients with high clinical suspicion of OSA were selected. Sixty-seven percent had high blood pressure controlled with medications, 27 % had diabetes, and all of them had high risk for OSA based on the Stop-Bang questionnaire results. After randomization was completed and patient records were obtained, 55 % of the sample exhibited PM records of acceptable quality and were included in the final analysis (Fig. 1).
The basal characteristics of the patients who were included and excluded on the basis of PM record quality are compared in Table 2. There were no statistically significant differences between the groups, and both groups were normally distributed.
The following intraclass coefficients of correlation, including all AHI values, exhibited statistically significant differences: AHI_PSG vs. AHI_STDLab (r = 0.92, [CI = 0.83–0.95] p = 0.0001), AHI_PSG vs. AHI_STDHome (r = 0.84, [CI = 0.69–0.92] p = 0.0001), and AHI_STDLab vs. AHI_STDHome (r = 0.85, [CI = 0.71–0.92] p = 0.0001).
Table 3 describes the Kappa index between the investigated methods according to the levels of severity. We found better concordance in the severe cases and a tendency toward better concordance when the test was performed simultaneously with PSG.
PM exhibited better diagnostic accuracy with high sensitivity in the severe cases. Specificity was good at the investigated cutoff points (Table 4).
A Bland-Altman visual analysis showed that PM had good agreement with PSG. We detected less variability of the AHI when both tests were performed simultaneously (Fig. 2). However, the patients always exhibited higher AHI values with PSG compared with PM.
According to the established analyses criteria of agreement, PM exhibited better agreement with PSG (87 %) when both tests were performed simultaneously in the lab (AHI_PSG vs. AHI_STDLab) than when PM was performed at home (65 %) (Table 5).
Discussion
Our results showed that although a significant number of PM records were lost, the test demonstrated good diagnostic accuracy for OSA in morbidly obese patients who were candidates for bariatric surgery. For the patients who exhibited a high probability of having OSA, PM was especially accurate when diagnosing severe cases and when the test was performed simultaneously with PSG.
Obesity is related to abnormalities in pulmonary function that worsen with recumbence. Excessive weight leads to increased airway resistance, reduced lung volume and impaired respiratory muscle function, especially during sleep; these abnormalities can affect the pulmonary gas exchange and lead to hypoxemia and hypercapnia [17]. The respiratory pattern of obese individuals becomes quite similar to the pattern observed in patients with chronic obstructive pulmonary disease (COPD) [18]. These factors could explain the loss of oximetry records in the present study. In a similar study that included patients with COPD plus OSA, Oliveira et al. [18] also found high record losses.
The present study showed similar losses in the tests performed with PM at home and the tests with PM that were performed at the sleep laboratory. It is important to emphasize that the tests performed at the laboratory were conducted for comparative purposes; technicians did not interfere, and online monitoring was not performed. Most of the losses were caused by problems with oximetry (50 %) and flow (31 %). The present losses were greater than the losses that have been previously reported in the literature, which vary from 3 to 18 % when PM is used in patients with OSA who have no comorbidities [19–21]. Santos-Silva et al. [15] used the same PM device to study patients with no comorbidities who exhibited a high clinical probability of OSA and had the same age range, but had lower BMI (28 ± 5 kg/m2); that study found that the PM results had good agreement with PSG for OSA diagnosis and exhibited a loss of approximately 10 % [19–21]. However, using the Stardust in patients with OSA plus COPD, Oliveira et al. [18] reported a loss of 61 % of the tests, which corroborates our results by showing that PM exhibits limitations in patients with comorbidities. Because no difference was observed in the present study between the analyzed and excluded patients with regard to demographic data and AHI severity, part of the losses may have been caused by the lower sensitivity of the Stardust oximeter compared with the oximeter used in the PSG.
A Bland-Altman analysis showed considerable variability in AHI results obtained from the PSG and STDHome tests. The mean difference value was 15.2, indicating an underestimation of AHI values. An analysis of the variability of the portable records showed that STDLab had fewer lost results compared with PM performed at home. Considering two standard deviations, the Bland-Altman plot reflects high variability in the literature [15, 22] and in our study.
However, by applying the criteria described by White et al. [16], we found a good rate of agreement, especially when the records were performed simultaneously. There was 87 % agreement when PM and PSG were performed on the same night; the underestimation rate was 10 %, and the overestimation rate was only 3 %. When the test was performed at home, it resulted in an OSA underdiagnoses rate of 32 %, which is higher than the rates obtained in the laboratory by Santos-Silva [15] (5 %) and White [16] (6.7 %). This result agrees with the Bland-Altman plot; an assessment of the portable monitoring system’s variability showed that the tests performed at the laboratory were better able to diagnose positive cases compared with the tests performed at home.
The lack of monitoring sleep characteristics like electroencephalogram and electromyogram using the PM when compared with the PSG may explain some of the divergent underdiagnoses results.
Limitations should be considered in the present study. One of the most important limitations comes from the final low number of valid patients. The second limitation concerns the selection of the participants, who were recruited at a single center and exhibited a high clinical probability of OSA. Third, the PM algorithm is associated with the possibility of losing records, which was more likely for the oximetry than for airflow. Fourth, we used the results of a single night when determining the success of the records. Addressing these limitations may require the authors to change their method for managing these patients, for example, by instituting repeated (e.g., two consecutive nights) home portable monitoring study nights and evaluating the use of other type III devices.
In conclusion, PM is an efficacious method for diagnosing OSA in obese patients who have a high clinical probability of the disease. The method displays good sensitivity and specificity in severe cases. It exhibits some advantages over PSG, as the patients can remain in their home environment and avoid the effects of spending a night at the laboratory. Additionally, the method represents simple, low-cost, and efficacious technology. Nevertheless, the high rate of data loss must be taken into account, and we were unable to anticipate the factors that were associated with record loss. Extreme obesity may remain a limiting factor for this test. The future development of domiciliary systems is promising, although new technologies for diagnosing OSA must be critically assessed.
References
Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–43. doi:10.1513/pats.200709-155MG.
Tufik S, Santos-Silva R, Taddei JA, et al. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–6. doi:10.1016/j.sleep.2009.10.005.
Lesser DJ, Haddad GG, Bush RA, et al. The utility of a portable recording device for screening of obstructive sleep apnea in obese adolescents. J Clin Sleep Med. 2012;8(3):271–7. doi:10.5664/jcsm.1912.
Carneiro G, Florio RT, Zanella MT, et al. Is mandatory screening for obstructive sleep apnea with polysomnography in all severely obese patients indicated. Sleep Breath. 2012;16(1):163–8. doi:10.1007/s11325-010-0468-7.
Chai-Coetzer CL, Antic NA, Rowland LS, et al. Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: a randomized trial. Jama. 2013;309(10):997–1004. doi:10.1001/jama.2013.1823.
Oliveira MG, Garbuio S, Treptow EC, et al. The use of portable monitoring for sleep apnea diagnosis in adults. Expert Rev Respir Med. 2014;8(1):123–32. doi:10.1586/17476348.2014.850421.
Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important. Curr Opin Anaesthesiol. 2009;22(3):405–11. doi:10.1097/ACO.0b013e32832a96e2.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5.
Hori T, Sugita Y, Koga E, et al. Sleep Computing Committee of the Japanese Society of Sleep Research Society. Proposed supplements and amendments to ’A Manual of StandardizedTerminology, Techniques and ScoringSystem for Sleep Stages of HumanSubjects’, the Rechtschaffen & Kales (1968) standard. Psychiatry Clin Neurosci. 2001;55(3):305–10.
Rahaghi F, Basner RC. Delayed diagnosis of obstructive sleep apnea: don’t ask don’t tell. Sleep Breath. 1999;3(4):119–24.
American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–84.
American Sleep Disorders Association Atlas Task Force. Recording and scoring leg movements. Sleep. 1993;16(8):748–59.
American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–89.
Hulley SB et al. Designing clinical research: an epidemiological approach. Second Edition. Philadelphia: Lippincott Williams & Wilkins; 2001.
Santos-Silva R, Sartori DE, Truksinas V, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32(5):629–36.
White DP, Gibb TJ, Wall JM, et al. Assessment of accuracy and analysis time of a novel device to monitor sleep and breathing in the home. Sleep. 1995;18(2):115–26.
Polese JF, Santos-Silva R, Kobayashi RF, et al. Portable monitoring devices in the diagnosis of obstructive sleep apnea: current status, advantages, and limitations. J Bras Pneumol. 2010;36(4):498–505.
Oliveira MG, Nery LE, Santos-Silva R, et al. Is portable monitoring accurate in the diagnosis of obstructive sleep apnea syndrome in chronic pulmonary obstructive disease. Sleep Med. 2012;13(8):1033–8. doi:10.1016/j.sleep.2012.06.011.
Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–47.
Man GC, Kang BV. Validation of a portable sleep apnea monitoring device. Chest. 1995;108(2):388–93.
Yin M, Miyazaki S, Ishikawa K. Evaluation of type 3 portable monitoring in unattended home setting for suspected sleep apnea: factors that may affect its accuracy. Otolaryngol Head Neck Surg. 2006;134(2):204–9.
de Oliveira AC T, Martinez D, Vasconcelos LF, et al. Diagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoring. Chest. 2009;135(2):330–6. doi:10.1378/chest.08–1859.
Acknowledgments
We would like to thank Laura Castro, MsC, and Altay Souza, PhD, for their valuable suggestions and statistical analyses.
Conflict of Interest
Márcia G. Oliveira, Erika C. Treptow, Cesar Fukuda, Luiz E. Nery, Rosana M. Valadares, Sérgio Tufik, Lia Bittencourt, and Sonia M. Togeiro had no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira, M.G., Treptow, E.C., Fukuda, C. et al. Diagnostic Accuracy of Home-Based Monitoring System in Morbidly Obese Patients with High Risk for Sleep Apnea. OBES SURG 25, 845–851 (2015). https://doi.org/10.1007/s11695-014-1469-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1469-6