Abstract
Obesity is linked to the development of cancer. Previous studies have suggested that there is a relationship between bariatric surgery and reduced cancer risk. Data sources were from Medline, Embase, and Cochrane Library. From 951 references, 13 studies met the inclusion criteria (54,257 participants). In controlled studies, bariatric surgery was associated with a reduction in the risk of cancer. The cancer incidence density rate was 1.06 cases per 1000 person-years within the surgery groups. In the meta-regression, we found an inverse relationship between the presurgical body mass index and cancer incidence after surgery (beta coefficient −0.2, P < 0.05). Bariatric surgery is associated with reduced cancer risk in morbidly obese people. However, considering the heterogeneity among the studies, conclusions should be drawn with care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Data from the National Health and Nutrition Examination Survey (NHANES) shows that the prevalence of obesity in adults is increasing every year in the USA [1], as well as worldwide [2]. Since 1960, the prevalence of adult obesity in the USA has nearly tripled, from 13 % in 1960–1962 to 36 % during 2009–2010. While US obesity rates have, overall, leveled off since 2003, the rates are still rising in some groups, and disparities persist: Non-Hispanic black, Hispanic, and Mexican American adults have higher rates of obesity than non-Hispanic white adults [3]. Severe obesity shortens life expectancy, is a significant cause of morbidity and mortality, and raises healthcare expenditures in North America [1]. In addition, obesity and weight gain increase the risk for several diseases, including cancer [4], and can lead to poor treatment outcomes and increased cancer-related mortality [5, 6]. The association between obesity and cancer risk involves metabolic and endocrine effects of obesity that induce the production of peptide and steroid hormones [7].
The treatment of obesity includes lifestyle changes (nutritional education, behavioral counseling, physical activity), pharmacological agents, and, in severe obesity, bariatric surgery [8, 9]. Bariatric surgery has been shown to produce significant long-term weight loss [10] and reduction of mortality rates [11] for patients with severe obesity. Although obesity is clearly linked to the development of cancer, to the best of our knowledge, it remains unclear whether weight loss obtained through bariatric surgery influences cancer incidence. It is also unknown if a relationship exists between body mass index (BMI) before surgery and cancer risk in the postoperative period. Previous studies suggest that there is a relationship between bariatric surgery and reduced cancer risk [12, 13]. A retrospective study, authored by McCawley et al.[12], that analyzed 1,482 women who underwent bariatric surgery found breast and endometrial cancers as the most prevalent cancers in their cohort and suggested that bariatric surgery may have decreased the development of cancer among these women. However, it is unknown whether the lower cancer rates following bariatric surgery were related to the metabolic changes associated with weight loss, or if lower BMIs following surgery resulted in earlier diagnosis and improved cancer treatment outcomes [14].
Accordingly, in order to summarize the relationship between postoperative weight loss and its potential association with cancer, we conducted a systematic review with a meta-analysis focused on the incidence of cancer in patients following bariatric surgery.
Methods
Protocol and Registration
This systematic review is reported in accordance with the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [15].
Eligibility Criteria
Studies were selected if they included both a cancer diagnosis following bariatric surgery as well as the following eligibility criteria: patients ≥18 years of age who were measured with a BMI ≥ 35 kg/m2. We tabulated all cancer cases as well as the adequacy of controls related to our eligibility criteria. Exclusion criteria for studies included patients who were diagnosed with cancer in the pre- or perioperative periods.
Information Sources
We searched the following electronic databases covering studies performed through January of 2014: MEDLINE (accessed by PubMed), EMBASE, and the Cochrane Central Register of Controlled Trials. We did not use limits for dates or language in conducting our search.
Search Strategy
We searched the terms abdominal fat / obesity, morbid / obesity / obesity, abdominal / adiposity / weight gain / intra-abdominal fat / overnutrition / bariatric surgery / bariatrics / gastroplasty / gastric balloon / gastric bypass / anastomosis, Roux-en-Y / biliopancreatic diversion / jejunoileal bypass / bariatric medicine / gastric banding / laparoscopic adjustable gastric banding / cancer and related terms. We used the search strategy that PubMed employed to create its cancer subset (see Appendix: PubMed search strategy).
Study Selection
Two reviewers independently analyzed the titles and abstracts retrieved from our literature search. All articles that failed to meet the inclusion criteria were excluded. All selected articles were analyzed, and eligible articles were identified. Disagreements between reviewers were resolved by a third reviewer’s opinion.
Data Extraction and Quality Assessment
Two reviewers independently extracted data from each study. Extracted data were as follows: year of publication, authors, geographic location of the first author, study design, the type of bariatric procedure, sample size, participant characteristics, cancer incidence, type of cancer, postsurgical period during which cancer was diagnosed, and deaths related to cancer. A third reviewer assessed all studies for completeness of criteria.
To avoid double counting patients who were included in multiple reports authored by the same individuals or working groups, patient recruitment periods were assessed and, if necessary, authors were contacted to provide clarity. We also contacted authors in cases where data relevant to our study were omitted from their publications.
The Newcastle-Ottawa Scale was adapted for our study, and it was used independently by two reviewers to assess the quality of the studies [16]. Assessment of the quality of nonrandomized studies, including case-control and cohort studies, is essential for properly understanding them. The Newcastle-Ottawa Scale [16] was developed to assess the quality of nonrandomized studies with its design, content, and ease of use directed to incorporate quality assessments in the interpretation of meta-analytic results. The scale allocates stars (maximum of nine), for quality of selection, comparability, exposure, and outcome of study participants.
Data Synthesis and Analysis
We calculated a pooled odds ratio (OR) to assess the association between incident cancer and bariatric surgery in the controlled studies. We used the random effects model in our meta-analyses, because we found significant heterogeneity among the studies.
We also used the surgery group from the controlled and uncontrolled studies to estimate the incidence density rate of cancer following bariatric surgery. To estimate this incidence density rate, we multiplied the total sample size by the follow-up period to calculate the denominator. We made logit transformations to address the asymmetrical distribution and weighted the logit model by the inverse of the logit’s variance. Also, we explored heterogeneity between studies by removing each study from the analysis to check if any particular study drove heterogeneity. Moreover, we performed predefined subgroup analyses according to age (dichotomized in two groups, <45.0 and >45.1 years old), and gender (dichotomized in two groups, <79.9 and >80.0 % of women), to assess if these variables were potential sources of heterogeneity. Furthermore, we performed meta-regression analyses to investigate other potential sources of heterogeneity. The variables used in this analysis were BMI and the time frame for a follow-up examination when cancer was diagnosed.
In our meta-analyses, we used the Cochran χ 2 and the I 2 tests to evaluate heterogeneity among studies. A P value below 0.05 was considered significant in the Cochran test [17]. In the I 2 test, values below 25 % reflected low heterogeneity, values between 25 and 50 % reflected moderate heterogeneity, and values exceeding 50 % reflected high heterogeneity [18].
We performed statistical analyses with Stata 11.0 software (Stata Corp, College Station, TX), and we used the second version of Comprehensive Meta-analysis™ software for our incidence meta-analysis.
Results
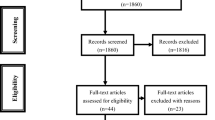
Overall, we identified 1,022 studies, from which we excluded 70 duplicates. After initial title and abstract screening, we excluded 823 citations, leaving 129 articles for retrieval. Full text assessment of these articles resulted in 21 eligible studies [10, 14, 19–33]. In cases where results for the same population were reported more than once, we selected the most recent and complete results, thus excluding eight additional articles [10, 14, 25, 27, 32–35]. Among the retrieved studies, there were no randomized clinical trials. We separated the remaining 13 articles, published between 1997 and 2011, into two groups: (1) controlled studies [12, 13, 19, 20] and (2) uncontrolled studies [21–24, 26, 28–31]. We excluded three uncontrolled studies from the meta-analysis [21, 29, 31] because these articles reported deaths caused by cancer but did not provide cancer incidence. Agreement between reviewers was estimated by the Kappa coefficient (Kappa = 0.835). Finally, we show a flowchart of study search and selection in Fig. 1.
Studies’ Characteristics
Table 1 shows the characteristics of four selected controlled studies, which included 11,087 patients who underwent bariatric surgery (surgery group) and 20,720 patients who did not undergo surgery (control group). Two of these studies were performed in the USA [12, 19], one in Canada [20], and one in Sweden [13]. The percentage of cancer-related mortality was reported in one study [19], totaling 0.62 % in the surgery group and 1.13 % in the control group.
Table 2 shows the characteristics of nine selected uncontrolled studies, which included 22,450 individuals in the surgery group. Five of these studies were performed in the USA [23, 24, 28, 30, 31], two in Sweden [22, 26], one in Australia [21], and one in Switzerland [29]. The percentage of cancer-related mortality ranged from 0.26 to 2.65 % in four studies [21, 23, 29, 31], and five studies did not report mortality [22, 24, 26, 28, 30].
Association Between Cancer Risk and Bariatric Surgery
Controlled studies showed that bariatric surgery is associated with a reduction in the risk of cancer (Fig. 2; OR 0.42; 95 % confidence interval [CI] 0.24, 0.73; I 2 = 93.3 %; P for heterogeneity <0.001). In an exploratory attempt to identify sources of heterogeneity among these studies, we removed one study [12] which was performed solely with women, though the results of this study were not different from the other studies (OR 0.53; CI 0.32, 0.88; I 2 = 91.5 %; P for heterogeneity = 0.014). When we removed another study [20], in which the control group was taken from a hospital population, no heterogeneity was observed and the association between bariatric surgery and low cancer risk was maintained (OR 0.74; CI 0.65, 0.85; I 2 = 0 %; P for heterogeneity < 0.512). The strategy above displays the high heterogeneity of the primary meta-analysis. After removing each study that eliminated heterogeneity, there was no need to perform sensitivity analysis or meta-regression in the controlled studies.
The cancer incidence density rate was 1.06 cases per 1,000 person-years (CI 0.64, 1.75 cases per 1,000 person-years; I 2 = 96.0 %; P for heterogeneity < 0.001) measured across nine studies, utilizing the surgery groups from the controlled and uncontrolled studies (Fig. 3). We know that this estimate is low, as the years subsequent to the follow-up period for cancer patients should be omitted from the denominator. Therefore, the real denominator should be lower than the value we used. To assess this impact on our results, we assumed that the cancer patients did not contribute to the denominator (the denominator was calculated by subtracting the number of cancer cases from the total number of patients, and then multiplying this difference by the average follow-up period, implying that cancers were diagnosed at follow-up times equal to zero). Using this method to calculate the denominator, the cancer incidence density was 1.08 cases per 1,000 person-years (CI 0.65, 1.80 cases per 1,000 person-years), and this implies that the real value of incidence is between 1.06 and 1.08 person-years. We excluded one study [12] in the meta-analysis that did not report data over the follow-up period.
We performed sensitivity analyses to explore heterogeneity, and we observed no heterogeneity upon removing each study. The results did not change when analyzed by age or gender in either surgery group both the controlled and uncontrolled studies, since these analyses resulted in no change in heterogeneity. In an additional attempt to identify sources of heterogeneity across the studies, we performed a meta-regression analysis using BMI and the time period to cancer diagnosis as covariates for the surgery group in both the controlled and uncontrolled studies. We found no difference in the results of our meta-regression that used time to cancer diagnosis as a covariate. However, in our meta-regression using the BMI as a covariate, we found a decrease in cancer incidence as BMI increased (beta coefficient −0.233, P < 0.05).
Quality Assessment
Although we achieved a reasonable degree of quality through our inclusion/exclusion criteria, we do note some variation between the quality assessments [16]. Based on a sample selection, three studies received four-star ratings [12, 13, 20], four studies received three-star ratings [19, 23, 24, 28], three studies received two-star ratings [26, 29, 30], and three studies received one-star ratings [21, 22, 31]. With respect to comparability, three studies received two-star ratings [13, 19, 20], one study received a one-star rating [12], and nine uncontrolled studies received no stars. This variation was due to differences in accounting for confounding factors. With respect to assessment quality, six studies received two-star ratings [21, 23, 24, 28, 30, 31] and seven studies received three-star ratings [12, 13, 19, 20, 22, 26, 29]. In total, four studies received high quality ratings of seven to nine stars [12, 13, 19, 20], five studies received medium quality ratings of five to six stars [23, 24, 26, 28, 29] and four studies received low quality ratings of four stars or below [21, 22, 30, 31] (Table 3).
Discussion
Our systematic review demonstrates that bariatric surgery is associated with a reduction in the incidence of cancer among morbidly obese patients. Importantly, this effect of bariatric surgery was found within both controlled and uncontrolled studies. Of the 13 studies included in our analyses, four controlled studies showed a significant reduction in the risk of cancer, with ORs ranging from 0.12 to 0.88. Data from the surgery group of controlled and uncontrolled studies displayed a cancer incidence density rate of 1.06 cases per 1,000 person-years in a postoperative follow-up period of 2 to 23 years.
Cancer rates in obese people are generally higher, as displayed in a study [36] of an Austrian population (5.43 cases per 1,000 person-years) and in a study [37] of a Swedish population (5.8 cases per 1,000 person-years), as well as in a systematic review and meta-analysis [38] of data from a global population (2.12 cases per 1,000 person-years). Therefore, the cancer incidence density rate of 1.06 cases per 1,000 person-years obtained in our meta-analysis displays that severely obese individuals undergoing bariatric surgery may reduce their risk of cancer to incidence density rates similar to those of nonobese people [38].
Reducing weight likely reduces high cancer rates associated with obesity [39], but the method employed to achieve target weight can change this perspective. For example, trials that used specific pharmacological interventions [40, 41] achieved weight reduction, but mortality/cardiovascular outcomes were increased, revealing the need for treatments that obtain both goals: weight loss and lower mortality rates. Moreover, no cancer incidence reduction was observed in population-wide weight reduction incidents, which were imposed by dietary restrictions in nonseverely obese people [42].
Although our meta-analysis showed that patients undergoing bariatric surgery have reduced cancer rates, a recent study [33] showed an increased risk of colorectal cancer following bariatric surgery. This study was not included in our meta-analysis due to duplicate reporting, and it is not clear that the mechanisms associated with bariatric surgery increase the colorectal cancer. However, rectal mucosal changes related to malabsorptive effects of the gastric bypass procedure could account for theses findings, and this study should stimulate research addressing colonoscopic evaluation following bariatric surgery [33].
Data from three cohorts showed that intentional (nonsurgical) weight loss is associated with lower cancer rates [39], but it is likely that multiple methods were used to achieve targeted weight loss in this study. Thus, it is difficult to draw precise conclusions about the most efficacious nonsurgical weight loss methods. Our results show that reducing body weight through bariatric surgery is associated with reduced cancer incidence rates, though we did not find mortality data specifically linked to cancer. Our findings are supported by Birks’ meta-analysis of studies that showed an association between lower cancer rates and people who intentionally lost weight through bariatric surgery [39]. Moreover, when examining the relationship between bariatric surgery and cancer rates, it is challenging to separate the effects of the surgery from the multiple associated changes it yields.
It is important to consider that bariatric surgery is more frequently performed on young subjects [43], while cancer is more frequently observed in aged people [44]. Moreover, the long lead time for the appearance of cancer cases may be the primary reason why there are very few studies associating bariatric surgery and cancer. However, obesity is a risk factor for cancer development. Thus, obesity likely precedes the diagnosis of several cancer cases [36]. Performing a surgical procedure of the magnitude of bariatric surgery raises awareness and possible diagnosis of cancer among these patients [13].
It remains unknown whether metabolic changes related to weight loss result in lower rates of cancer development. It is also unknown if a lower BMI simply allows for better assessment and treatment, or if it is actually related to lower cancer incidence. These data are supported by well-known pathophysiological disturbances related to obesity, such as chronic inflammation [45] and hormonal changes [7]. Pro-inflammatory cytokines act on tissues and cells, resulting in cancer development through direct and indirect effects on innate and adaptive immune cells, imbalances in tissue homeostasis, and increased oxidative stress [46]. People with obesity have increased estrogen levels, due to the conversion of circulating androgens by increased aromatase activity in peripheral adipocytes, increased adrenal and ovarian secretion of hormones, and decreased progesterone production due to decreased ovulation [7]. In addition, increased insulin levels cause the inhibition of sex hormone-binding globulin synthesis by the liver, increasing free steroid hormone levels. Overall, the increased unopposed estrogen can promote cancer in hormonally responsive tissues [7, 47]. Therefore, in both obese and cancer populations, there is an established mechanistic association between inflammation and hormonal imbalance [48]. Reversal of these inflammatory and hormonal disturbances should be expected with weight loss from bariatric surgery, as decreases in both oxidative stress and systemic inflammatory markers were reported [49], and there is consistent evidence that the incidence of cancer is reduced [39, 50]. Beyond obesity risk, the excess of visceral adiposity, type 2 diabetes, insulin resistance, chronic inflammation, and metabolic syndrome are all associated with cancer, independent of body size [51].
A strength of our systematic review is its inclusion of uncontrolled studies, adding information to the research provided by Birks et al [39]. Traditionally, controlled studies are viewed as providing higher quality evidence than uncontrolled studies. However, the controls for bariatric patients are likely imperfect, as it is difficult to find appropriate matches of their clinical conditions. Thus, patients undergoing bariatric surgery may comprise a medically fitter population as compared to those who did not undergo the surgery.
We used a variety of strategies to explore the high heterogeneity levels observed in our meta-analyses, investigating potential sources of variation among the studies. In the controlled studies, we accounted for heterogeneity by removing the study of McCawley et al. [12], which excluded men, and also by removing the study of Christou et al. [20], which used a hospital population as its control group. Upon removing these two studies from the meta-analysis, no heterogeneity was observed (I 2 = 0 %). Although the specific population characteristics of these studies accounted for high heterogeneity, these two studies were strictly inside the previously determined inclusion criteria, and thus, they were included in our primary meta-analysis.
In the surgery group from the controlled and uncontrolled studies, we observed no reduction in heterogeneity upon removing each study or in performing sensitivity analysis. Further, the meta-regression analysis showed a decrease in cancer incidence when high presurgical BMI values were present. This is in accordance with the results of Renehan et al. [38], as their meta-analysis displayed an increased risk of cancer with increased BMI. We would anticipate a larger magnitude of intervention effects in populations with higher presurgical derangements [52]. Finally, the high levels of heterogeneity we observe may be due to different study designs, differences in the populations studied, or differences in a variety of other characteristics (e.g., genetic background, varied bariatric procedures) across the studies. Indeed, it is likely that one or more of these factors which were beyond our control, account for a portion of the heterogeneity across the studies.
Limitations
Limitations are present in this study. In the absence of randomized controlled trials, we dealt with distinct data that expressed cancer incidence and death registries. As is inherent in observational studies, data from registries can be biased, so the information we compiled in this meta-analysis may also have a bias. Therefore, our derived estimates may potentially influence our results. Although such bias would impact both surgical and nonsurgical samples, the control groups were carefully selected in two studies [13, 19]. Due to observational designs, the surgery group may have been healthier than the patients who were not offered surgery and were used as controls. In addition, the generalization of our findings is somewhat hampered by the geographic origin of our included studies, as they were primarily performed in the USA and Europe.
Among the limitations we note in bariatric surgery and cancer literature, we could not explore the potential effects of different bariatric procedures, which promote distinct action mechanisms on various target populations. Likewise, we were unable to obtain BMI and weight data at the time of cancer diagnosis in the studies within our review. Risk factors associated with cancer such as family history, smoking, gender, physical inactivity, alcoholism, and malnutrition were also unavailable. In conjunction with surgery outcomes, we underscore that pre- and post-surgery dietary patterns could contribute to our analyses. Moreover, the number of cancer cases within our review were too low to allow analyses by organ or type, especially those cancers related to obesity and smoking. Finally, the follow-up analysis and documentation following bariatric surgery is often poor, and patients may not disclose a cancer diagnosis during follow-up appointments.
Conclusion
Bariatric surgery is associated with reduced cancer risk in morbidly obese people. Our study is unique, because it is the first publication to include a meta-analysis of cancer incidence following bariatric surgery, providing preliminary validation to a positive association between bariatric surgery and reduced cancer rates. However, considering some of the limitations cited above, conclusions should be drawn with care. We suggest that variables associated with cancer should be measured in prospective bariatric surgery trials, and that cancer rates should be assessed as a primary outcome.
References
Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA : J Am Med Assoc. 2010;303(3):235–41. Epub 2010/01/15.
Rokholm B, Baker JL, Sorensen TI. The levelling off of the obesity epidemic since the year 1999—a review of evidence and perspectives. Obes Rev: Off J Int Assoc Study Obes. 2010;11(12):835–46. Epub 2010/10/27.
May AL, Freedman D, Sherry B, Blanck HM. Obesity—United States, 1999-2010. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2013;62 Suppl 3:120-8. Epub 2013/11/23.
Samanic C, Chow WH, Gridley G, et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17(7):901–9.
Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu Rev Nutr. 2012;32:311–42. Epub 2012/05/01.
Kaidar-Person O, Bar-Sela G, Person B. The two major epidemics of the twenty-first century: obesity and cancer. Obes Surg. 2011;21(11):1792–7. Epub 2011/08/16.
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91.
Eckel RH. Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358(18):1941–50.
Colquitt JL, Picot J, Loveman E, et al. Surgery for obesity. Cochrane Database Syst Rev. 2009;2, CD003641.
Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240(3):416–23. discussion 23–4; Epub 2004/08/21.
Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes (Lond) (2005). 2008;32 Suppl 7:S93–7.
McCawley GM, Ferriss JS, Geffel D, et al. Cancer in obese women: potential protective impact of bariatric surgery. J Am Coll Surg. 2009;208(6):1093–8. Epub 2009/05/30.
Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–62. Epub 2009/06/27.
Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61. Epub 2007/08/24.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA : J Am Med Assoc. 2000;283(15):2008–12.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp[cited 2013 Feb 05] [updated 02/05/2013; cited 2013 Feb 05, 2013]; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. Epub 2003/09/06.
Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity. 2009;17(4):796–802.
Christou NV, Lieberman M, Sampalis F, et al. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis: Off J Am Soc Bariatric Surg. 2008;4(6):691–5. Epub 2008/11/26.
Clough A, Layani L, Shah A, et al. Laparoscopic gastric banding in over 60s. Obes Surg. 2011;21(1):10–7. Epub 2010/05/22.
Forsell P, Hellers G. The Swedish Adjustable Gastric Banding (SAGB) for morbid obesity: 9 year experience and a 4-year follow-up of patients operated with a new adjustable band. Obes Surg. 1997;7(4):345–51. Epub 1997/08/01.
Gagne DJ, Papasavas PK, Maalouf M, et al. Obesity surgery and malignancy: our experience after 1500 cases. Surg Obes Relat Dis: Off J Am Soc Bariatric Surg. 2009;5(2):160–4. Epub 2008/10/14.
Gusenoff JA, Koltz PF, O'Malley WJ, et al. Breast cancer and bariatric surgery: temporal relationships of diagnosis, treatment, and reconstruction. Plast Reconstr Surg. 2009;124(4):1025–32. Epub 2009/11/26.
Marsk R, Freedman J, Tynelius P, et al. Antiobesity surgery in Sweden from 1980 to 2005: a population-based study with a focus on mortality. Ann Surg. 2008;248(5):777–81. Epub 2008/10/25.
Ostlund MP, Lu Y, Lagergren J. Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Ann Surg. 2010;252(6):972–6. Epub 2010/06/24.
Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. Epub 2007/08/24.
Srikanth MS, Fox SR, Oh KH, et al. Renal cell carcinoma following bariatric surgery. Obes Surg. 2005;15(8):1165–70. Epub 2005/10/04.
Steffen R, Potoczna N, Bieri N, et al. Successful multi-intervention treatment of severe obesity: a 7-year prospective study with 96 % follow-up. Obes Surg. 2009;19(1):3–12. Epub 2008/09/17.
Sugerman HJ, Sugerman EL, Wolfe L, et al. Risks and benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234(1):41–6. Epub 2001/07/20.
Sultan S, Gupta D, Parikh M, et al. Five-year outcomes of patients with type 2 diabetes who underwent laparoscopic adjustable gastric banding. Surg Obes Relat Dis: Off J Am Soc Bariatric Surg. 2010;6(4):373–6. Epub 2010/07/16.
Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. JAMA : J Am Med Assoc. 2012;308(11):1132–41. Epub 2012/09/20.
Derogar M, Hull MA, Kant P, et al. Increased risk of colorectal cancer after obesity surgery. Ann Surg. 2013;258(6):983–8. Epub 2013/03/09.
Burza MA, Pirazzi C, Maglio C, et al. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig liver Dis : Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2012;44(12):1037–41. Epub 2012/06/19.
Maglio C, Ericson U, Burza MA, et al. The IRS1 rs2943641 variant and risk of future cancer among morbidly obese individuals. J Clin Endocrinol Metab. 2013;98(4):E785–9. Epub 2013/02/19.
Rapp K, Schroeder J, Klenk J, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93(9):1062–7. Epub 2005/10/20.
Lukanova A, Bjor O, Kaaks R, et al. Body mass index and cancer: results from the Northern Sweden Health and Disease Cohort. Int J Cancer J int Cancer. 2006;118(2):458–66. Epub 2005/07/29.
Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. Epub 2008/02/19.
Birks S, Peeters A, Backholer K, et al. A systematic review of the impact of weight loss on cancer incidence and mortality. Obes Rev: Off J Int Assoc Study Obes. 2012;13(10):868–91. Epub 2012/06/08.
Topol EJ, Bousser MG, Fox KA, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet. 2010;376(9740):517–23. Epub 2010/08/17.
James WP, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–17. Epub 2010/09/08.
Franco M, Bilal U, Ordunez P, et al. Population-wide weight loss and regain in relation to diabetes burden and cardiovascular mortality in Cuba 1980-2010: repeated cross sectional surveys and ecological comparison of secular trends. BMJ. 2013;346:f1515. Epub 2013/04/11.
DeMaria EJ, Pate V, Warthen M, et al. Baseline data from American Society for Metabolic and Bariatric Surgery-designated bariatric surgery centers of excellence using the bariatric outcomes longitudinal database. Surgery Obes Relat Dis: Off J Am Soc Bariatric Surg. 2010;6(4):347–55. Epub 2010/02/24.
Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA: Cancer J Clin. 2010;60(5):277–300. Epub 2010/07/09.
Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. Epub 2006/04/15.
de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. Epub 2006/01/07.
Modesitt SC, Huang B, Shelton BJ, et al. Endometrial cancer in Kentucky: the impact of age, smoking status, and rural residence. Gynecol Oncol. 2006;103(1):300–6. Epub 2006/04/25.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. Epub 2002/12/20.
Ashrafian H, Ahmed K, Rowland SP, et al. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer. 2011;117(9):1788–99. Epub 2011/04/22.
Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women's Health Study. Int J Obes Relat Metab Disord: J Int Assoc Study Obes. 2003;27(12):1447–52. Epub 2003/11/25.
Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–11. Epub 2012/10/25.
Unick JL, Beavers D, Jakicic JM, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care. 2011;34(10):2152–7. Epub 2011/08/13.
Acknowledgments
This study was supported by a grant from The Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES). We thank Dr. Lars Sjostrom, who provided complementary data from his study. We also thank Dr. Rodrigo Ribeiro, who helped with our statistical analyses.
Conflict of Interest
The authors have no potential conflicts of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials disclosed in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Casagrande, D.S., Rosa, D.D., Umpierre, D. et al. Incidence of Cancer Following Bariatric Surgery: Systematic Review and Meta-analysis. OBES SURG 24, 1499–1509 (2014). https://doi.org/10.1007/s11695-014-1276-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1276-0